Self-assembly and hydrogelation from multicomponent coassembly of pentafluorobenzyl-phenylalanine and pentafluorobenzyl-diphenylalanine†
Shu-Min Hsu,
Fang-Yi Wu,
Tsung-Sheng Lai,
Yu-Chun Lin and
Hsin-Chieh Lin*
Department of Materials Science and Engineering, National Chiao Tung University, Hsinchu, 300, Taiwan, Republic of China. E-mail: hclin45@nctu.edu.tw; Fax: +886-3-5724727; Tel: +886-3-5731949
First published on 23rd February 2015
Abstract
To screen the possibility of forming self-assembled hydrogels under physiological pH, various molar ratios of the hydrogelators based on pentafluorobenzyl-phenylalanine (PFB-F) and pentafluorobenzyl-diphenylalanine (PFB-FF) were studied. The equimolar ratio of PFB-F and PFB-FF formed coassembled nanofibers and a self-supporting hydrogel at the physiological pH of 7.4. The spectroscopic characterization of the blend gel indicates that π–π interactions and hydrogen-bonding interactions are the major driving forces behind the coassembly. In addition, we also prove that the blend hydrogel is biocompatible, thus making it a scaffolding material for biomedical applications. In this work, the utility of two distinct hydrogelators to promote coassembly under physiological condition expands the repertoire of noncovalent interactions that can be used in the development of sophisticated noncovalent biomaterials.
Self-assembled hydrogels provide useful nano-sized scaffolds for biomedical applications.1–5 Amino-acid derivatives and peptides are attractive in this regard, as they mimic certain aspects of the natural extracellular matrix, such as their nanofibrous features and high hydration.4 A hydrogel self-assembled from aromatic peptide amphiphiles is an ideal candidate material for the development of smart, soft and nano-sized biomaterials due to its synthetic customizability,5 and it has been used in the applications of tissue scaffolding,6,7 cell culture,8 drug delivery,9,10 and wound healing.11,12 Recently, more efforts have been paid to fabricate self-assembled hydrogels by mixing two or more aromatic peptide amphiphiles to adjust their mechanical and morphological properties.13,14 For example, Ulijn et al. have demonstrated the coassembled peptide materials combine functional and structural components and are potentially useful as tunable matrices for protein immobilization that can be exploited in biocatalysis.13 Nilsson et al. have proven that equimolar mixtures of Fmoc-Phe and Fmoc-F5-Phe, which possess side-chain aromatic groups with complementary quadrupole interactions, readily coassemble to form two-component hydrogels where Fmoc-Phe alone fails to self-assemble.14 In this work, we report that the equimolar ratio of PFB-F and PFB-FF efficiently forms coassembled nanofibers and hydrogels under the physiological pH of 7.4. In addition, the study of cell survival ratio suggests the blend hydrogel is biocompatible, thus making it a potentially useful scaffolding material for biomedical applications.
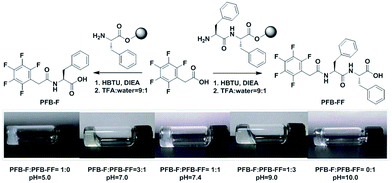
Molecules of PFB-F and PFB-FF have been synthesized by solid phase peptide synthesis (SPPS) and their chemical structures are shown in Fig. 1.3 The self-assembled hydrogels were prepared by a sequential change in pH. The 1 wt% hydrogels of PFB-F and PFB-FF efficiently formed at pH 5.0 and 10.0, respectively. To explore characteristics of PFB-capped hydrogels, we have investigated sol–gel transition of PFB-F and PFB-FF with various pH values (pH = 3–10), concentrations (0.25–2 wt%) and blend ratios (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) (see Fig. S1† for details). We found that all the hydrogels with different blend ratios formed at 1 wt% and their gel images of PFB-capped hydrogels were shown in Fig. 1. For further study, we chose the blend gel in each case with a specific pH value at 1 wt% because of its shorter gelation time compared with those of different pH values (Fig. 1). Notably, the gelation times for different blend ratios were measured which reveal that the co-assembling hydrogelations are much time-consuming than those of single components (Fig. S2†). The appearances of the hydrogels are transparent for a 3
0) (see Fig. S1† for details). We found that all the hydrogels with different blend ratios formed at 1 wt% and their gel images of PFB-capped hydrogels were shown in Fig. 1. For further study, we chose the blend gel in each case with a specific pH value at 1 wt% because of its shorter gelation time compared with those of different pH values (Fig. 1). Notably, the gelation times for different blend ratios were measured which reveal that the co-assembling hydrogelations are much time-consuming than those of single components (Fig. S2†). The appearances of the hydrogels are transparent for a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 or 0
3 or 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 blend of PFB-F and PFB-FF, while semi-translucent for pure PFB-F. Interestingly, the increase of the molar ratio of PFB-FF in the mixture would result in higher pH value for efficient hydrogelation (Table 1). Importantly, we found that an equimolar mixture of PFB-F and PFB-FF could efficiently coassemble to form a self-assembled hydrogel under physiological pH.
1 blend of PFB-F and PFB-FF, while semi-translucent for pure PFB-F. Interestingly, the increase of the molar ratio of PFB-FF in the mixture would result in higher pH value for efficient hydrogelation (Table 1). Importantly, we found that an equimolar mixture of PFB-F and PFB-FF could efficiently coassemble to form a self-assembled hydrogel under physiological pH.
PFB-F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFB-FFa PFB-FFa |
Appr.b (pH) | G′, G′′ (Pa) | Critical strain (%) | Fiber diameter (nm) |
|---|---|---|---|---|
a PFB-F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PFB-FF: a blend of PFB-F and PFB-FF.b TG: transparent gel; SG: semi-translucent gel. PFB-FF: a blend of PFB-F and PFB-FF.b TG: transparent gel; SG: semi-translucent gel. |
||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
SG (5.0) | 3.0 × 103, 1.5 × 102 | 0.63 | 31.0 ± 5 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
TG (7.0) | 2.8 × 103, 5.5 × 102 | 0.57 | 14.7 ± 3, 6.2 ± 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
SG (7.4) | 3.0 × 103, 2.0 × 102 | 0.71 | 8.3 ± 2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
TG (9.0) | 2.6 × 104, 4.5 × 103 | 0.64 | 7.7 ± 2 |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
TG (10.0) | 3.4 × 104, 8.0 × 103 | 0.89 | 5.0 ± 1 |
The microscopic nanostructures of the PFB-capped hydrogels were measured by transmission electron microscopy (TEM) and presented in Fig. 2. The TEM analysis indicated that the PFB-capped hydrogels consisted of a fibrous network and these nanofibers entangle to trap water in the gel. The PFB-F gel showed a thicker fiber diameter (31.0 nm) among the hydrogels, while the PFB-FF with the relatively thinner fiber diameter (Table 1). A 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 blend of PFB-F and PFB-FF leads to the formation of uneven nanofibers with the diameters of 14.7 and 6.2 nm. Notably, the presence of two types of fibrous nanostructures in the gel of a 3
1 blend of PFB-F and PFB-FF leads to the formation of uneven nanofibers with the diameters of 14.7 and 6.2 nm. Notably, the presence of two types of fibrous nanostructures in the gel of a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 blend of PFB-F and PFB-FF may be attributed to the partial co-assembling behavior of PFB-F and PFB-FF. For most of the molecules, the nanoscale self-sorting is possible since multi-gelator gels may independently assemble their own fibrous nanostructures in the mixture as reported recently by Smith et al.15,16 Furthermore, when increasing the ratio of PFB-FF (1
1 blend of PFB-F and PFB-FF may be attributed to the partial co-assembling behavior of PFB-F and PFB-FF. For most of the molecules, the nanoscale self-sorting is possible since multi-gelator gels may independently assemble their own fibrous nanostructures in the mixture as reported recently by Smith et al.15,16 Furthermore, when increasing the ratio of PFB-FF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), the TEM images showed uniform nanostructures with a homogeneous diameter of ∼8 nm (Fig. 2c and d and Table 1).
3), the TEM images showed uniform nanostructures with a homogeneous diameter of ∼8 nm (Fig. 2c and d and Table 1).
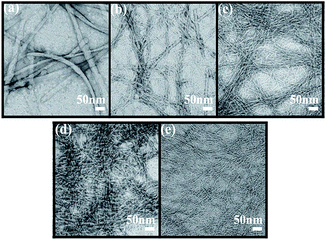 | ||
Fig. 2 TEM images in different blend hydrogels of PFB-F and PFB-FF at 1 wt%. (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, (b) 3 0, (b) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (c) 1 1, (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (d) 1 1, (d) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and (e) 0 3 and (e) 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
The spectroscopic characterization of the gel states of hydrogelators provides relevant evidence regarding the intermolecular interactions in the assemblies.17–19 Therefore, the spectroscopic characterization of different blend hydrogels of PFB-F and PFB-FF were then studied. The UV-vis absorption spectra of the five PFB-capped hydrogels displayed absorption bands at ca. 260 nm, corresponding to π–π* transitions of the typical aromatic rings (Fig. 3a). Notably, a new absorption band at 285 nm (275–305 nm) was observed and its intensity was increased with increasing the ratio of PFB-FF, thus indicating the additional Phe in PFB-FF may enhance the π–π interactions in the gel. The emission spectra of the five materials were obtained with an excitation wavelength of 260 nm under the gelation conditions, all materials showed red-shifted and broad emissions in gel state (ca. 360 nm) with respect to the corresponding emissions in solution (∼310 nm),3 which is attributed to aggregate formation in gel state. In addition, we found that the emission intensity would increase continuously as the ratio of the PFB-FF increased (Fig. 3b). The circular dichroism (CD) spectra were shown in Fig. 3c, the hydrogel of PFB-F revealed bisignate Cotton effects, with a negative part at 260 nm and a positive part at 285 nm. With increasing the ratio of PFB-FF, the bisignate signal would disappear, and instead of a strong positive CD signal at 260 nm. As shown in Fig. 3d, the FT-IR spectra of a pure PFB-F and a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 blend of PFB-F and PFB-FF hydrogels showed that there are no secondary structures formed in the hydrogels, suggesting the flexibility of PFB-F hydrogelator does not allow the single amino acid to interlock in a β-sheet-like conformation.3 With increasing the ratio of PFB-FF, the spectra of the 1
1 blend of PFB-F and PFB-FF hydrogels showed that there are no secondary structures formed in the hydrogels, suggesting the flexibility of PFB-F hydrogelator does not allow the single amino acid to interlock in a β-sheet-like conformation.3 With increasing the ratio of PFB-FF, the spectra of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the 1
1 and the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 blend of PFB-F and PFB-FF as well as pure PFB-FF gels showed two peaks located around 1656 cm−1, 1630 cm−1 and a less intense peak around 1545 cm−1, indicating the formation of β-sheet-like structure in the assemblies.20
3 blend of PFB-F and PFB-FF as well as pure PFB-FF gels showed two peaks located around 1656 cm−1, 1630 cm−1 and a less intense peak around 1545 cm−1, indicating the formation of β-sheet-like structure in the assemblies.20
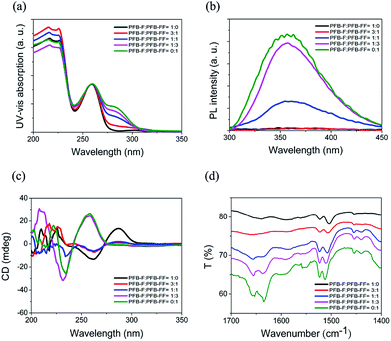 | ||
| Fig. 3 (a) UV-vis, (b) Fluorescent emission, (c) CD and (d) FT-IR spectra in different blend hydrogels of PFB-F and PFB-FF at 1 wt% in water under their hydrogelation conditions. | ||
The mechanical properties of the PFB-capped hydrogels were obtained by oscillatory rheometry. In comparison of blend hydrogels with different molar ratios, the viscoelastic properties of PFB-capped hydrogels are determined at the concentration of 1 wt% and the results are collected in Fig. 4a and b and Table 1. The storage moduli of all the hydrogels are higher than their loss moduli, thus indicating these materials formed viscoelastic gels. The average storage modulus of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 blend was 3.0 kPa which supports the mass of a cell (∼0.1 kPa). Therefore, it is possibly to be used as scaffolding material for tissue engineering.21,22 Moreover, the strain sweeps results of the gels are shown in Fig. 4c and d and their critical strains of the blend gels are collected in Table 1.
1 blend was 3.0 kPa which supports the mass of a cell (∼0.1 kPa). Therefore, it is possibly to be used as scaffolding material for tissue engineering.21,22 Moreover, the strain sweeps results of the gels are shown in Fig. 4c and d and their critical strains of the blend gels are collected in Table 1.
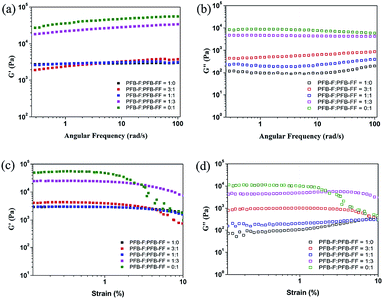 | ||
| Fig. 4 Frequency and strain sweeps of different blend hydrogels for PFB-F and PFB-FF at 1 wt%. (a and c) G′ and (b and d) G′′. | ||
Based on the success of the blend hydrogel formed under physiological pH, we further examined the biocompatibility of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 blend material. The biocompatibility of a 1
1 blend material. The biocompatibility of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 blend of PFB-F and PFB-FF was examined using colorimetric MTT assay.23 In Fig. 5a, we examined the viability of CTX TNA2 cells, the results indicate the 1
1 blend of PFB-F and PFB-FF was examined using colorimetric MTT assay.23 In Fig. 5a, we examined the viability of CTX TNA2 cells, the results indicate the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 blend gel of PFB-F and PFB-FF was biocompatible because the 50% inhibition (IC50) are higher than 500 μM after 72 h, and its survival ratio is higher than 80%. As a potential drug carrier, we used MCF-7 cell line as a model to evaluate the cell compatibility of the material. In Fig. 5b, the experiments revealed that after being incubated the MCF-7 cells with the blend material (10–500 μM) for 72 h, cells that were grown in liquid medium showed the proliferation capacities with high survival ratio (above 80% at 500 μM).24 These observations illustrate the potentially useful of this material in the applications of 3D cell culturing and drug delivery.
1 blend gel of PFB-F and PFB-FF was biocompatible because the 50% inhibition (IC50) are higher than 500 μM after 72 h, and its survival ratio is higher than 80%. As a potential drug carrier, we used MCF-7 cell line as a model to evaluate the cell compatibility of the material. In Fig. 5b, the experiments revealed that after being incubated the MCF-7 cells with the blend material (10–500 μM) for 72 h, cells that were grown in liquid medium showed the proliferation capacities with high survival ratio (above 80% at 500 μM).24 These observations illustrate the potentially useful of this material in the applications of 3D cell culturing and drug delivery.
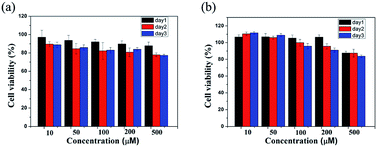 | ||
Fig. 5 Viability data of (a) CTX TNA2 and (b) MCF-7 cells incubated with 10–500 μM of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 blend of PFB-F and PFB-FF after 24, 48 and 72 h. 1 blend of PFB-F and PFB-FF after 24, 48 and 72 h. | ||
In summary, we have studied various molar ratios of PFB-F and PFB-FF to screen the possibility of forming self-assembled hydrogel under physiological pH. We found that equimolar ratio of PFB-F and PFB-FF efficiently formed coassembled nanofibers and the hydrogel at the pH of 7.4. The spectroscopic characterization of the blend gel indicates the π–π interactions and hydrogen-bonding interactions are the major driving forces behind the coassembly. Additionally, the results of cell survival ratio of CTX TNA2 and MCF-7 cells suggest the blend hydrogel is biocompatible, thus making it a potentially useful scaffolding material for biomedical applications such as drug delivery and tissue engineering. In this study, the successful usage of two distinct hydrogelators to promote the coassembly under physiological pH expands the repertoire of noncovalent interactions that can be used in the development of sophisticated noncovalent biomaterials.
Acknowledgements
This study was supported financially by the National Science Council of the Republic of China, Taiwan (grant NSC 102-2113-M-009-006-MY2); the “Aim for the Top University” program of National Chiao Tung University; and the Ministry of Education, Taiwan, R.O.C. We thank Jhong-Hua Lin and Yu-Tang Huang for help with gelation tests.Notes and references
- S. Fleming and R. V. Ulijn, Chem. Soc. Rev., 2014, 43, 8150–8177 RSC.
- A. Mahler, M. Reches, M. Rechter, S. Cohen and E. Gazit, Adv. Mater., 2006, 18, 1365–1370 CrossRef CAS.
- S.-M. Hsu, Y.-C. Lin, J.-W. Chang, Y.-H. Liu and H.-C. Lin, Angew. Chem., Int. Ed., 2014, 53, 1921–1927 CrossRef CAS.
- S. Zhang, Nat. Biotechnol., 2003, 21, 1171–1178 CrossRef CAS.
- F. Zhao, M. L. Ma and B. Xu, Chem. Soc. Rev., 2009, 38, 883–891 RSC.
- M. Zhou, A. M. Smith, A. K. Das, N. W. Hodson, R. F. Collins, R. V. Ulijn and J. E. Gough, Biomaterials, 2009, 30, 2523–2530 CrossRef CAS.
- L. A. Haines, K. Rajagopal, B. Ozbas, D. A. Salick, D. J. Pochan and J. P. Schneider, J. Am. Chem. Soc., 2005, 127, 17025–17029 CrossRef CAS.
- V. Jayawarna, M. Ali, T. A. Jowitt, A. F. Miller, A. Saiani, J. E. Gough and R. V. Ulijn, Adv. Mater., 2006, 18, 611–614 CrossRef CAS.
- H. Komatsu, S. Matsumoto, S. Tamaru, K. Kaneko, M. Ikeda and I. Hamachi, J. Am. Chem. Soc., 2009, 131, 5580–5585 CrossRef CAS.
- H. M. Wang, J. Wei, C. B. Yang, H. Y. Zhao, D. X. Li, Z. N. Yin and Z. M. Yang, Biomaterials, 2012, 33, 5848–5853 CrossRef CAS.
- Z. Yang, G. Liang, M. Ma, A. S. Abbah, W. W. Lu and B. Xu, Chem. Commun., 2007, 843, 843–845 RSC.
- X. Li, Y. Kuang, H.-C. Lin, Y. Gao, J. Shi and B. Xu, Angew. Chem., Int. Ed., 2011, 50, 9365–9369 CrossRef CAS.
- G. Scott, S. Roy, Y. M. Abul-Haija, S. Fleming, S. Bai and R. V. Ulijn, Langmuir, 2013, 29, 14321–14327 CrossRef CAS.
- D. M. Ryan, T. M. Doran and B. L. Nilsson, Langmuir, 2011, 27, 11145–11156 CrossRef CAS.
- J. R. Moffat and D. K. Smith, Chem. Commun., 2009, 316–318 RSC.
- M. M. Smith and D. K. Smith, Soft Matter, 2011, 7, 4856–4860 RSC.
- A. M. Smith, R. J. Williams, C. Tang, P. Coppo, R. F. Collins, M. L. Turner, A. Saiani and R. V. Ulijn, Adv. Mater., 2008, 20, 37–41 CrossRef CAS.
- M. Ma, Y. Kuang, Y. Gao, Y. Zhang, P. Gao and B. Xu, J. Am. Chem. Soc., 2010, 132, 2719–2728 CrossRef CAS.
- C. Tang, R. V. Ulijn and A. Saiani, Langmuir, 2011, 27, 14438–14449 CrossRef CAS.
- E. Goormaghtigh, J. M. Ruysschaert and V. Raussens, Biophys. J., 2006, 90, 2946–2957 CrossRef CAS.
- C. Yan and D. Pochan, Chem. Soc. Rev., 2010, 39, 3528–3540 RSC.
- L. Haines-Butterick, K. Rajagopal, M. Branco, D. Salick, R. Rughani, M. Pilarz, M. S. Lamm, D. J. Pochan and J. P. Schneider, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 7791–7796 CrossRef CAS.
- M. V. Berridge and A. S. Tan, Arch. Biochem. Biophys., 1993, 303, 474–482 CrossRef CAS.
- K. Reichenbächer, H. I. Süss and J. Hulliger, Chem. Soc. Rev., 2005, 34, 22–30 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra01020a |
| This journal is © The Royal Society of Chemistry 2015 |