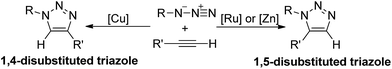Mechanism and selectivity for zinc-mediated cycloaddition of azides with alkynes: a computational study†
Yingzi Li‡
a,
Xiaotian Qi‡a,
Yu Leiab and
Yu Lan*a
aSchool of Chemistry and Chemical Engineering, Chongqing University, Chongqing 400030, China. E-mail: lanyu@cqu.edu.cn; Fax: +86-23-88288267; Tel: +86-18680805840
bDepartment of Emergency, First Hospital, Jilin University, Changchun, China
First published on 28th May 2015
Abstract
Density functional theory (DFT) method B3LYP with a dispersion term (B3LYP-D3BJ) has been used to clarify the regioselectivity of zinc mediated 1,3-dipolar cycloaddition of azides and alkynes. Computational results indicate that the dipolar cycloaddition takes place via a concerted five-membered-ring transition state, leading to a 1,5-disubstituted 1,2,3-triazole product, which is consistent with the experiment reported by Greaney's group. The coordination of imidazole ligand to zinc is reversible, and the regioselectivity is irrelevant to the coordination of imidazole ligand. Moreover, substituent effect of alkynes has also been studied. Finally, distortion–interaction analysis along the reaction pathways and frontier molecular orbital theory are used to explain the reactivity and 1,5-regioselectivity.
Introduction
1,3-Dipolar cycloadditions of azides and alkynes, which have been known for more than a century,1 represent the most direct route to construct 1,2,3-triazoles,2 and have been widely applied in biochemistry and material science.3 Many efforts have been made to promote the development of this type of reaction. During 1950s–1970s, Huisgen and co-workers systematically studied the uncatalytic thermal cycloadditions of azides and alkynes.4 In recent years, transition metal assisted 1,3-dipolar cycloadditions are emerged as a powerful strategy to synthesis 1,2,3-triazoles since the introduction of metal could improve the reactivity and control the regioselectivity.5 As shown in Scheme 1, when substituted alkynes and azides are employed as reactants, either 1,4- or 1,5-disubstituted 1,2,3-triazoles would be formed through 1,3-dipolar cycloaddition depending on what metal (Cu, Ru or Zn) is added.6The copper(I) catalyzed azide–alkyne cycloadditions (CuAAC) to selectively yield 1,4-disubstituted 1,2,3-triazoles, which is first reported by Meldal,6b Fokin, and Sharpless,6a individually, attract lots of attention due to the mild conditions, wide substrate scopes, and vast ranges of triazole application.7 Kinetic and mechanistic studies of CuAAC suggest that the active species contain two copper ions, one or two acetylenes, and one azide.8 Moreover, the π complexation of copper acetylide towards another copper ion also plays a critical role in positioning the substrates appropriately for the cycloaddition reaction, as well as activating the reacting acetylene towards nucleophilic attack by the azide.9
While Cu(I) catalysis has provided a reliable method for the construction of 1,4-disubstituted 1,2,3-triazoles,10 general methods for the synthesis of 1,5-disubstituted regioisomers are relatively scarce.11 Fokin in collaboration with Jia6a,6c reported some Ru(II)-catalyzed azide–alkyne cycloadditions (RuAAC) for the generation of 1,5-disubstituted 1,2,3-triazoles in moderate yields. Nonetheless, considerable limitations still exist in this approach, due to the strong base required, the utilization of alkalis12 or magnesium acetylides,10a,13 and the noble metal ruthenium.6c–e
Recently, Greaney reported a zinc mediated 1,3-dipolar cycloaddition of azides and alkynes, which regioselectively generates 1,5-substituted 1,2,3-triazoles at room temperature.14 The unique regioselectivity obtained under mild condition draws our attention to investigate the detailed mechanism. In nowadays chemistry field, density functional theory (DFT) calculation, which has been extensively used for the mechanistic study of CuAAC and RuAAC,6c,d,15,16 is regarded as a powerful tool to reveal the mechanism of 1,3-dipolar cycloadditions.17 Herein, we report our DFT calculations towards the mechanism and regioselectivity research of this Zn-mediated azide–alkyne cycloaddition, including frontier molecular orbital (FMO) theory18 and distortion–interaction energy analysis19 to provide interpretation for the regioselectivity.
Computational methods
All the DFT calculations were carried out with the Gaussian 09 series of programs.20 DFT method B3LYP21 with a standard 6-311+G(d) basis set (SDD22 basis set for zinc atom) was used for geometry optimizations. Harmonic vibration frequency calculations were performed for all stationary points to confirm them as a local minima or transition structure and to derive the thermochemical corrections for the enthalpies and free energies. On the other hand, the addition of dispersion interaction into B3LYP functional could describe the long-range interactions better, thus an atom–atom additive damped empirical potential of the form –f(R)C6/R6, which named B3LYP-D3BJ, was employed in our work to give more accurate energy information.23 The solvent effects were considered by B3LYP-D3BJ method calculated single point energies with a standard 6-311+G(d) basis set (SDD basis set for zinc atom) on the gas-phase stationary points with a PCM solvation model.24 Furthermore, the FMOs for some important intermediates were calculated using the B3LYP/6-311+G(d) level of theory.Two kinds of free energies are given in the free energy profiles, i.e. the G(gas phase)B3LYP and the G(THF)B3LYP-D3BJ, which were obtained by eqn (1) and eqn (2), respectively. Gcorrection is the thermochemical corrections for the Gibbs free energies. E(gas phase)B3LYP is the single point energy calculated at B3LYP/6-311+G(d) level in gas phase, and E(THF)B3LYP-D3BJ is the single point energy calculated at B3LYP-D3BJ/6-311+G(d) level in THF based on gas phase stationary point.
| G(gas phase)B3LYP = E(gas phase)B3LYP + Gcorrection | (1) |
| G(THF)B3LYP-D3BJ = E(THF)B3LYP-D3BJ + Gcorrection | (2) |
In this work, the intrinsic reaction coordinates (IRCs)25 of the transition states for zinc mediate dipolar cycloadditions between ethyl(phenylethynyl)zinc and azidobenzene were used to calculate the reaction pathway at the B3LYP/6-311+G(d) level of theory. The distortion energy (ΔEdis) was set to the energy difference between the energy of distorted ethyl(phenylethynyl)zinc and azidobenzene parts along the reaction pathway and the energy of fully optimized ethyl(phenylethynyl)zinc and azidobenzene parts by single point energy calculations at the B3LYP/6-311+G(d) level of theory. The interaction energy (ΔEint) was set to the energy difference between the single point energy of the geometry on the reaction coordinate and the energy of the relative distorted ethyl(phenylethynyl)zinc and azidobenzene parts. When the distortion, interaction, and total energies are plotted, the distance between the center of the two neutral nitrogen atoms in azidobenzene and the center of two reacting carbon atoms in ethyl(phenylethynyl)zinc is used to represent the reaction coordinate.
Results and discussion
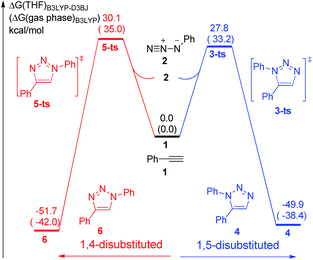
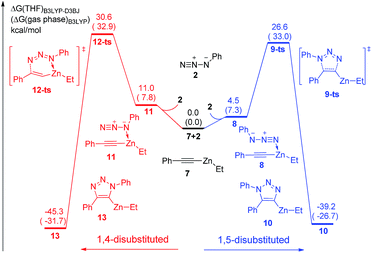
The uncatalytic 1,3-dipolar cycloadditions between azidobenzene and phenylacetylene are initially put forward. Computational results (Fig. 1) suggest that the activation free energy of corresponding transition state 5-ts is 30.1 kcal mol−1, which is consistent with Fokin's result calculated at B3LYP/6-311G+(2d,2p) level of theory.8b In another case, the barrier for the generation of 1,5-disubstituted 1,2,3-triazole via transition state 3-ts is 27.8 kcal mol−1, which is 2.3 kcal mol−1 lower compared with 1,4-disubstituted one.As shown in Fig. 2, ethyl(phenylethynyl)zinc 7, which could be easily formed from phenylacetylene and diethylzinc due to the higher acidity of phenylacetylene comparing with generated ethane, is chose as the reactant to study the reactivity of zinc mediated 1,3-dipolar cycloaddition. Coordination of 7 to the neutral nitrogen of azidobenzene 2 forms intermediate 8 with 4.5 kcal mol−1 endothermic. The 1,3-dipolar cycloaddition to form 1,5-diphenyl-4-(ethyl)zinc-1,2,3-triazole 10 takes place irreversibly via a concerted transition state 9-ts with an overall activation free energy of 26.6 kcal mol−1. The geometry information of transition state 9-ts is shown in Fig. 6. The two forming carbon–nitrogen bond lengths are 1.96 Å and 2.35 Å, respectively. The distance between nitrogen and zinc is 2.90 Å, which indicates none direct interaction between azidobenzene and zinc. In another case, coordination of the nucleophilic nitrogen atom of azidobenzene with 7 forms intermediate 11 with 11.0 kcal mol−1 endothermic. The corresponding cycloaddition to form 1,4-regioisomer could occur via transition state 12-ts with an overall barrier of 30.6 kcal mol−1. Intrinsic reaction coordinate (IRC) calculation of transition state 9-ts and 12-ts have proved it is the accurate transition state which connect the reactant and product. The nitrogen–zinc bond length of 2.22 Å in transition state 12-ts (Fig. 6) shows that when the neutral nitrogen–carbon bond is forming, the nucleophilic nitrogen still coordinates with zinc. The calculations indicate that when azidobenzene reacts with ethyl(phenylethynyl)zinc 7, the forming rate of 1,5-diphenyl-1,2,3-triazole is fast compared with the formation of 1,4-regiomer.
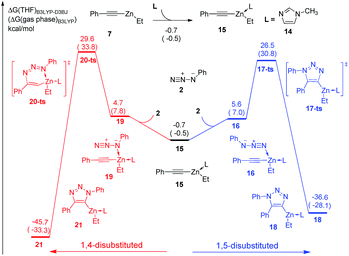
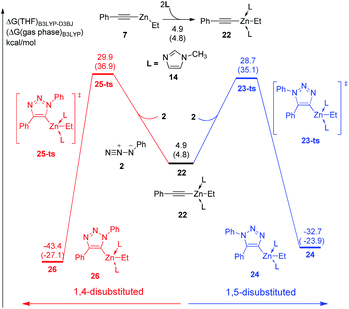
In the experiment, N-methylimidazole is added as a co-catalyst for zinc-mediated cycloaddition. Therefore, the ligand effects of N-methylimidazole towards reactivity and regioselectivity are considered theoretically. As shown in Fig. 3, The coordination of one molecular N-methylimidazole to ethyl(phenylethynyl)zinc 7 results in 0.7 kcal mol−1 exothermic, and forms intermediate 15. Subsequently, intermediate 16 or 19 would be formed by the coordination of azidobenzene 2 with neutral or nucleophilic nitrogen atom with 6.3 kcal mol−1 or 5.4 kcal mol−1 endothermic, respectively. 1,5-Disubstituted product 18 could be formed via transition state 17-ts with a barrier of 26.5 kcal mol−1. The relative free energy of transition 17-ts is 0.1 kcal mol−1 lower than that of transition state 9-ts, which indicates that the cycloaddition for the formation of 1,5-diphenyl-1,2,3-triazole cannot be assisted by the coordination of one N-methylimidazole ligand. As contrast, the activation energy of transition state 20-ts for the formation of 1,4-disubstituted regioisomer 21 is 29.6 kcal mol−1, which is 3.1 kcal mol−1 higher comparing with that of transition state 17-ts. According to this data, the ratio of 1,5-substituted 1,2,3-triazole to 1,4-regioisomer should be 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Therefore, the generation of 1,4-disubstituted regioisomer 21 is unfavorable, which is consistent with the experiment results. Theoretical calculations indicate that the regioselectivity is irrelevant to the coordination of N-methylimidazole ligand. The geometry information of transition state 20-ts is shown in Fig. 6. The nitrogen–zinc bond length of 2.39 Å in transition state 20-ts, which is 0.17 Å longer than the corresponding bond length in transition state 12-ts, indicates that the coordination of N-methylimidazole would weaken the interaction between zinc and azidobenzene in transition state.
1. Therefore, the generation of 1,4-disubstituted regioisomer 21 is unfavorable, which is consistent with the experiment results. Theoretical calculations indicate that the regioselectivity is irrelevant to the coordination of N-methylimidazole ligand. The geometry information of transition state 20-ts is shown in Fig. 6. The nitrogen–zinc bond length of 2.39 Å in transition state 20-ts, which is 0.17 Å longer than the corresponding bond length in transition state 12-ts, indicates that the coordination of N-methylimidazole would weaken the interaction between zinc and azidobenzene in transition state.
Moreover, two equivalent N-methylimidazole involved pathway was also calculated. As shown in Fig. 4, the coordination of second N-methylimidazole ligand is 5.6 kcal mol−1 endothermic. Although the barrier of dipolar cycloaddition via transition state 23-ts is only 23.8 kcal mol−1 from intermediate 22, the overall activation free energy for the formation of complex 24 is 29.4 kcal mol−1, which is 2.2 kcal mol−1 higher than that for transition state 17-ts. The activation free energy for the generation of relative 1,4-regioisomer with two ligands is 30.6 kcal mol−1, which is 1.2 kcal mol−1 higher than that for the formation of 1,5-disubstituted product due to the endothermic coordination of second ligand. Consequently, when dipolar cycloaddition of ethyl(phenylethynyl)zinc and azidobenzene to form 1,4- or 1,5-diphenyltriazole takes place, at most one N-methylimidazole ligand coordinates with zinc.
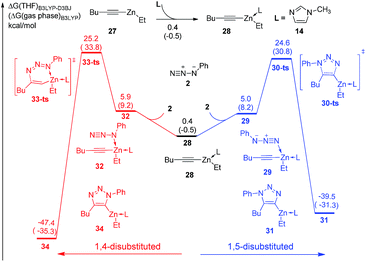
Substituent effects have also been considered in the theoretical study. As shown in Fig. 5, ethylhexynylzinc complex 27 is chosen to investigate the reactivity and regioselectivity of alkyl substituted alkynes instead of aryl substituent. The concerted [3 + 2] cycloaddition to form 1,5-disubstituted product 30 could take place via transition state 29-ts with an overall barrier of 24.6 kcal mol−1. In another case, the barrier for the formation of 1,4-disubstituted product 34 via transition state 33-ts is 25.2 kcal mol−1, which is 0.6 kcal mol−1 higher than that for the formation of 1,5-regioisomer. The B3LYP-D3BJ calculations indicate that the reactivity of alkyl substituted alkynes is similar with aryl substituted substrate, while the regioselectivity of alkyl substituted alkynes is lower, which is consistent with experimental observations.
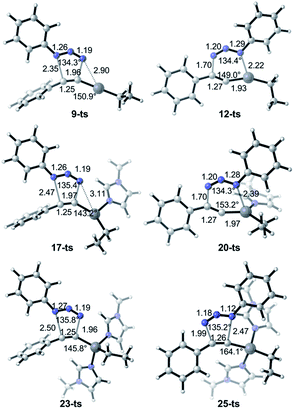 | ||
| Fig. 6 B3LYP optimized geometries of transition structures 9-ts, 12-ts, 17-ts, 20-ts, 23-ts and 25-ts. The values of bond length are given by Å. | ||
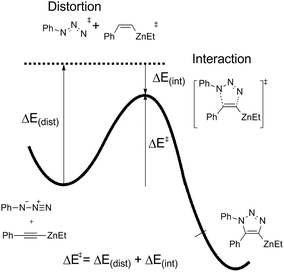
The distortion–interaction energy analysis, which is reported as a powerful model to explain reactivity trends for bimolecular reactions,26 is employed to study this 1,3-dipolar cycloaddition. As shown in Fig. 7, the total activation energy (ΔE‡) is decomposed into the sum of distortion energy (ΔEdis) and interaction energy (ΔEint) between distorted reactants.
Table 1 shows the distortion energies and interaction energies of the transition states 9-ts, 12-ts, 17-ts, 20-ts, 23-ts, and 25-ts. Comparing with transition states 9-ts and 12-ts, the distortion energy of transition state 12-ts is 11.0 kcal mol−1 higher than that of transition state 9-ts, however the interaction energy of transition state 12-ts is 10.7 kcal mol−1 lower due to the coordination of nucleophilic nitrogen atom with zinc. Therefore, the relative energy of transition state 12-ts is 2.3 kcal mol−1 higher than that of transition state 9-ts. The coordination of one imidazole ligand decreases the binding energy of nitrogen with zinc in six-membered transition state, therefore the distortion energy of transition state 20-ts is 1.3 kcal mol−1 lower than that of transition state 12-ts. Moreover, the distortion energy of transition state 17-ts is 1.9 kcal mol−1 lower than that of 9-ts because of the stronger frontier molecular orbital interaction, which will be discussed later. Therefore, the overall activation energy of transition state 17-ts is 3.1 kcal mol−1 lower than that of transition state 20-ts. Comparing with five-membered-ring type transition states 23-ts and 25-ts, the higher distortion energy of 25-ts leads to a higher activation energy.
| ΔEdis | ΔEint | ΔE‡ | ΔΔG‡ | |
|---|---|---|---|---|
| 9-ts | 29.9 | −14.6 | 15.3 | 26.6 |
| 12-ts | 40.9 | −23.9 | 17.0 | 30.6 |
| 17-ts | 28.0 | −14.6 | 13.4 | 26.5 |
| 20-ts | 39.6 | −24.9 | 14.7 | 29.6 |
| 23-ts | 25.7 | −14.7 | 11.0 | 28.7 |
| 25-ts | 29.6 | −17.0 | 12.6 | 29.9 |
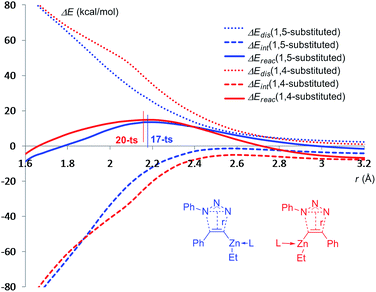
To further shed light on the regioselectivity for the cycloaddition of ethyl(phenylethynyl)zinc and azidobenzene with one imidazole ligand, the distortion and interaction energies along the reaction pathways for the formation of 1,5-disubstituted product 18 and the corresponding 1,4-regioisomer 21 have been calculated,27 using the distances between dipole and dipolarophile as a reaction coordinate.28 As shown in Fig. 8, as long as the distance between ethyl(phenylethynyl)zinc and azidobenzene are longer than 2.6 Å, the distortion energies for the two pathways are essentially the same. As the distance decreases below 2.6 Å, the distortion energy of the pathway for the formation of 1,4-disubstituted product climbs higher than for the formation of 1,5-regioisomer, because when the six-membered-ring transition state 20-ts is forming, ethyl(phenylethynyl)zinc part should be folded. For the formation of 1,4-disubstituted product 21, when the distance decreases below 2.1 Å, the six-membered-ring type geometry begins to transfer to five-membered-ring. Therefore, the gradient of distortion energy is decreased. Comparing with the red line and blue line, the relative energy of 20-ts is higher than that of 17-ts due to the higher distortion energy because of the stronger distortion of ethyl(phenylethynyl)zinc.
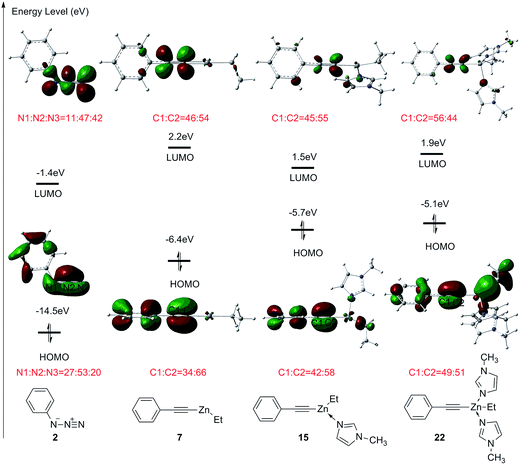
FMO theory is employed to further investigate the interaction between ethyl(phenylethynyl)zinc and azidobenzene.17b,27a The molecular orbitals and orbital energies of dipole 2, and phenylethynylzinc 7, 15, and 22 are shown in Fig. 9. The energy levels of highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) for the ground state of azidobenzene 2 is −14.5 eV and −1.4 eV, respectively. The gap between the HOMO of phenylethynylzinc 7 and the LUMO of azidobenzene 2 is 7.3 eV lower than that between the HOMO of azidobenzene 2 and LUMO of phenylethynylzinc. Therefore, in the transition state of dipolar cycloaddition between ethyl(phenylethynyl)zinc and azidobenzene, the reactivity and regioselectivity are majorly depending on the orbital interaction between the HOMO of azidobenzene 2 and LUMO of phenylethynylzinc. When phenylethynylzinc 7 is coordinated with one or two imidazole ligands, the energy level of HOMO is increased due to the electron-donating effect of additional ligands, consequently, the reactivity is increased. However, the endothermic for the coordination of second imidazole leads to a higher overall barrier for this type reaction. The weight of the atomic orbitals in the ground state molecular orbitals also has been calculated to explain the selectivity. In azidobenzene 2, the weight of LUMO on N3 atom is higher than that on N1 atom. In one imidazole coordinated phenylethynylzinc 15, the weight of HOMO on C2 atom is higher than that on C1 atom. Therefore, when azidobenzene 2 reacts with phenylethynylzinc 15, 1,5-disubstituted 1,2,3-triazole is supposed to be the major product.
Conclusions
The DFT method B3LYP with a dispersion term (B3LYP-D3BJ) has been used to clarify the regioselectivity for the synthesis of 1,5-substituted 1,2,3-triazoles through zinc mediated 1,3-dipolar cycloaddition. Computational results suggest that only one N-methylimidazole ligand coordinates to the metal center during the reaction between ethyl(phenylethynyl)zinc and azidobenzene. 1,5-Substituted 1,2,3-triazole is formed through a five-membered-ring transition state with a lower activation free energy compared with the generation of 1,4-regioisomer via a six-membered-ring transition state. Moreover, substituent effect of alkynes has also been considered. B3LYP-D3BJ calculations show that alkyl substituted ethynylzinc owns the same reactivity as ethyl(phenylethynyl)zinc, while the regioselectivity is lower, however, B3LYP gives a higher regioselectivity, which is consistent with experimental observation. Beside, distortion–interaction analysis along the reaction pathways provides explanation for the reactivity and regioselectivity. The lower distortion energy in transition state leads to a lower activation energy for the generation of 1,5-substituted product. Furthermore, FMO analysis reveals the coordination of N-methylimidazole ligand towards zinc would increase the HOMO of ethyl(phenylethynyl)zinc, thereby improving the reactivity.Acknowledgements
This work was supported by the National Science Foundation of China (Grants 21372266, and 51302327), and the Foundation of 100 Young Chongqing University (Project 0903005203191). We are also thankful for the project (no. 106112015CDJZR228806) supported by the Fundamental Research Funds for the Central Universities (Chongqing University).Notes and references
- A. Michael, J. Prakt. Chem., 1893, 48, 94 CrossRef PubMed.
- (a) R. Huisgen, 1,3-Dipolar Cycloaddition Chemistry, ed. A. Padwa, Wiley, New York, 1984, pp. 1–176 Search PubMed; (b) Synthetic Applications of 1,3 Dipolar Cycloaddition Chemistry toward Heterocycles and Natural Products, ed. A. Padwa and W. H. Pearson, Wiley, New York, 2002 Search PubMed; (c) W. Lwowski, in 1,3-Dipolar Cycloaddition Chemistry, ed. A. Padwa, Wiley, New York, 1984, vol. 1, p. 559 Search PubMed; (d) X.-Z. Cheng, W. Liu, Z.-D. Huang and C.-X. Kuang, Chin. Chem. Lett., 2003, 24, 764 CrossRef PubMed.
- (a) J. P. Collman, N. K. Devaraj and C. E. D. Chidsey, Langmuir, 2004, 20, 1051 CrossRef CAS; (b) A. E. Speers, G. C. Adam and B. F. Cravatt, J. Am. Chem. Soc., 2003, 125, 4686 CrossRef CAS PubMed; (c) Q.-H. Li, Y. Ding and N.-W. Huang, Chin. Chem. Lett., 2014, 25, 1469 CrossRef CAS PubMed.
- (a) R. Huisgen, Proc. Chem. Soc., 1961, 357 Search PubMed; (b) R. Huisgen, Angew. Chem., Int. Ed. Engl., 1963, 2, 565 CrossRef PubMed; (c) R. Huisgen, Angew. Chem., Int. Ed. Engl., 1963, 2, 633 CrossRef PubMed; (d) R. Huisgen, Angew. Chem., Int. Ed. Engl., 1968, 7, 321 CrossRef CAS PubMed; (e) R. Huisgen, G. Mloston and E. Langhals, J. Am. Chem. Soc., 1986, 108, 6401 CrossRef CAS.
- (a) C. Girard, E. Önen, M. Aufort, S. Beauvière, E. Samson and J. Herscovici, Org. Lett., 2006, 8, 1689 CrossRef CAS PubMed; (b) V. O. Rodionov, V. V. Fokin and M. G. Finn, Angew. Chem., 2005, 117, 2250 (Angew. Chem., Int. Ed., 2005, 44, 2210) CrossRef PubMed; (c) M. Hu, J. Li and S. Q. Yao, Org. Lett., 2008, 10, 5529 CrossRef CAS PubMed; (d) C. Chowdhury, A. K. Sasmal and P. K. Dutta, Tetrahedron Lett., 2009, 50, 2678 CrossRef CAS PubMed; (e) J. T. Fletcher, S. E. Walz and M. E. Keeney, Tetrahedron Lett., 2008, 49, 7030 CrossRef CAS PubMed; (f) P. Zhong and S.-R. Guo, Chin. J. Chem., 2004, 22, 1183 CrossRef CAS PubMed; (g) Q. Luo, G. Jia, J. Sun and Z. Lin, J. Org. Chem., 2014, 79, 11970–11980 CrossRef CAS PubMed.
- (a) V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS; (b) C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057 CrossRef PubMed; (c) L. Zhang, X. Chen, P. Xue, H. H. Y. Sun, I. D. Williams, K. B. Sharpless, V. V. Fokin and G. Jia, J. Am. Chem. Soc., 2005, 127, 15998 CrossRef CAS PubMed; (d) B. C. Boren, S. Narayan, L. K. Rasmussen, L. Zhang, H. Zhao, Z. Lin, G. Jia and V. V. Fokin, J. Am. Chem. Soc., 2008, 130, 8923 CrossRef CAS PubMed; (e) M. Lamberti, G. C. Fortman, A. Poater, J. Broggi, A. M. Z. Slawin, L. Cavallo and S. P. Nolan, Organometallics, 2012, 31, 756 CrossRef CAS; (f) L.-J. Li, Y.-Q. Zhang, Y. Zhang, A.-L. Zhu and G.-S. Zhang, Chin. Chem. Lett., 2014, 25, 1161 CrossRef CAS PubMed.
- (a) G. C. Tron, T. Pirali, R. A. Billington, P. L. Canonico, G. Sorba and A. A. Genazzani, Med. Res. Rev., 2008, 28, 278 CrossRef CAS PubMed; (b) J.-F. Lutz and Z. Zarafshani, Adv. Drug Delivery Rev., 2008, 60, 958 CrossRef CAS PubMed; (c) J. E. Moses and A. D. Moorhouse, Chem. Soc. Rev., 2007, 36, 1249 RSC; (d) V. V. Fokin, ACS Chem. Biol., 2007, 2, 775 CrossRef CAS PubMed; (e) J. A. Johnson, J. T. Koberstein, M. G. Finn and N. J. Turro, Macromol. Rapid Commun., 2008, 29, 1052 CrossRef CAS PubMed; (f) M. Meldal and C. W. Tornøe, Chem. Rev., 2008, 108, 2952 CrossRef CAS PubMed; (g) B. Lewandowski, G. De Bo, J. W. Ward, M. Papmeyer, S. Kuschel, M. J. Aldegunde, P. M. E. Gramlich, D. Heckmann, S. M. Goldup, D. M. D'Souza, A. E. Fernandes and D. A. Leigh, Science, 2013, 339, 189 CrossRef CAS PubMed; (h) P. Thirumurugan, D. Matosiuk and K. Jozwiak, Chem. Rev., 2013, 113, 4905 CrossRef CAS PubMed.
- (a) M. Ahlquist and V. V. Fokin, Organometallics, 2007, 26, 4389 CrossRef CAS; (b) F. Himo, T. Lovell, R. Hilgraf, V. V. Rostovtsev, L. Noodleman, K. B. Sharpless and V. V. Fokin, J. Am. Chem. Soc., 2005, 127, 210 CrossRef CAS PubMed; (c) D. Cantillo, M. Ávalos, R. Babiano, P. Cintas, J. L. Jiménez and J. C. Palacios, Org. Biomol. Chem., 2011, 9, 2952 RSC.
- (a) G. Pampaloni, R. Peloso, C. Graiff and A. Tiripicchio, Organometallics, 2005, 24, 819 CrossRef CAS; (b) X. Yang and P. Knochel, Synlett, 2004, 13, 2303 Search PubMed; (c) R. R. Conry, A. A. Tipton, W. S. Striejewske, E. Erkizia, M. A. Malwitz, A. Caffaratti and J. A. Natkin, Organometallics, 2004, 23, 5210 CrossRef CAS; (d) J. M. Kok, B. W. Skelton and A. H. White, J. Cluster Sci., 2004, 15, 365 CrossRef CAS.
- (a) A. Krasiński, V. V. Fokin and K. B. Sharpless, Org. Lett., 2004, 6, 1237 CrossRef PubMed; (b) B.-Y. Lee, S. R. Park, H. B. Jeon and K. S. Kim, Tetrahedron Lett., 2006, 47, 5105 CrossRef CAS PubMed; (c) I. Jlalia, H. Elamair, F. Meganem, J. Herscovici and C. Girard, Tetrahedron Lett., 2008, 49, 6756 CrossRef CAS PubMed.
- (a) D.-R. Hou, T.-C. Kuan, Y.-K. Li, R. Lee and K.-W. Huang, Tetrahedron, 2010, 66, 9415 CrossRef CAS PubMed; (b) A. H. Yap and S. M. Weinreb, Tetrahedron Lett., 2006, 47, 3035 CrossRef CAS PubMed; (c) M. M. Majireck and S. M. Weinreb, J. Org. Chem., 2006, 71, 8680 CrossRef CAS PubMed; (d) J. R. Johansson, P. Lincoln, B. Nordén and N. Kann, J. Org. Chem., 2011, 76, 2355 CrossRef CAS PubMed.
- (a) S. W. Kwok, J. R. Fotsing, R. J. Fraser, V. O. Rodionov and V. V. Fokin, Org. Lett., 2010, 12, 4217 CrossRef CAS PubMed; (b) M. E. Meza-Aviña, M. K. Patel, C. B. Lee, T. J. Dietz and M. P. Croatt, Org. Lett., 2011, 13, 2984 CrossRef PubMed; (c) L. Wu, Y. Chen, M. Tang, X. Song, G. Chen, X. Song and Q. Lin, Synlett, 2012, 23, 1529 CrossRef CAS PubMed; (d) L. Hong, W. Lin, F. Zhang, R. Liu and X. Zhou, Chem. Commun., 2013, 49, 5589 RSC.
- A. Akao, T. Tsuritani, S. Kii, K. Sato, N. Nonoyama, T. Mase and N. Yasuda, Synlett, 2007, 1, 31 CrossRef.
- C. D. Smith and M. F. Greaney, Org. Lett., 2013, 15, 4826 CrossRef CAS PubMed.
- (a) M. Ahlquist and V. V. Fokin, Organometallics, 2007, 26, 4389 CrossRef CAS; (b) B. F. Straub, Chem. Commun., 2007, 3868 RSC.
- (a) L. K. Rasmussen, B. C. Boren and V. V. Fokin, Org. Lett., 2007, 9, 5337 CrossRef CAS PubMed; (b) S. Oppilliart, G. Mousseau, L. Zhang, G. Jia, P. Thuéry, B. Rousseau and J.-C. Cintrat, Tetrahedron, 2007, 63, 8094 CrossRef CAS PubMed.
- (a) F. Risitano, G. Grassi, F. Foti, G. Bruno and A. Rotondo, Heterocycle, 2003, 60, 857 CrossRef CAS; (b) D. H. Ess and K. N. Houk, J. Am. Chem. Soc., 2008, 130, 10187 CrossRef CAS PubMed; (c) H. Zhao, X. Li, X. Ran and W. Zhang, J. Mol. Struct.: THEOCHEM, 2004, 683, 207 CrossRef CAS PubMed.
- (a) K. Fukui, J. Org. Chem., 1971, 4, 57 CAS; (b) M. Burdisso, R. Gandolfi, S. Quartieri and A. Rastelli, Tetrahedron, 1987, 43, 159 CrossRef CAS; (c) H. Y. Yoo and K. N. Houk, J. Am. Chem. Soc., 1997, 119, 2877 CrossRef CAS.
- (a) K. Kitaura and K. Morokuma, Int. J. Quantum Chem., 1976, 10, 325 CrossRef CAS PubMed; (b) S. Nagase and K. Morokuma, J. Am. Chem. Soc., 1978, 100, 1666 CrossRef CAS; (c) K. N. Houk, R. W. Gandour, R. W. Strozier, N. G. Rondan and L. A. Paquette, J. Am. Chem. Soc., 1979, 101, 6797 CrossRef CAS; (d) R. D. J. Froese, J. M. Coxon, S. C. West and K. Morokuma, J. Org. Chem., 1997, 62, 6991 CrossRef CAS; (e) N. Koga, T. Ozawa and K. Morokuma, J. Phys. Org. Chem., 1990, 3, 519 CrossRef CAS PubMed; (f) J. M. Coxen, S. T. Grice, R. G. A. R. Maclagan and D. Q. McDonald, J. Org. Chem., 1990, 55, 3804 CrossRef; (g) J. M. Coxon, R. D. J. Rroese, B. Ganguly, A. P. Marchand and K. J. Morokuma, Synlett, 1999, 11, 1681 CrossRef PubMed; (h) M. Avalos, R. Babiano, J. L. Bravo, P. Cintas, J. Jim-enez, J. Palacios and M. A. Silva, J. Org. Chem., 2000, 65, 6613 CrossRef CAS; (i) K. Geetha, T. C. Dinadayalane and G. N. Sastry, J. Phys. Org. Chem., 2003, 16, 298 CrossRef CAS PubMed; (j) M. Manoharan and P. Venuvanalingam, J. Chem. Soc., Perkin Trans., 1997, 2, 1799 RSC; (k) K. Kavitha, M. Manoharan and P. Venuvanalingam, J. Org. Chem., 2005, 70, 2528 CrossRef CAS PubMed; (l) K. Kavitha and P. Venuvanalingam, Int. J. Quantum Chem., 2005, 104, 67 CrossRef PubMed; (m) P. Blowers, L. Ford and R. Masel, J. Phys. Chem. A, 1998, 102, 9267 CrossRef CAS.
- M. J. Frisch, et al., Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT, 2009. The full author list is shown in ESI† Search PubMed.
- (a) A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS PubMed; (b) C. Lee, W. Yang and R. G. Parr, Phys. Rev. B, 1988, 37, 785 CrossRef CAS.
- (a) D. Liu, S. Tang, H. Yi, X. Qi, Y. Lan and A. Lei, Chem.–Eur. J., 2014, 20, 15605–15610 CrossRef CAS PubMed; (b) O. Gutierrez, J. C. Tellis, D. N. Primer, G. A. Molander and M. C. Kozlowski, J. Am. Chem. Soc., 2015, 137, 4896 CrossRef CAS PubMed.
- S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456 CrossRef CAS PubMed.
- A. V. Marenich, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. B, 2009, 113, 6378 CrossRef CAS PubMed.
- (a) K. Fukui, Acc. Chem. Res., 1981, 14, 363 CrossRef CAS; (b) H. P. Hratchian and H. B. Schlegel, in Theory and Applications of Computational Chemistry: The First 40 Years, ed. C. E. Dykstra, G. Frenking, K. S. Kim and G. Scuseria, Elsevier, Amsterdam, 2005, p. 195 Search PubMed; (c) H. P. Hratchian and H. B. Schlegel, J. Chem. Phys., 2004, 120, 9918 CrossRef CAS PubMed; (d) H. P. Hratchian and H. B. Schlegel, J. Chem. Theory Comput., 2005, 1, 61 CrossRef CAS.
- D. H. Ess and K. N. Houk, J. Am. Chem. Soc., 2007, 129, 10646 CrossRef CAS PubMed.
- (a) Y. Lan, S. E. Wheeler and K. N. Houk, J. Chem. Theory Comput., 2011, 7, 2104 CrossRef CAS; (b) S. Liu, Y. Lei, X. Qi and Y. Lan, J. Phys. Chem., 2014, 118, 2638 CrossRef CAS PubMed.
- ESI†.
Footnotes |
| † Electronic supplementary information (ESI) available: Cartesian coordinates and energies of all reported structures and full authorship of Gaussian 09. See DOI: 10.1039/c5ra02703a |
| ‡ Yingzi Li and Xiaotian Qi contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |