Characterization of phenolic compounds and antioxidant properties of Glycyrrhiza glabra L. rhizomes and roots
Natália Martinsab,
Lillian Barros*a,
Montserrat Dueñasc,
Celestino Santos-Buelga c and
Isabel C. F. R. Ferreira*a
c and
Isabel C. F. R. Ferreira*a
aMountain Research Centre (CIMO), ESA, Polytechnic Institute of Bragança, Campus de Santa Apolónia, Apartado 1172, 5301-855 Bragança, Portugal. E-mail: iferreira@ipb.pt; lillian@ipb.pt; Fax: +351-273-325405; Fax: +351-273-325405; Tel: +351-273-303219 Tel: +351-273-303903
bCEB, Centre of Biological Engineering, LIBRO – Laboratório de Investigação em Biofilmes Rosário Oliveira, University of Minho, 4710-057 Braga, Portugal
cGIP-USAL, Faculty of Pharmacy, University of Salamanca, Campus Miguel de Unamuno, 37007 Salamanca, Spain
First published on 12th March 2015
Abstract
The present work aims to characterize and quantify the phenolic composition and to evaluate the antioxidant activity of Glycyrrhiza glabra L. (commonly known as licorice) rhizomes and roots. The antioxidant potential of its methanol/water extract could be related to flavones (mainly apigenin derivatives), flavanones (mainly liquiritin derivatives), a methylated isoflavone and a chalcone, identified in the extract. Lipid peroxidation inhibition was the most pronounced antioxidant effect (EC50 = 0.24 ± 0.01 μg mL−1 and 22.74 ± 2.42 μg mL−1 in TBARS and β-carotene/linoleate assays, respectively), followed by free radical scavenging activity (EC50 = 111.54 ± 6.04 μg mL−1) and, finally, reducing power (EC50 = 128.63 ± 0.21 μg mL−1). In this sense, licorice extract could be used as a source of antioxidants for the pharmaceutical, cosmetic and/or food industries.
1. Introduction
Environmental factors, such as pollution, smoking, certain drugs, poor diet, sedentary lifestyle and stress-inducing agents, are considered the main external aggressors for human bodies, increasing cell deterioration and, in the long term, contributing to aging and several diseases/disorders. Furthermore, the normal metabolism also produces high quantities of oxidant molecules, through different chemical reactions. Commonly known as free radicals, these substances are highly reactive molecules containing one or more unpaired electrons in atomic or molecular orbitals that can join with cellular components and destroy them.1–3Plants are widely used to improve health and even to treat various diseases. Currently, there are several studies evidencing these natural matrices as rich sources of biomolecules, which provide numerous health benefits.4–6 Antioxidant phytochemicals are a good example of these biomolecules, being considered important contributors to protect cells and DNA, once neutralize reactive molecules and even prevent a cascade of reactions that lead to degenerative processes such as aging, neurodegenerative diseases, cancer, cardiovascular diseases, cataracts, rheumatism, ulcers, or atherosclerosis, among others.1,2,7–11
Among antioxidants, phenolic compounds have been considered important promoters of health and wellbeing, acting as free radical scavengers, metal chelators, singlet oxygen quenchers, inhibitors of lipid peroxidation as well as modulators of the formation of pro-oxidant and pro-inflammatory molecules (leukotrienes, 5-LOX, cytokines).12–14
Glycyrrhiza glabra L. (Fabaceae), commonly known as licorice, is widely recommended as emollient, for upper respiratory tract infections and dermal affections, as anti-inflammatory, antiulcer, antibacterial, antifungal, antiviral, anti-allergic, and immunostimulant, among other benefits.15–18 Its antioxidant properties have also been reported, either in aqueous,7,19,20 ethanol,20–22 methanol20,23–25 or methanol/water26,27 extracts. There are several studies that focused on the phenolic characterization of Glycyrrhiza sp.23,24,26,27 Nevertheless, information on the quantification of these compounds is scarce.
The aim of this work was to characterize and quantify the phenolic composition and evaluate the antioxidant properties in methanol/water extracts of Glycyrrhiza glabra L. (rhizomes and roots).
2. Materials and methods
2.1. Samples
Dried rhizomes and roots of Glycyrrhiza glabra L. were supplied by Soria Natural (Garray – Soria, Spain). The samples were obtained in the autumn 2012 and certified as clean products, with monitored parameters for pesticides, herbicides, heavy metals and radioactivity. For each analysis, three different samples were used and the assays were performed in triplicate.2.2. Standards and reagents
Methanol was of analytical grade purity and supplied by Pronalab (Lisbon, Portugal). 2,2-Diphenyl-1-picrylhydrazyl (DPPH) was obtained from Alfa Aesar (Ward Hill, MA, USA). HPLC-grade acetonitrile was obtained from Merck KgaA (Darmstadt, Germany). Formic and acetic acids were purchased from Prolabo (VWR International, France). The phenolic compound standards were from Extrasynthese (Genay, France). Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Water was treated in a Milli-Q water purification system (TGI Pure Water Systems, Greenville, SC, USA).2.3. Extraction procedure
The extraction was performed by stirring the sample (1 g) with 30 mL of methanol/water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v) at 25 °C and 150 rpm for 1 h, and filtered through Whatman no. 4 paper. The final residue was then extracted with an additional 30 mL portion of the extraction solvents mixture. The combined extracts were evaporated at 35 °C under reduced pressure (rotary evaporator Büchi R-210, Flawil, Switzerland) and then lyophilized (FreeZone 4.5, Labconco, Kansas City, MO, USA). The lyophilized extracts were re-dissolved in methanol/water (80
20, v/v) at 25 °C and 150 rpm for 1 h, and filtered through Whatman no. 4 paper. The final residue was then extracted with an additional 30 mL portion of the extraction solvents mixture. The combined extracts were evaporated at 35 °C under reduced pressure (rotary evaporator Büchi R-210, Flawil, Switzerland) and then lyophilized (FreeZone 4.5, Labconco, Kansas City, MO, USA). The lyophilized extracts were re-dissolved in methanol/water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v), performing a stock solution with a concentration of 20 mg mL−1, from which several dilutions were prepared.
20, v/v), performing a stock solution with a concentration of 20 mg mL−1, from which several dilutions were prepared.
2.4. Analysis of phenolic compounds
Phenolic compounds were determined by HPLC (Hewlett-Packard 1100, Agilent Technologies, Santa Clara, USA) as previously described by the authors.28 Double online detection was carried out in the diode array detector (DAD) using 280 nm and 370 nm as preferred wavelengths and in a mass spectrometer (MS) connected to the HPLC system via the DAD cell outlet. Peaks were tentatively identified based on their UV-vis and mass spectra and comparison with data reported in the literature. Quantification was performed from the areas of the peaks recorded at 280 and 370 nm using calibration curves (1–100 μg mL−1) obtained with phenolic standards of the same group. The results were expressed in mg per g of extract.2.5. Evaluation of antioxidant activity
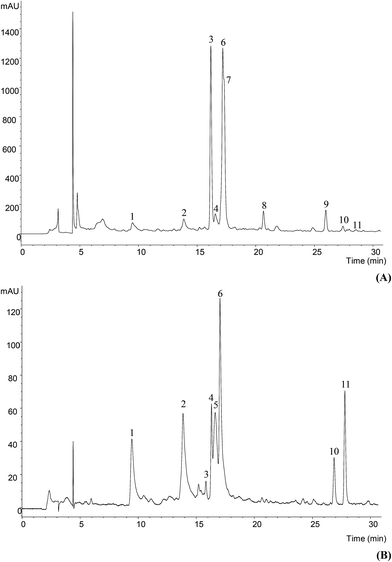 | ||
| Fig. 1 Phenolic profile of Glycyrrhiza glabra L. methanol/water extract at 280 nm (A) and 370 nm (B). | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (w/v) brain tissue homogenate, which was centrifuged at 3000g for 10 min. An aliquot (0.1 mL) of the supernatant was incubated with the different solution concentrations (0.2 mL) in the presence of FeSO4 (10 μM; 0.1 mL) and ascorbic acid (0.1 mM; 0.1 mL) at 37 °C for 1 h. The reaction was stopped by the addition of trichloroacetic acid (28% w/v, 0.5 mL), followed by thiobarbituric acid (TBA, 2%, w/v, 0.38 mL), and the mixture was then heated at 80 °C for 20 min. After centrifugation at 3000g for 10 min to remove the precipitated protein, the colour intensity of the malondialdehyde (MDA)–TBA complex in the supernatant was measured by its absorbance at 532 nm. The inhibition ratio (%) was calculated using the following formula: inhibition ratio (%) = [(A − B)/A] × 100%, where A and B were the absorbance of the control and the compound solution, respectively.32 The extract concentration providing 50% of antioxidant activity (EC50) was calculated from the graph of TBARS formation inhibition against extract concentrations. Trolox was used as positive control.
2 (w/v) brain tissue homogenate, which was centrifuged at 3000g for 10 min. An aliquot (0.1 mL) of the supernatant was incubated with the different solution concentrations (0.2 mL) in the presence of FeSO4 (10 μM; 0.1 mL) and ascorbic acid (0.1 mM; 0.1 mL) at 37 °C for 1 h. The reaction was stopped by the addition of trichloroacetic acid (28% w/v, 0.5 mL), followed by thiobarbituric acid (TBA, 2%, w/v, 0.38 mL), and the mixture was then heated at 80 °C for 20 min. After centrifugation at 3000g for 10 min to remove the precipitated protein, the colour intensity of the malondialdehyde (MDA)–TBA complex in the supernatant was measured by its absorbance at 532 nm. The inhibition ratio (%) was calculated using the following formula: inhibition ratio (%) = [(A − B)/A] × 100%, where A and B were the absorbance of the control and the compound solution, respectively.32 The extract concentration providing 50% of antioxidant activity (EC50) was calculated from the graph of TBARS formation inhibition against extract concentrations. Trolox was used as positive control.3. Results and discussion
3.1. Characterization of the phenolic compounds
The phenolic profile of Glycyrrhiza glabra, obtained after methanol/water extraction, and recorded at 280 and 370 nm is shown in Fig. 1; compound characteristics and tentative identities are presented in Table 1. Eleven compounds were detected corresponding to the groups of flavones, flavanones and chalcones, as well as a possible isoflavone.| Peak | Rt (min) | λmax (nm) | Molecular ion [M − H]− (m/z) | MS2 (m/z) | Identification | Quantification (mg g−1) |
|---|---|---|---|---|---|---|
| a n.q. not quantified. | ||||||
| 1 | 9.5 | 336 | 593 | 473(25), 383(12), 353(23) | Apigenin-6,8-di-C-glycoside | 0.61 ± 0.04 |
| 2 | 13.9 | 336 | 563 | 443(13), 413(4), 323(4), 311(3), 293(3) | Apigenin 2′′-O-pentosyl-6-C-hexoside | 0.99 ± 0.04 |
| 3 | 16.2 | 272, sh316 | 549 | 429(23), 417(15), 255(29) | Liquiritigenin apiosyl-glucoside isomer | 4.41 ± 0.10 |
| 4 | 16.3 | 272/320 | 577 | 559(5), 503(12), 415(5) | (Iso)violanthin | 0.48 ± 0.01 |
| 5 | 16.6 | 334 | 445 | 283(100), 268(10) | Methyl apigenin-O-hexoside | 0.84 ± 0.02 |
| 6 | 17.1 | 276, sh316 | 549 | 429(3), 417(15), 255(29) | Liquiritigenin apiosyl-glucoside isomer | 4.02 ± 0.04 |
| 7 | 17.3 | 276, sh318 | 549 | 429(5), 417(11), 255(55) | Liquiritigenin apiosyl-glucoside isomer | 3.85 ± 0.02 |
| 8 | 20.7 | 284, sh336 | 565 | 271(100) | Naringenin-7-O-apiosylglucoside | 0.43 ± 0.02 |
| 9 | 26.0 | 252, sh300 | 561 | 267(100), 252(10) | Formononetin-7-O-apiosylglucoside | 1.23 ± 0.02 |
| 10 | 26.7 | 362 | 549 | 417(5), 255(59) | (Neo)licuroside | 0.14 ± 0.01 |
| 11 | 27.8 | 250, sh292, 372 | 591 | 297(100), 282(46) | Unknown (chalcone derivative) | n.q |
| Total phenolic compounds | 17.00 ± 0.09 | |||||
Compound 1 presented a pseudomolecular ion [M − H]− at m/z 593, releasing MS2 fragment ions at m/z 443 (loss of 120 u), 383 (apigenin + 113 u) and 353 (apigenin + 83 u), whereas no relevant fragments derived from the loss of complete hexosyl (−162 u) or pentosyl residues (−132 u) were detected. This fragmentation behaviour is characteristic of di-C-glycosylated flavones.33 The compound was tentatively identified as apigenin-6,8-di-C-glucoside (vicenin-2) owing to its previous description in Traditional Chinese Medicine Formulae containing Glycyrrhiza roots and rhizomes.34,35
Compound 2 presented a pseudomolecular ion [M − H]− at m/z 563. A compound with the same mass was reported in licorice (dried roots and rhizomes of Glycyrrhiza species) by Xu et al. (2013) and identified as the di-C-glycosylflavone isoschaftoside (i.e., 6-C-arabinopyranosyl-8-C-glucopyranosylapigenin). However, the MS2 fragmentation pattern of the compound observed in our samples would not match such a structure, but it points to the pentosyl residue is O-attached to a C-glycosylating hexose. This assumption is supported by the characteristic fragment detected at m/z 413 ([M − 150]−), which according to33 would be typical from that type of substitution. Further, the fragment ion at m/z 443 ([M − 120]−) supported the presence of a C-attached hexose, whilst the absence of an ion [(M − H) − 90]− suggested a 6-C attachment.33 The pentose should not be attached on positions 6′′, 4′′ or 3′′ of the hexose, otherwise the fragment [(M − H) − 120]− would not be produced. As for the rest of fragment ions, the one at m/z 323 [(M − H) − 150 − 90]− would result from the partial loss of the C-attached hexose from the ion at m/z 413, whereas those at m/z 311 [aglycone + 41]− and 293 [aglycone + 41 − 18]− are associated to mono-C-glycosyl derivatives O-glycosylated on 2′′.36 All in all, peak 2 was tentatively assigned as apigenin 2′′-O-pentosyl-6-C-hexoside.
Compound 4 showed a pseudomolecular ion ([M − H]− at m/z 577) and a UV spectrum coherent the C-glycosylflavones commonly reported in Glycyrrhiza species isoviolanthin (apigenin-6-C-rhamnoside-8-C-glucoside)34,35,37–39 or violanthin (apigenin-6-C-glucoside-8-C-rhamnoside).34,35 The data obtained in this study do not allow to conclude about the precise pattern of sugar substitution, so that the compound was just identified as (iso)violanthin. Compound 5 also corresponded to another flavone that was tentatively assigned as a methylapigenin O-hexoside based on its UV and mass spectral data.
Compounds 3, 6, 7 and 10 presented the same pseudomolecular ion [M − H]− at m/z 549, all of them releasing a main MS2 fragment at m/z 255, from the loss of 132 + 162 u (pentosyl + hexosyl residues), pointing to the correspond different apiosyl-glucosides of (iso)liquiritigenin, consistently reported to occur as major flavonoids in licorice.34,35,37–43 The fragmentation patterns do not allow to distinguish between liquiritigenin (a flavanone) and isoliquiritigenin (a chalcone), so that they were assigned as derived from one or another based on their UV spectra, showing maxima at 272–276 nm plus a shoulder around 316–318 nm (peaks 3, 6 and 7) or 362 nm (peak 10), respectively. Liquiritin apioside (i.e., liquiritigenin 4′-O-apiosyl-glucoside) has been widely reported to occur in Glycyrrhiza species,34,35,37–45 although other isomers have also been described, such as liquiritigenin 7-O-apiosyl-glucoside40,42,43 and liquiritigenin-7-O-apiosyl-4′-O-glucoside.34 The results obtained herein do not allow concluding about the precise location of the sugar moieties, so that they were just identified as liquiritigenin apiosyl-glucoside isomers. Furthermore, as the carbon at position 2 is asymmetric the possibility of different stereoisomers may be also envisaged.
As previously indicated, compound 10 should correspond to a derivative of the chalcone isoliquiritigenin bearing pentosyl + hexosyl residues. Two main isomers possessing that structure have been widely reported in Glycyrrhiza species: licuroside (also designed as licuraside; isoliquiritigenin-4′-O-apiosyl-glucoside) and neolicuroside (isoliquiritigenin-4-O-apiosyl-glucoside).34,35,37,39,41–43,45 As for the liquiritigenin derivatives, it was not possible to conclude about the precise location of the glycosyl groups, so that compound 10 was assigned as (neo)licuroside.
Compound 8 presented a pseudomolecular ion [M − H]− at m/z 565 releasing a fragment ion at m/z 271 (−294 u, loss of a pentosyl and hexosyl moieties), and a UV spectrum coherent with a flavanone. These characteristics match the structure of naringenin-7-O-apiosyl-glucoside reported in radix Glycyrrhizae by Wang et al. (2014), so that this identity was tentatively assumed for the compound.
Compound 9 ([M − H]− at m/z 561) was tentatively identified as glycyroside (i.e., formononetin-7-O-apiosylglucoside) owing to the previous identification of that isoflavone in radix Glycyrrhizae by Wang et al. (2014). The presence of formononetin derivatives in Glycyrrhiza species has also been reported by various authors.35,41,42,45,46
Finally, it was not possible to identify compound 11 with a pseudomolecular ion [M − H]− at m/z 591 that released two fragments at m/z 297 (−294, loss of a pentosyl and hexosyl moieties) and 282 (further loss of −15 u of a methyl residue), although its UV spectra with a maximum at 372 nm pointed to a chalcone aglycone.
Among the eleven phenolic compounds detected, liquiritin apioside isomers were the most abundant. Many papers have been published profiling phenolic compounds in G. glabra samples from different origins and using different extraction methodologies, some of them cited in the previous discussion.34,35,37–46 However, from all of them, only Montoro et al. (2011) presented quantitative results, although they cannot be compared with ours results since they are expressed differently (mg g−1 of dry plant), thus these authors revealed liquiritin apioside as the main flavonoid present in their sample, which is in agreement with the sample studied herein. In our case, the results were expressed in mg g−1 of extract in order to relate the amounts of phenolic compounds found in the extract to the antioxidant activity. Therefore this study will add new data related to the quantification of these compounds, which are scarce in literature.
3.2. Evaluation of antioxidant activity
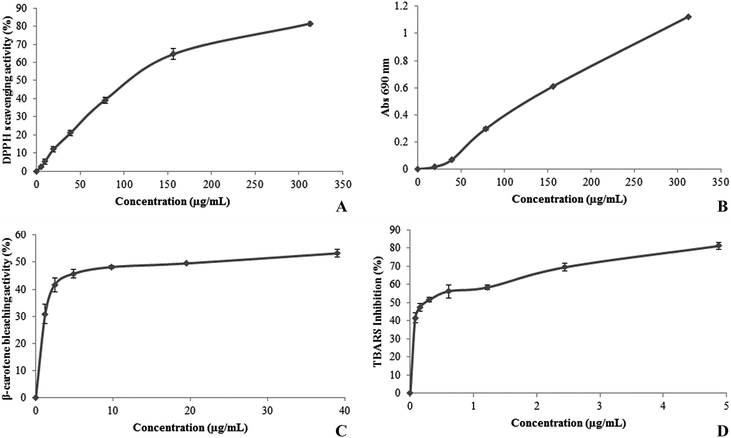
Fig. 1 shows the results of the antioxidant potential of the licorice extract using different assays: DPPH radical scavenging activity (RSA), reducing power (RP), β-carotene bleaching inhibition (CBI) and lipid peroxidation inhibition (LPI) in brain cell homogenates. The most pronounced effect was observed for LPI assay (EC50 = 0.24 ± 0.01 μg mL−1), followed by CBI (EC50 = 22.74 ± 2.42 μg mL−1). RSA and RP presented higher EC50 values (meaning lower antioxidant activity), respectively, 111.54 ± 6.04 μg mL−1 and 128.63 ± 0.21 μg mL−1.The LPI capacity, accessed by using the TBARS assay, measures the malondialdehyde (MDA) formed as the split product of an endoperoxide of unsaturated fatty acids resulting from oxidation of a lipid substrate. The MDA is reacted with thiobarbituric acid (TBA) to form a pink pigment (TBARS) that is measured spectrophotometrically at 532 nm.32
| MDA + TBA → MDA − TBA2 |
| MDA + TBA + A → MDA + TBA2 |
This procedure involves two distinct steps: the substrate is oxidized with the addition of a transition metal ion such as copper or iron or a free radical source such as 2,2′-azobis (2-amidinopropane) dihydrochloride, and then the extent of oxidation is determined by addition of TBA and spectrophotometric measurement of the product (MDA–TBA2). Oxidation is inhibited by the addition of an antioxidant and, therefore, a reduction in the absorbance is observed. In the present experiment, the studied methanol/water extract exerted strong inhibitory effects of lipid oxidation (e.g., exponential inhibition of TBARS formation, being these effects achieved at extremely low concentrations), which is in agreement with the results obtained by Jiang et al.47 that reported the efficacy of licorice ethanolic extract to prevent lipid oxidation and protect sensory attributes of ground pork.
Concerning to the CBI assay, and taking into account the basis of the method, β-carotene undergoes a rapid discoloration in the absence of an antioxidant since the free linoleic acid radical attacks the β-carotene molecule, which loses the double bonds and, consequently, loses its characteristic orange colour. Antioxidants can donate hydrogen atoms to quench radicals and prevent decolourization of carotenoids,48 through the following reactions: β-carotene–H (orange) + ROO˙ → β-carotene˙ (bleached) + ROOH β-carotene–H (orange) + ROO˙ + AH → β-carotene–H (orange) + ROOH + A˙
The decolourization of β-carotene can be monitored by spectrophotometry at 470 nm.49 Regarding the obtained results for the CBI activity of the studied methanol/water extract, a more pronounced effect (EC50 = 23 μg mL−1) was observed than the one reported by Ercisli et al. (2008) for ethanolic extracts of licorice roots collected in Turkey (EC58 = 75 μg mL−1). The results reported by these authors ranged between 28.3% (25 μg mL−1) and 88.7% (800 μg mL−1).
2,2-Diphenyl-1-picrylhydrazyl radical (DPPH), a stable organic nitrogen radical which presents a deep purple colour, allows the determination and quantification of the reducing capacity of antioxidants toward DPPH. Representing the DPPH radical by X˙ and the donor molecule by AH (being mainly phenolic compounds, they are proton donators), the primary reaction is:
| X˙ + AH → XH + A˙ |
In the present reaction, XH is the reduced form and A˙ is the free radical produced in this first step. This latter radical will then undergo further reactions, which control the overall stoichiometry, that is, the number of molecules of DPPH reduced (decolorized) by one molecule of the reductant.50 When a solution of DPPH˙ is mixed with a substance that can donate a hydrogen atom, the reduced form of the radical is generated accompanied by loss of colour. Upon reduction, the colour of DPPH˙ solution fades and this colour change is conveniently monitored measuring the absorbance decrease at 515–528 nm.51 Thus, by using the present assay, the free radicals scavenger effect of licorice was accessed. The RSA obtained for the studied methanol/water extract (EC50 = 112 μg mL−1) was similar to some of the values reported by Cheel et al. (2012) for similar extracts prepared from samples harvested at different times (February – EC70 = 100 μg mL−1, May – EC60 = 30 μg mL−1, August – EC50 = 50 μg mL−1, November – EC50 = 30 μg mL−1), and by Cheel et al. (2010) for extracts obtained by infusion (EC49 = 100 μg mL−1). However, it was lower than the RSA described by Tohma & Gulçin (2010) for aqueous (EC52 = 62 μg mL−1) and ethanol (EC54 = 50 μg mL−1) extracts obtained from roots of Turkish licorice samples.
RP assay, widely used due to its specificity to access the electron-donating potential of antioxidants, and consequent reduction of yellow ferric form to blue ferrous form.52,53 Antioxidant species Fe(III) or Fe(CN)63−, when in the present of composite ferricyanide reagent, favors its reduction, and either Fe(II) or Fe(CN)64− is formed, and combining with a reagent component – Prussian blue, KFe[Fe(CN)6], a coloured product is produced. In this sense, by using Fe3+ in conjunction with Fe(CN)63−, while oxidizing agent, any of the follow two reaction pair could occurs, despite the ending coloured product to be the same:54
| Fe3+ + antioxidant ⇆ Fe2+ + oxidized antioxidant, |
| Fe2+ + Fe(CN)63− ⇆ Fe[Fe(CN)6]−. |
The resultant blue colour is linearly correlated with the total reducing potential of electron-donating antioxidants, being measured spectrophotometrically at 700 nm.55 The RP value obtained in the present study (EC50 = 129 μg mL−1) was similar to the one described by Tohma & Gulçin (2010) for aqueous (EC45 = 62 mg mL−1) and ethanolic (EC76 = 50 mg mL−1) extracts.
Numerous reports have confirmed the association between phenolic compounds and bioactive properties. Regarding G. glabra, flavonoids saponins, coumarins, and stilbenoids have been related with its bioactive properties. Until now, licochalcone A, B, C, D and echinatin, some isoflavones and derivatives, such as glabridin, an isoflavan, hispaglabridin A, hispaglabridin B and 4′-O-methylglabridin, but also some chalcones, namely isoprenylchalcone derivative and isoliquiritigenin, were described as possessing potent antioxidant effects, not only inhibiting lipid peroxidation but also acting as radical scavengers and oxidative process preventers.18,27,56,57 Regarding our study, it is feasible to attribute the antioxidant potential observed for the tested extract to the most abundant phenolic compounds identified, namely liquiritigenin apiosyl-glucosides. Nevertheless, it is important to highlight that plant extracts are usually much more effective than isolated compounds, as it was proved by Cheel et al. (2010) for the case of licorice aqueous extract. The authors verified that, despite in some assays licorice extract evidenced a weak antioxidant activity, the major components identified (liquiritin and glycyrrhizin) presented negligible or even no effects.
Overall, licorice extract could be used as a source of antioxidants for pharmaceutical, cosmetic and/or food industries. Regarding its antioxidants contribution in daily diet, further studies are necessary in order to elucidate the mechanisms of in vivo antioxidant action, bioavailability and involved metabolic pathways.
Acknowledgements
The authors are grateful to Foundation for Science and Technology (FCT, Portugal) for N. Martins grant (SFRH/BD/87658/2012), L. Barros researcher contract under “Programa Compromisso com Ciência – 2008” and financial support to the research center CIMO (strategic project PEst-OE/AGR/UI0690/2011).References
- M. Valko, D. Leibfritz, J. Moncol, M. T. D. Cronin, M. Mazur and J. Telser, Int. J. Biochem. Cell Biol., 2007, 39, 44–84 CrossRef CAS PubMed.
- R. K. Chaturvedi and M. F. Beal, Free Radical Biol. Med., 2013, 63, 1–29 CrossRef CAS PubMed.
- M. Gilca, I. Stoian, V. Atanasiu and B. Virgolici, J. Postgrad. Med., 2007, 53, 207–213 CrossRef CAS PubMed.
- E. Agradi, E. Vegeto, A. Sozzi, G. Fico, S. Regondi and F. Tomè, Phytother. Res., 2006, 20, 670–675 CrossRef CAS PubMed.
- E. A. Palombo, J. Evidence-Based Complementary Altern. Med., 2011, 2011, 1–15 CrossRef PubMed.
- R. R. Mendonça-Filho, in Modern Phytomedicine. Turning Medicinal Plants into Drugs, Wiley-VCH, 2006, pp. 1–24 Search PubMed.
- J. Cheel, P. Van Antwerpen, L. Tůmová, G. Onofre, D. Vokurková, K. Zouaoui-Boudjeltia, M. Vanhaeverbeek and J. Nève, Food Chem., 2010, 122, 508–517 CrossRef CAS PubMed.
- R. P. Singh, S. Sharad and S. Kapur, J. Indian Acad. Clin. Med., 2004, 5, 218–225 Search PubMed.
- M. Carocho and I. C. F. R. Ferreira, Food Chem. Toxicol., 2013, 51, 15–25 CrossRef CAS PubMed.
- M. Carocho and I. C. F. R. Ferreira, Anti-Cancer Agents Med. Chem., 2013, 13, 1236–1258 CrossRef CAS.
- B. Halliwell, Nutr. Rev., 2012, 70, 257–265 CrossRef PubMed.
- L. Rubió, M.-J. Motilva and M.-P. Romero, Crit. Rev. Food Sci. Nutr., 2013, 53, 943–953 CrossRef PubMed.
- J. Dai and R. J. Mumper, Molecules, 2010, 15, 7313–7352 CrossRef CAS PubMed.
- J. S. Ramkissoon, M. F. Mahomoodally, N. Ahmed and A. H. Subratty, Asian Pac. J. Trop. Med., 2013, 6, 561–569 CrossRef CAS.
- G. Calapai and M. Delbò, Eur. Med. Agency – Sci. Med. Heal., 2012, 1–40 Search PubMed.
- V. S. Jatav, S. K. Singh, P. Khatri and A. K. Sharma, Int. J. Pharm. Res., 2011, 1, 170–185 Search PubMed.
- M. T. Murray, The healing power of herbs, Random House, New York, NY, 2nd edn, 2004 Search PubMed.
- R. Kaur, H. Kaur and A. S. Dhindsa, Int. J. Pharma Sci. Res., 2013, 4, 2470–2477 Search PubMed.
- G. H. Naik, K. I. Priyadarsini, J. G. Satav, M. M. Banavalikar, D. P. Sohoni, M. K. Biyani and H. Mohan, Phytochemistry, 2003, 63, 97–104 CrossRef CAS.
- H. S. Tohma and I. Gulçin, Int. J. Food Prop., 2010, 13, 657–671 CrossRef CAS.
- J. Vaya, P. A. Belinky and M. Aviram, Free Radical Biol. Med., 1997, 23, 302–313 CrossRef CAS.
- S. Ercisli, I. Coruh, A. Gormez, M. Sengul and S. Bilen, Ital. J. Food Sci., 2008, 20, 91–100 CAS.
- I. Khalaf, L. Vlase, D. Lazãr, A. Corciovã, B. Ivãnescu and M. I. Lazãr, Farmacia, 2010, 58, 416–421 CAS.
- L. Siracusa, A. Saija, M. Cristani, F. Cimino, M. D'Arrigo, D. Trombetta, F. Rao and G. Ruberto, Fitoterapia, 2011, 82, 546–556 CrossRef CAS PubMed.
- S. D. Angelo, A. Morana, A. Salvatore, V. Zappia and P. Galletti, J. Med. Food, 2009, 12, 1326–1333 CrossRef PubMed.
- Y.-J. Li, J. Chen, Y. Li, Q. Li, Y.-F. Zheng, Y. Fu and P. Li, J. Chromatogr. A, 2011, 1218, 8181–8191 CrossRef CAS PubMed.
- J. Cheel, L. Tůmová, C. Areche, P. Van Antwerpen, J. Nève, K. Zouaoui-Boudjeltia, A. S. Martin, I. Vokřál, V. Wsól and J. Neugebauerová, Acta Physiol. Plant., 2012, 35, 1337–1349 CrossRef PubMed.
- L. Barros, C. T. Alves, M. Dueñas, S. Silva, R. Oliveira, A. M. Carvalho, M. Henriques, C. Santos-Buelga and I. C. F. R. Ferreira, Ind. Crops Prod., 2013, 44, 104–110 CrossRef CAS PubMed.
- T. Hatano, H. Kagawa, T. Yasuhara and T. Okuda, Chem. Pharm. Bull., 1988, 36, 2090–2097 CrossRef CAS.
- M. Oyaizu, Jpn. J. Nutr., 1986, 44, 307–315 CrossRef CAS.
- S. Mi-Yae, K. Tae-Hun and S. Nak-Ju, Food Chem., 2003, 82, 593–597 CrossRef.
- T. B. Ng, F. Liu and Z. Wang, Life Sci., 2000, 66, 709–723 CrossRef CAS.
- F. Ferreres, B. M. Silva, P. B. Andrade, R. M. Seabra and M. A. Ferreira, Phytochem. Anal., 2003, 14, 352–359 CrossRef CAS PubMed.
- S. Wang, L. Chen, J. Leng, P. Chen, X. Fan and Y. Cheng, J. Pharm. Biomed. Anal., 2014, 98, 22–35 CrossRef CAS PubMed.
- Q. Yin, P. Wang, A. Zhang, H. Sun, X. Wu and X. Wang, J. Sep. Sci., 2013, 36, 1238–1246 CrossRef CAS PubMed.
- F. Ferreres, C. Sousa, P. Valentão, P. B. Andrade, R. M. Seabra and Á. Gil-Izquierdo, J. Agric. Food Chem., 2007, 55, 10187–10193 CrossRef CAS PubMed.
- M. A. Farag, A. Porzel and L. A. Wessjohann, Phytochemistry, 2012, 76, 60–72 CrossRef CAS.
- P. Montoro, M. Maldini, M. Russo, S. Postorino, S. Piacente and C. Pizza, J. Pharm. Biomed. Anal., 2011, 54, 535–544 CrossRef CAS PubMed.
- Y. Wang, S. He, X. Cheng, Y. Lu, Y. Zou and Q. Zhang, J. Pharm. Biomed. Anal., 2013, 80, 24–33 CrossRef CAS PubMed.
- W. C. Liao, Y.-H. Lin, T.-M. Chang and W.-Y. Huang, Food Chem., 2012, 132, 2188–2193 CrossRef CAS PubMed.
- X. Qiao, M. Ye, C. Xiang, Q. Wang, C.-F. Liu, W.-J. Miao and D. Guo, J. Chromatogr. A, 2012, 1258, 84–93 CrossRef CAS PubMed.
- T. Xu, M. Yang, Y. Li, X. Chen, Q. Wang, W. Deng, X. Pang, K. Yu, B. Jiang, S. Guan and D. Guo, Rapid Commun. Mass Spectrom., 2013, 27, 2297–2309 CrossRef CAS PubMed.
- C. Simmler, T. Jones, J. R. Anderson, D. C. Nikolić, R. B. van Breemen, D. D. Soejarto, S.-N. Chen and G. F. Pauli, Phytochem. Anal., 2014, 25, 378–388 CrossRef CAS.
- M. Ye, S.-H. Liu, Z. Jiang, Y. Lee, R. Tilton and Y.-C. Cheng, Rapid Commun. Mass Spectrom., 2007, 21, 3593–3607 CrossRef CAS PubMed.
- R. Simons, J.-P. Vincken, E. J. Bakx, M. A. Verbruggen and H. Gruppen, Rapid Commun. Mass Spectrom., 2009, 23, 3083–3093 CrossRef CAS PubMed.
- J. Xie, W. Wang, Y. Zhang, Y. Bai and Q. Yang, J. Pharm. Biomed. Anal., 2007, 45, 450–455 CrossRef CAS PubMed.
- J. Jiang, X. Zhang, A. D. True, L. Zhou and Y. L. Xiong, J. Food Sci., 2013, 78, C1686–C1694 CrossRef CAS PubMed.
- I. P. Kaur and T. Geetha, Mini-Rev. Med. Chem., 2006, 6, 305–312 CrossRef CAS.
- A. R. Ndhlala, M. Moyo and J. V. Staden, Molecules, 2010, 15, 6905–6930 CrossRef CAS.
- P. Molyneux, Songklanakarin J. Sci. Technol., 2004, 26, 211–219 CAS.
- A. Karadag, B. Ozcelik and S. Samim, Food Analytical Methods, 2009, 2, 41–60 CrossRef.
- I. F. F. Benzie, W. Y. Chung and J. J. Strain, J. Nutr. Biochem., 1999, 10, 146–150 CrossRef CAS.
- F. S. Reis, A. Martins, L. Barros and I. C. F. R. Ferreira, Food Chem. Toxicol., 2012, 50, 1201–1207 CrossRef CAS PubMed.
- K. I. Berker, K. Güçlü, I. Tor and R. Apak, Talanta, 2007, 72, 1157–1165 CrossRef CAS PubMed.
- D. Huang, B. Ou and R. L. Prior, J. Agric. Food Chem., 2005, 53, 1841–1856 CrossRef CAS PubMed.
- M. N. Asl and H. Hosseinzadeh, Phytother. Res., 2008, 22, 709–724 CrossRef CAS PubMed.
- Y. W. Chin, H. A. Jung, Y. Liu, B. N. Su, J. A. Castoro, W. J. Keller, M. A. Pereira and A. D. Kinghorn, J. Agric. Food Chem., 2007, 55, 4691–4697 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |