An efficient heterogenized palladium catalyst for N-alkylation of amines and α-alkylation of ketones using alcohols†
Tuan Thanh Dang,
Siah Pei Shan,
Balamurugan Ramalingam and
Abdul Majeed Seayad*
Organic Chemistry, Institute of Chemical and Engineering Sciences (ICES), 8 Biomedical Grove, #07-01 Neuros, Singapore 138665. E-mail: abdul_seayad@ices.a-star.edu.sg
First published on 6th May 2015
Abstract
A silica supported palladium–NiXantphos complex is reported as an efficient and a high turnover heterogeneous catalyst for the N-alkylation of amines and the α-alkylation of ketones using readily available alcohols under neat conditions at 120–140 °C following hydrogen borrowing strategy. The catalyst is easily separable and offers negligible amount of palladium leaching (<0.01 ppm). A high turnover number of about 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for the N-alkylation of amines and 4400 for the α-alkylation of ketones were achieved in the respective single batch reactions. The catalyst is recyclable up to four times without appreciable change in catalytic performance.
000 for the N-alkylation of amines and 4400 for the α-alkylation of ketones were achieved in the respective single batch reactions. The catalyst is recyclable up to four times without appreciable change in catalytic performance.
Introduction
C–N and C–C bond forming reactions are two indispensable transformations in organic synthesis as well as pharmaceutical and fine chemical industries.1 Conventional alkylation methods that utilize reactive and toxic alkyl halides have been in practice for the past several decades as the most convenient method for the synthesis of alkylated amines and α-alkylated ketones.2 This method often produces over-alkylated products causing selectivity issues that not only reduces the overall yield of the desired product but also poses separation issues apart from generating toxic halogenated waste, which needs further downstream processing. Under this context, utilization of readily available and less-toxic alcohols as alkylating agents following the recently developed catalytic hydrogen borrowing strategy3 offers a green and sustainable alternative method for the direct alkylation of amines and ketones.Ru4 and Ir5 remain to be the preferred choice of metals for developing promising catalysts for the direct alkylation of amines using alcohols under homogeneous reaction conditions. Other metals such as Rh,6 Au,7 Cu,8 Fe,9 Pd10 and Os11 were also reported as alternative catalysts. Although, these developments in homogeneous catalysts are remarkable achieving turn-over-numbers (TON) up to 1000,10a several heterogeneous catalysts were also reported12 to address the catalyst-product separation and recycle issues. Apart from the requirement of stringent conditions, and limited substrate scope with most of these heterogeneous catalyst systems, the main drawback is the low turnover numbers, which limit their potential applications.
Supported metal nanoparticles, oxides and hydroxides of Ru,13 Ir,14 Au,15 Ag,16 Pd,17 Ni,18 Cu,19 Fe,20 and Mn,21 as well as unmodified magnetite22 were reported to be promising heterogeneous catalysts for N-alkylation using various alcohols at temperatures of 100–180 °C to achieve 50–98% yields. An improved TON of about 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 was reported using Au/TiO2 catalyst at 180 °C for 96 h.15e Under similar condition, a TON of 570 was reported using Pd/MgO17e as a solid catalyst for the mono-alkylation of aniline in trifluorotoluene. Bimetallic heterogeneous catalysts23 were also reported for N-alkylation of amines using alcohols as well as diols. The metal leaching and the recyclability of many of these heterogeneous catalysts are not well reported.
800 was reported using Au/TiO2 catalyst at 180 °C for 96 h.15e Under similar condition, a TON of 570 was reported using Pd/MgO17e as a solid catalyst for the mono-alkylation of aniline in trifluorotoluene. Bimetallic heterogeneous catalysts23 were also reported for N-alkylation of amines using alcohols as well as diols. The metal leaching and the recyclability of many of these heterogeneous catalysts are not well reported.
Heterogenization of active homogeneous catalysts is another approach24 to circumvent the separation and recyclability problems associated with efficient homogeneous catalysts. Accordingly, heterogenization of an active N-heterocyclic carbene (NHC) iridium complex on mesoporous silica (SBA-15)14b was reported for the N-alkylation of amines and β-alkylation of secondary alcohols in the presence of 50 mol% a base. Though the catalyst gave high yields at 110 °C for 24–48 h and was recyclable, the catalyst loading was high (1.5 mol%) that limits the TON (<70). Recently, we have developed a reusable ruthenium catalyst13a for the N-alkylation of amines at 120 °C under batch (5 h) and continuous flow conditions (30 min residence-time). In our continued effort to develop efficient heterogeneous catalyst systems, herein we report a silica supported-palladium NiXantphos complex as an efficient and high turnover catalyst for the alcohol activation towards N- and C-alkylation reactions.
Results and discussion
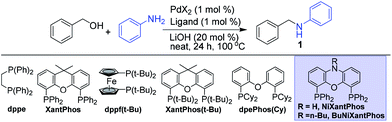
In our previous work,10a we have reported an in situ formed homogeneous palladium catalyst from PdCl2 and a bidentate phosphine ligand such as dppe, Xantphos(t-Bu), etc., as an efficient catalyst for the N-alkylation of amines under relatively mild operating conditions. In order to select a suitable bidentate ligand for the facile anchoring on readily available and inexpensive solid supports, such as silica, to develop the corresponding heterogeneous analogue, we have further screened other promising bidendate ligands for the N-alkylation of aniline using benzyl alcohol and the representative results are given in Table 1. We have observed that Xantphos(t-Bu) is still one of the best bidendate ligands screened giving near quantitative yield of the N-alkylated product at 100 °C for 24 h (entry 3). The corresponding commercially available NiXantphos, which is easy to anchor through the free N–H group, gave a promising yield of 86% (entry 6). To our delight, quantitative yield was achieved (entry 7) when the free N–H group of NiXantphos was protected using alkyl groups such as n-Bu to mimic the tethering group for anchoring to the solid support. Under homogeneous conditions, PdCl2 gave higher performance compared to that of Pd(OAc)2 (entry 7 vs. 8). Encouraged by these results, NiXantphos was selected as the potential ligand to anchor on to the readily available solid support such as silica to prepare the heterogenized palladium catalyst.| Entry | PdX2 | Ligand | Product, 1b (%) |
|---|---|---|---|
a Reaction condition: aniline (2 mmol), benzyl alcohol (3 equiv.), catalyst (1 mol%, Pd/L = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), LiOH (20 mol%) 100 °C, 24 h.b NMR yield using mesitylene as the internal standard. 1), LiOH (20 mol%) 100 °C, 24 h.b NMR yield using mesitylene as the internal standard. |
|||
| 1 | PdCl2 | dppe | 96 |
| 2 | PdCl2 | Xantphos | 34 |
| 3 | PdCl2 | Xantphos(t-Bu) | >99 |
| 4 | PdCl2 | dpePhos(Cy) | 95 |
| 5 | PdCl2 | dppf(t-Bu) | 89 |
| 6 | PdCl2 | NiXantphos | 86 |
| 7 | PdCl2 | BuNiXantphos | >99 |
| 8 | Pd(OAc)2 | BuNiXantphos | 83 |
Immobilization of NiXantphos on solid supports was reported earlier for the rhodium catalyzed hydrofomylation25 and for the palladium catalyzed allylic alkylation26 of diethyl 2-methylmalonate. Adopting a similar procedure, we have prepared silica supported Pd–NiXantphos catalysts in a modified one-pot protocol as depicted in Scheme 1. The alkylation of NiXantphos with 3-chloropropyl(trimethoxy)silane afforded the intermediate alkylated Si–Pr–NiXantphos, which was subsequently anchored on to silica to obtain Si–Pr–NiXantphos/SiO2. Addition of palladium(II) precursor such as PdCl2 or Pd(OAc)2 in toluene at 80 °C resulted in the formation of the solid Pd–Si–Pr–NiXantphos/SiO2 catalysts A and B, which were filtered under argon and washed with dry toluene to remove impurities.
The silica supported palladium catalysts were characterized by solid state NMR, XPS and ICP methods. For example, catalyst B gave 13C NMR signals in the region 10–50 ppm and 110–150 ppm in the solid state NMR spectrum (Fig. 1) indicating the presence of aliphatic and aromatic carbons corresponding to that of the Si–Pr–NiXantphos ligand. The presence of 3d5/2 peak at 337.21 eV in XPS spectra corresponds to that of Pd(II)–phosphine complex. The amount of palladium present in the catalysts A and B were found to be 2.31 wt% and 2.23 wt% respectively by ICP analysis.
To our surprise, we observed that only very small amount of palladium is required to achieve good yield of the N-alkylated product when using the solid catalysts A or B. Accordingly, up to 65% yield was observed with 0.043 mol% of palladium at 100 °C for 24 h that provided a high TON of 1511 as shown in Table 2 (entry 1) for the N-alkylation of aniline using benzyl alcohol with catalyst A. Under these conditions, catalyst B gave better results with a TON of 1928 (entry 2) and was selected for further optimization. Upon increasing the temperature to 120 °C, quantitative yield with an improved TON of 2381 was observed. Decreasing LiOH from 20 mol% to 10 mol% had only a marginal decrease in the catalytic performance. Decreasing the catalyst loading to 0.021 mol% and 0.0105 mol% of palladium gave further improved TON of 4666 and 7429 respectively (entries 5 and 6).
| Entry | Cat. (mg, Pd mol%) | LiOH (equiv.) | Temp. (°C) | Product, 1b (%) | TON |
|---|---|---|---|---|---|
| a Reaction condition: aniline (2 mmol), benzyl alcohol (3 equiv.), LiOH (20 mol%) 120 °C, 24 h.b NMR yield using mesiltylene as an internal standard. No alkylation reaction was observed in the absence of catalyst A or B. | |||||
| 1 | A (4, 0.043) | 0.2 | 100 | 65 | 1511 |
| 2 | B (4, 0.042) | 0.2 | 100 | 81 | 1928 |
| 3 | B (4, 0.042) | 0.2 | 120 | >99 | 2381 |
| 4 | B (4, 0.042) | 0.1 | 120 | 95 | 2262 |
| 5 | B (2, 0.021) | 0.2 | 120 | 98 | 4666 |
| 6 | B (1, 0.0105) | 0.2 | 120 | 78 | 7429 |
After achieving the high TON, we have evaluated the substrate scope and limitations using the catalyst B for the N-alkylation of various amines using primary and secondary alcohols. In most cases, the desired products were obtained in good to excellent isolated yields as given in Table 3. Aniline and 4-methylaniline were benzylated to the corresponding mono substituted anilines 1 and 2 in 94% and 98% isolated yields respectively at 120 °C. Less reactive and challenging substrates required slightly higher temperature or increased catalyst loading. Electron deficient anilines having substituents such as Cl, F and CF3 were benzylated at 130 °C for 24 h to give the corresponding products 3, 4 and 5 in excellent yields up to 99%. Electron rich linear aliphatic amines such as n-hexyl amine and n-octyl amine were also benzylated under similar conditions to afford the secondary amines 6 (94%) and 7 (86%) in excellent yields. α-Branched amines were also found to be good substrates and the corresponding alkylated products 8 and 9 were obtained in 82% and 88% yields respectively. Less reactive aliphatic alcohols such as n-octanol and 2-ethyl-1-butanol could also be used as electrophilic partner for obtaining the secondary amines 10–12 in 75–83% yields.
Involvement of unfavorable iminium intermediate makes the cyclic amines generally less reactive under hydrogen borrowing conditions. However, the current heterogeneous Pd catalyst promotes the alkylation of cyclic amines such as piperidine, pyrrolidine, azepane, morpholine, tetralin and N-methyl piperazine to the corresponding teritary amines 13–20 in 75–92% isolated yields. The alkylation of amines with challenging secondary alcohols such as 1-phenyl ethanol, 2-octanol and cyclohexanol also gave the corresponding alkylated products 21, 22 and 23 in very good isolated yields (81–87%) in the case of cyclohexylamine as the substrate. With aromatic amines such as aniline, the alkylated product 24 was obtained in 53% isolated yield when cyclohexanol was used as the alkylating agent. The alkylation of piperidine with 2-octanol resulted in 65% isolated yield of the desired product 25. In most cases, higher TON was observed compared to the literature reports.
Transition metals catalyzed α-alkylation of ketones using alcohols following hydrogen borrowing strategy also attracted a greater attention in recent years as it provides an environmentally attractive protocol compared to the classical alkylation methods.27 Most of the catalysts reported were based on Ru and Ir complexes and required stoichiometric amount of an inorganic base to achieve very good yields. Palladium was also explored as an alternative catalyst for α-alkylation using alcohols. For example, a homogeneous Pd–NHC catalyst28 was reported for the α-alkylation of acetophenone using benzyl alcohol at 125 °C giving a mixture of alcohol and ketone products in 20% and 56%, respectively. A heterogeneous Pd catalyst (Pd/AlOOH)29 was reported that gave a TON of up to 490 for the α-alkylation of ketones using a variety of alcohols at 80 °C. Palladium nanoparticles supported on viologen30 was reported under solvent free condition, however the TON was found to be very low (<20). Recently, Pd/C was reported as a catalyst in water as the solvent at temperatures of 140–160 °C in the presence of 1–3 equivalents of base giving up to 93% yield with a TON of 46.31
We have explored the potential use of the silica supported palladium Ni-Xantphos catalyst B for the α-alkylation of ketones in the presence of sub-stoichiometric amounts of base under solventless conditions. As given in Table 4, α-alkylation of methyl ketones were achieved at 140 °C in the presence of only 20 mol% of LiOH as a base providing excellent yields of the α-alkylated ketones 26–37 with a TON of up to 467. Under these pre-optimized conditions acetophenone gave 1,3-diphenylpropan-1-one (26) in 83% yield, while 98% and 96% yields were achieved with 4-methyl (27) and penta-methyl (28) substituted acetophenones respectively, when benzyl alcohol was used as the alkylating agent. Unprotected hydroxy group was tolerated and the corresponding alkylated product 29 was obtained in 81% yield. Other aromatic methyl ketones such as naphthyl, ferrocenyl and furanyl ketones were also excellent substrates and the corresponding benzylated products 30–32 were obtained in 74–98% yield. Excellent yields were achieved in the case of cyclic (33) and acyclic (34) ketones. Less reactive aliphatic alcohol such as 1-octanol was also found to be a very good alkylating agent and the alkylated products 35 and 36 were obtained in 67% and 73%, respectively. In addition, the alkylation of acetophenone with substituted benzyl alcohol such as 3,5-dimethylbenzyl alcohol afforded the ketone product 37 in 75% isolated yield.
| a Reaction condition: ketone (2 mmol, 1 equiv.), alcohol (6 mmol, 3 equiv.), LiOH (20 mol%), catalyst B (20 mg, 0.21 mol% of Pd, TON, 319–467). |
|---|
 |
Further, we have investigated the recyclability of the silica supported palladium–Ni-Xantphos catalyst B by taking N-alkylation of aniline using benzyl alcohol at 120 °C for 24 h as the representative reaction. 4 mg (0.042 mol%) of catalyst B was used for easy handling and the catalyst was recovered by simple filtration under argon and reused for subsequent reactions. Unfortunately, the yield of the mono-alkylated amine decreased to 59% for the first recycle and 22% in the second recycle compared to 99% in the first reaction (Fig. 2). This may be due to the decomposition of the Pd(II) catalyst either in presence of water produced in the reaction or in contact with small amount of air during the recycling procedure.
Improved recyclability was observed when the reaction was carried out in the presence of molecular sieves (50 mg) to partially absorb the water produced during the reaction, providing 99%, 98% and 45% yields respectively, in first, second and third runs.32 Though the addition of molecular sieves improved the recyclability, higher amount of solids in the reaction mixture may not be very much practicable. Hence, we decided to improve the stability of the catalyst by increasing the ligand to palladium ratio on the silica support and two new catalysts C (0.95 wt% Pd) and D (0.52 wt% Pd) with ligand/Pd ratios of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 6
1 and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 respectively were synthesized. Both the catalysts C and D were found to be active and not only gave near quantitative yields under the standard reaction conditions, but also provided higher recyclability compared to that of the catalyst B (Fig. 2). The catalyst D with a ligand/Pd ratio of 6
1 respectively were synthesized. Both the catalysts C and D were found to be active and not only gave near quantitative yields under the standard reaction conditions, but also provided higher recyclability compared to that of the catalyst B (Fig. 2). The catalyst D with a ligand/Pd ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was found to be more stable and could be recycled up to four times without significant loss in activity as shown in Fig. 2. A total TON of about 16
1 was found to be more stable and could be recycled up to four times without significant loss in activity as shown in Fig. 2. A total TON of about 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 was achieved after 6 runs using the catalyst D. The palladium leaching in each run was found to be less than 0.01 ppm.32 In order to further investigate the stability of the catalyst D, the representative N-alkylation of aniline with benzyl alcohol was carried out with very low catalyst loading (2 mg, 0.00196 mol%) for 5 mmol of substrate and a surprisingly high TON of about 46
000 was achieved after 6 runs using the catalyst D. The palladium leaching in each run was found to be less than 0.01 ppm.32 In order to further investigate the stability of the catalyst D, the representative N-alkylation of aniline with benzyl alcohol was carried out with very low catalyst loading (2 mg, 0.00196 mol%) for 5 mmol of substrate and a surprisingly high TON of about 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 was achieved in 72 h of reaction with 91% yield.32 A high TON of about 4400 was also achieved in the case of α-alkylation of 4-methyl acetophenone with benzyl alcohol using 0.0146 mol% catalyst D for 5 mmol of substrate, providing 65% yield in 96 h.32
000 was achieved in 72 h of reaction with 91% yield.32 A high TON of about 4400 was also achieved in the case of α-alkylation of 4-methyl acetophenone with benzyl alcohol using 0.0146 mol% catalyst D for 5 mmol of substrate, providing 65% yield in 96 h.32
The catalytic cycle may be initiated by the formation of an in situ formed palladium alkoxide species on the silica support and proceeds through hydrogen borrowing mechanism similar to that reported for the homogeneous palladium catalyst.10a
Conclusions
In conclusion, we have reported silica supported palladium–NiXantphos as an efficient and high turnover heterogeneous catalyst for the N-alkylation of amines and α-alkylation of ketones using a variety of alcohols including challenging aliphatic and secondary alcohols as alkylating agents under solvent free conditions. The catalyst can be readily recovered from the reaction mixture for subsequent runs and offers a very low palladium leaching of less than 0.01 ppm. The catalyst provided very high turnover numbers (TON) compared to most of the reported catalysts and a remarkably high TON of up to 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 was achieved in a single batch reaction for the N-alkylation of amine using alcohols. The catalyst can be recycled up to four times without appreciable change in catalytic performance. A high TON of about 4400 was also achieved for the α-alkylation of ketone in the presence of only 20 mol% of base under solventless conditions. Though the supported palladium catalysts B, C and D provided similar catalytic performance in a single batch reaction, the catalyst D having higher ligand to Pd ratio is superior and is preferred in terms of stability and recyclability.
000 was achieved in a single batch reaction for the N-alkylation of amine using alcohols. The catalyst can be recycled up to four times without appreciable change in catalytic performance. A high TON of about 4400 was also achieved for the α-alkylation of ketone in the presence of only 20 mol% of base under solventless conditions. Though the supported palladium catalysts B, C and D provided similar catalytic performance in a single batch reaction, the catalyst D having higher ligand to Pd ratio is superior and is preferred in terms of stability and recyclability.
Experimental
General procedure for the one-pot preparation of solid catalysts
10 mL of dry toluene, 55.2 mg of NiXantphos (0.1 mmol), NaH (0.3 mmol) were charged under argon-atmosphere in a Schlenk tube followed by additions of (3-chloropropyl)triethoxysilane (0.3 mmol). The reaction tube was closed with the Teflon stopper and was heated to 80 °C under stirring for 12 h. 250 mg of dry SiO2 was introduced in to the reaction mixture and stirring continued at 80 °C for another 12 h. The reaction mixture was cooled to room temperature and the calculated amount of Pd precursor (Pd(OAc)2) was introduced under argon and the stirring continued at 80 °C for another 4 h. The reaction mixture was allowed to cool to RT and the brown solid catalyst was collected by filtration and washed with toluene (3 times, 20 mL) under argon atmosphere. The solid catalyst was dried in a vacuum oven at 50 °C overnight, collected and stored under argon atmosphere.General procedure for the N-alkylation of amine with alcohol
Catalyst B (2–20 mg), LiOH (20.0 mol%), alcohol (6.0 mmol), and amine (2.0 mmol) were charged under argon-atmosphere in a Radleys carrousel reaction tube. The reaction tube was closed with the Teflon stopper and was heated to the desired temperature under stirring. After the desired reaction time, the reaction mixture was allowed to cool to room temperature and diluted with toluene (10 mL). SiO2 (400 mg) was added into the crude mixture. The organic solvent was removed under vacuum and the product was purified by column chromatography.General procedure for the α-alkylation of ketones with alcohols
Catalyst B (20 mg), LiOH (20.0 mol%), alcohol (6.0 mmol), and ketone (2.0 mmol) were charged under argon-atmosphere in a Radleys carrousel reaction tube. The reaction tube was closed with the Teflon stopper and was heated to the desired temperature under stirring. After the desired reaction time, the reaction mixture was allowed to cool to room temperature and diluted with toluene (10 mL). SiO2 (400 mg) was added into the crude mixture. The organic solvent was removed under vacuum and the product was purified by column chromatography.Acknowledgements
This work was funded by “GSK—Singapore Partnership for Green and Sustainable manufacturing and the Institute of Chemical and Engineering Sciences (ICES), Agency for Science, Technology and Research (A*STAR), Singapore.Notes and references
- (a) A. de Meijere and F. Diederich, in Metal-Catalyzed Cross-Coupling Reactions, Wiley-VCH, Weinheim, 2004 Search PubMed; (b) Modern amination methods, ed. A. Ricci, Wiley-VCH, Weinheim, 2000 Search PubMed; (c) A. R. Cartolano and G. A. Vedage, in Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Inc, New York, 2004, vol. 2, pp. 476–498 Search PubMed; (d) Amino Group Chemistry: From Synthesis to the Life Sciences, ed. A. Ricci, Wiley-VCH, Weinheim, 2008 Search PubMed; (e) Amines: Synthesis, Properties, and Applications, ed. S. A. Lawrence, Cambridge University, Cambridge, 2006 Search PubMed.
- (a) F. A. Carey and R. J. Sundberg, in Advanced Organic Chemistry, Part B: Reaction and Synthesis, Springer, 5th edn, 2007 Search PubMed; (b) M. B. Smith and J. March, March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, Wiley, 6th edn, 2007 Search PubMed; (c) T. C. Nugenta and M. El-Shazlya, Adv. Synth. Catal., 2010, 352, 753–819 CrossRef PubMed; (d) S. Gomez, J. A. Peters and T. Maschmeyer, Adv. Synth. Catal., 2002, 344, 1037–1057 CrossRef CAS; (e) R. N. Salvatore, C. H. Yoon and K. W. Jung, Tetrahedron, 2001, 57, 7785–7811 CrossRef CAS; (f) D. M. Hodgson and A. Charlton, Tetrahedron, 2014, 70, 2207–2236 CrossRef CAS PubMed.
- For reviews see: (a) D. Hollmann, ChemSusChem, 2014, 7, 2411–2413 CrossRef CAS PubMed; (b) M. Pera-Titus and F. Shi, ChemSusChem, 2014, 7, 720–722 CrossRef CAS PubMed; (c) S. Bahn, S. Imm, L. Neubert, M. Zhang, H. Neumann and M. Beller, ChemCatChem, 2011, 3, 1853–1864 CrossRef PubMed; (d) G. E. Dobereiner and R. H. Crabtree, Chem. Rev., 2010, 110, 681–703 CrossRef CAS PubMed; (e) G. Guillena, D. J. Ramon and M. Yus, Chem. Rev., 2010, 110, 1611–1641 CrossRef CAS PubMed; (f) A. J. A. Watson and J. M. J. Williams, Science, 2010, 329, 635–636 CrossRef CAS PubMed; (g) T. D. Nixon, M. K. Whittlesey and J. M. J. Williams, Dalton Trans., 2009, 753–762 RSC; (h) M. H. S. A. Hamid, P. A. Slatford and J. M. J. Williams, Adv. Synth. Catal., 2007, 349, 1555–1575 CrossRef CAS PubMed.
- Recent references on ruthenium based homogeneous catalysts: (a) S. P. Shan, X. Xiaoke, B. Gnanaprakasam, T. T. Dang, B. Ramalingam, H. V. Huynh and A. M. Seayad, RSC Adv., 2015, 5, 4434–4442 RSC; (b) R. Ramachandran, G. Prakash, S. Selvamurugan, P. Viswanathamurthi, J. G. Malecki, W. Linert and A. Gusev, RSC Adv., 2015, 5, 11405–11422 RSC; (c) V. R. Jumde, L. Gonsalvi, A. Guerriero, M. Peruzzini and M. Taddei, Eur. J. Org. Chem., 2015, 1829–1833 CrossRef CAS PubMed; (d) S. Demir, F. Coskun and I. Ozdemir, J. Organomet. Chem., 2014, 755, 134–140 CrossRef CAS PubMed; (e) R. Ramachandran, G. Prakash, S. Selvamurugan, P. Viswanathamurthi, J. G. Malecki and V. Ramkumar, Dalton Trans., 2014, 7889–7902 RSC; (f) N. J. Oldenhuis, V. M. Dong and Z. B. Guan, J. Am. Chem. Soc., 2014, 136, 12548–12551 CrossRef CAS PubMed; (g) A. Rodriguez-Barzano, J. D. A. Fonseca, A. J. Blacker and P. C. McGowan, Eur. J. Inorg. Chem., 2014, 1974–1983 CrossRef CAS PubMed; (h) F. Xie, M. M. Chen, X. T. Wang, H. F. Jiang and M. Zhang, Org. Biomol. Chem., 2014, 12, 2761–2768 RSC; (i) A. E. Putra, Y. Oe and T. Ohta, Eur. J. Org. Chem., 2013, 6146–6151 CrossRef PubMed; (j) W. Baumann, A. Spannenberg, J. Pfeffer, T. Haas, A. Kockritz, A. Martin and J. Deutsch, Chem.–Eur. J., 2013, 19, 17702–17706 CrossRef CAS PubMed; (k) D. Pingen, O. Diebolt and D. Vogt, ChemCatChem, 2013, 5, 2905–2912 CrossRef CAS PubMed; (l) M. Zhang, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2013, 52, 597–601 CrossRef CAS PubMed; (m) S. Agrawal, M. Lenormand and B. Martin-Matute, Org. Lett., 2012, 14, 1456–1459 CrossRef CAS PubMed; (n) F. E. Fernandez, M. C. Puerta and P. Valerga, Organometallics, 2012, 31, 6868–6879 CrossRef CAS; (o) F. Li, J. J. Xie, H. X. Shan, C. L. Sun and L. Chen, RSC Adv., 2012, 2, 8645–8652 RSC; (p) J. Y. Luo, M. Y. Wu, F. H. Xiao and G. J. Deng, Tetrahedron Lett., 2011, 52, 2706–2709 CrossRef CAS PubMed; (q) S. Imm, S. Baehn, M. Zhang, L. Neubert, H. Neumann, F. Klasovsky, J. Pfeffer, T. Haas and M. Beller, Angew. Chem., Int. Ed., 2011, 50, 7599–7603 CrossRef CAS PubMed; (r) A. J. A. Watson, A. C. Maxwell and J. M. J. Williams, J. Org. Chem., 2011, 76, 2328–2331 CrossRef CAS PubMed; (s) Y. Liu, W. Chen, C. Feng and G. J. Deng, Chem.–Asian J., 2011, 6, 1142–1146 CrossRef CAS PubMed; (t) S. Baehn, S. Imm, L. Neubert, M. Zhang, H. Neumann and M. Beller, Chem.–Eur. J., 2011, 17, 4705–4708 CrossRef CAS PubMed; (u) S. Imm, S. Baehn, L. Neubert, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2010, 49, 8126–8129 CrossRef CAS PubMed; (v) S. Baehn, S. Imm, K. Mevius, L. Neubert, A. Tillack, J. M. J. Williams and M. Beller, Chem.–Eur. J., 2010, 16, 3590–3593 CrossRef CAS PubMed; (w) B. Sundararaju, Z. Tang, M. Achard, G. V. M. Sharma, L. Toupet and C. Bruneau, Adv. Synth. Catal., 2010, 352, 3141–3146 CrossRef CAS PubMed; (x) D. Pingen, C. Muller and D. Vogt, Angew. Chem., Int. Ed., 2010, 49, 8130–8133 CrossRef CAS PubMed.
- Recent references on iridium based homogeneous catalysts: (a) J. Lee, S. Muthaiah and S. H. Hong, Adv. Synth. Catal., 2014, 356, 2653–2660 CrossRef CAS PubMed; (b) P. Qu, C. Sun, J. Ma and F. Li, Adv. Synth. Catal., 2014, 356, 447–459 CrossRef CAS PubMed; (c) C. Crotti, E. Farnetti, S. Licen, P. Barbieri and G. Pitacco, J. Mol. Catal. A: Chem., 2014, 382, 64–70 CrossRef CAS PubMed; (d) Y. Zhang, C.-S. Lim, D. S. B. Sim, H.-J. Pan and Y. Zhao, Angew. Chem., Int. Ed., 2014, 53, 1399–1403 CrossRef CAS PubMed; (e) S. Ruch, T. Irrgang and R. Kempe, Chem.–Eur. J., 2014, 20, 13279–13285 CrossRef CAS PubMed; (f) S. Wockel, P. Plessow, M. Schelwies, M. K. Brinks, F. Rominger, P. Hofmann and M. Limbach, ACS Catal., 2014, 4, 152–161 CrossRef; (g) Y.-H. Chang, Y. Nakajima and F. Ozawa, Organometallics, 2013, 32, 2210–2215 CrossRef CAS; (h) A. Wetzel, S. Woeckel, M. Schelwies, M. K. Brinks, F. Rominger, P. Hofmann and M. Limbach, Org. Lett., 2013, 15, 266–269 CrossRef CAS PubMed; (i) F. Li, C. L. Sun, H. X. Shan, X. Y. Zou and J. J. Xie, ChemCatChem, 2013, 5, 1543–1552 CrossRef CAS PubMed; (j) Y. H. Chang, Y. Nakajima and F. Ozawa, Organometallics, 2013, 32, 2210–2215 CrossRef CAS; (k) J. Q. Li and P. G. Andersson, Chem. Commun., 2013, 49, 6131–6133 RSC; (l) L. L. R. Lorentz-Petersen, L. U. Nordstrom and R. Madsen, Eur. J. Org. Chem., 2012, 6752–6759 CrossRef CAS PubMed; (m) Y. Obora, S. Ogawa and N. Yamamoto, J. Org. Chem., 2012, 77, 9429–9433 CrossRef CAS PubMed; (n) F. Li, Q. Kang, H. Shan, L. Chen and J. Xie, Eur. J. Org. Chem., 2012, 5085–5092 CrossRef CAS PubMed; (o) S. Michlik, T. Hille and R. Kempe, Adv. Synth. Catal., 2012, 354, 847–862 CrossRef CAS PubMed; (p) A. Bartoszewicz, R. Marcos, S. Sahoo, A. K. Inge, X. D. Zou and B. Martin-Matute, Chem.–Eur. J., 2012, 18, 14510–14519 CrossRef CAS PubMed; (q) F. Li, Q. K. Kang, H. X. Shan, L. Chen and J. J. Xie, Eur. J. Org. Chem., 2012, 5085–5092 CrossRef CAS PubMed; (r) F. Li, H. X. Shan, L. Chen, Q. K. Kang and P. Zou, Chem. Commun., 2012, 48, 603–605 RSC; (s) R. Kawahara, K.-I. Fujita and R. Yamaguchi, Adv. Synth. Catal., 2011, 353, 1161–1168 CrossRef CAS PubMed; (t) I. Cumpstey, S. Agrawal, K. E. Borbas and B. Martin-Matute, Chem. Commun., 2011, 47, 7827–7829 RSC; (u) X. Gong, H. Zhang and X. W. Li, Tetrahedron Lett., 2011, 52, 5596–5600 CrossRef CAS PubMed; (v) W. X. Zhang, X. C. Dong and W. L. Zhao, Org. Lett., 2011, 13, 5386–5389 CrossRef CAS PubMed; (w) J. Norinder and A. Boerner, ChemCatChem, 2011, 3, 1407–1409 CrossRef CAS PubMed; (x) M. A. Berliner, S. P. A. Dubant, T. Makowski, K. Ng, B. Sitter, C. Wager and Y. S. Zhang, Org. Process Res. Dev., 2011, 15, 1052–1062 CrossRef CAS; (y) O. Saidi, A. J. Blacker, G. W. Lamb, S. P. Marsden, J. E. Taylor and J. M. Williams, Org. Process Res. Dev., 2010, 14, 1046–1049 CrossRef CAS; (z) S. Michlik and R. Kempe, Chem.–Eur. J., 2010, 16, 13193–13198 CrossRef CAS PubMed.
- P. Satyanarayana, G. M. Reddy, H. Maheswaran and M. L. Kantam, Adv. Synth. Catal., 2013, 355, 1859–1867 CrossRef CAS PubMed.
- (a) H. Yang, R. Mao, C. Luo, C. Lu and G. Cheng, Tetrahedron, 2014, 70, 8829–8835 CrossRef CAS PubMed; (b) V. Terrasson, S. Marque, M. Georgy, J. M. Campagne and D. Prim, Adv. Synth. Catal., 2006, 348, 2063–2067 CrossRef CAS PubMed.
- (a) A. Martinez-Asencio, D. J. Ramon and M. Yus, Tetrahedron, 2011, 67, 3140–3149 CrossRef CAS PubMed; (b) A. Martinez-Asencio, D. J. Ramon and M. Yus, Tetrahedron Lett., 2010, 51, 325–327 CrossRef CAS PubMed; (c) F. Shi, M. K. Tse, X. J. Cui, D. Gordes, D. Michalik, K. Thurow, Y. Q. Deng and M. Beller, Angew. Chem., Int. Ed., 2009, 48, 5912–5915 CrossRef CAS PubMed; (d) X. J. Cui, F. Shi, M. K. Tse, D. Gordes, K. Thurow, M. Beller and Y. Q. Deng, Adv. Synth. Catal., 2009, 351, 2949–2958 CrossRef CAS PubMed.
- (a) T. Yan, B. L. Feringa and K. Barta, Nat. Commun., 2014, 5, 5602 CrossRef CAS PubMed; (b) A. J. Rawlings, L. J. Diorazio and M. Wills, Org. Lett., 2015, 17, 1086–1089 CrossRef CAS PubMed; (c) X. J. Cui, F. Shi, Y. Zhang and Y. Q. Deng, Tetrahedron Lett., 2010, 51, 2048–2051 CrossRef CAS PubMed; (d) J.-J. Yu, L.-M. Wang, F.-L. Guo, J.-Q. Liu, Y. Liu and N. Jiao, Synth. Commun., 2011, 41, 1609–1616 CrossRef CAS PubMed; (e) M. Bala, P. K. Verma, U. Sharma, N. Kumar and B. Singh, Green Chem., 2013, 15, 1687–1693 RSC.
- (a) T. T. Dang, B. Ramalingam, S. P. Shan and A. M. Seayad, ACS Catal., 2013, 3, 2536–2540 CrossRef CAS; (b) A. Martinez-Asencio, M. Yus and D. J. Ramon, Synthesis, 2011, 3730–3740 CAS.
- (a) M. Bertoli, A. Chouale, D. G. Gusev, A. J. Lough, Q. Major and B. Moore, Dalton Trans., 2011, 8941–8949 RSC; (b) M. A. Esteruelas, N. Honczek, M. Olivan, E. Onate and M. Valencia, Organometallics, 2011, 30, 2468–2471 CrossRef CAS.
- K.-I. Shimizu, Catal. Sci. Technol., 2015, 5, 1412–1427 CAS.
- (a) S. Pei Shan, D. T. Tuan, A. M. Seayad and B. Ramalingam, ChemCatChem, 2014, 6, 808–814 CrossRef PubMed; (b) A. Peeters, L. Claes, I. Geukens, I. Stassen and D. De Vos, Appl. Catal., A, 2014, 469, 191–197 CrossRef CAS PubMed; (c) K. Yamaguchi, J. L. He, T. Oishi and N. Mizuno, Chem.–Eur. J., 2010, 16, 7199–7207 CrossRef CAS PubMed; (d) R. Cano, D. J. Ramon and M. Yus, J. Org. Chem., 2011, 76, 5547–5557 CrossRef CAS PubMed.
- (a) A. M. Rasero-Almansa, A. Corma, M. Iglesias and F. Sanchez, ChemCatChem, 2014, 6, 1794–1800 CrossRef CAS PubMed; (b) D. Wang, X.-Q. Guo, C.-X. Wang, Y.-N. Wang, R. Zhong, X.-H. Zhu, L.-H. Cai, Z.-W. Gao and X.-F. Hou, Adv. Synth. Catal., 2013, 355, 1117–1125 CrossRef CAS PubMed; (c) R. Cano, M. Yus and D. J. Ramon, Chem. Commun., 2012, 48, 7628–7630 RSC.
- (a) A. Corma, J. Navas and M. J. Sabater, Chem.–Eur. J., 2012, 18, 14150–14156 CrossRef CAS PubMed; (b) L. He, Y. Qian, R. S. Ding, Y. M. Liu, H. Y. He, K. N. Fan and Y. Cao, ChemSusChem, 2012, 5, 621–624 CrossRef CAS PubMed; (c) T. Ishida, R. Takamura, T. Takei, T. Akita and M. Haruta, Appl. Catal., A, 2012, 413–414, 261–266 CrossRef CAS PubMed; (d) C.-H. Tang, L. He, Y.-M. Liu, Y. Cao, H.-Y. He and K.-N. Fan, Chem.–Eur. J., 2011, 17, 7172–7177 CrossRef CAS PubMed; (e) L. He, X. B. Lou, J. Ni, Y. M. Liu, Y. Cao, H. Y. He and K. N. Fan, Chem.–Eur. J., 2010, 16, 13965–13969 CrossRef CAS PubMed.
- (a) H. H. Liu, G. K. Chuah and S. Jaenicke, J. Catal., 2012, 292, 130–137 CrossRef CAS PubMed; (b) K. Shimizu, K. Shimura, M. Nishimura and A. Satsuma, ChemCatChem, 2011, 3, 1755–1758 CrossRef CAS PubMed; (c) K. Shimizu, M. Nishimura and A. Satsuma, ChemCatChem, 2009, 1, 497–503 CrossRef CAS PubMed.
- (a) R. Cano, M. Yus and D. J. Ramon, Tetrahedron, 2011, 67, 8079–8085 CrossRef CAS PubMed; (b) Y. Zhang, X. J. Qi, X. J. Cui, F. Shi and Y. Q. Deng, Tetrahedron Lett., 2011, 52, 1334–1338 CrossRef CAS PubMed; (c) Y. Shiraishi, K. Fujiwara, Y. Sugano, S. Ichikawa and T. Hirai, ACS Catal., 2013, 3, 312–320 CrossRef CAS; (d) M. Ousmane, G. Perrussel, Z. Yan, J. M. Clacens, F. De Campo and M. Pera-Titus, J. Catal., 2014, 309, 439–452 CrossRef CAS PubMed; (e) A. Corma, T. Ródenas and M. J. Sabater, Chem.–Eur. J., 2010, 16, 254–260 CrossRef CAS PubMed.
- (a) A. Mehta, A. Thaker, V. Londhe and S. R. Nandan, Appl. Catal., A, 2014, 478, 241–251 CrossRef CAS PubMed; (b) K.-I. Shimizu, N. Imaiida, K. Kon, S. M. A. H. Siddiki and A. Satsuma, ACS Catal., 2013, 3, 998–1005 CrossRef CAS; (c) F. Alonso, P. Riente and M. Yus, Acc. Chem. Res., 2011, 44, 379–391 CrossRef CAS PubMed; (d) S. Ueno, K. Usui and R. Kuwano, Synlett, 2011, 1303–1307 CrossRef CAS PubMed.
- (a) F. Santoro, R. Psaro, N. Ravasio and F. Zaccheria, RSC Adv., 2014, 4, 2596–2600 RSC; (b) M. Dixit, M. Mishra, P. A. Joshi and D. O. Shah, Catal. Commun., 2013, 33, 80–83 CrossRef CAS PubMed; (c) F. Santoro, R. Psaro, N. Ravasio and F. Zaccheria, ChemCatChem, 2012, 4, 1249–1254 CrossRef CAS PubMed; (d) J. M. Perez, R. Cano, M. Yus and D. J. Ramon, Eur. J. Org. Chem., 2012, 4548–4554 CrossRef CAS PubMed; (e) J. L. He, K. Yamaguchi and N. Mizuno, Chem. Lett., 2010, 39, 1182–1183 CrossRef CAS; (f) P. R. Likhar, R. Arundhathi, M. L. Kantam and P. S. Prathima, Eur. J. Org. Chem., 2009, 5383–5389 CrossRef CAS PubMed.
- C. Gonzalez-Arellano, K. Yoshida, R. Luque and P. L. Gai, Green Chem., 2010, 12, 1281–1287 RSC.
- X. Yu, C. Liu, L. Jiang and Q. Xu, Org. Lett., 2011, 13, 6184–6187 CrossRef CAS PubMed.
- R. Martinez, D. J. Ramon and M. Yus, Org. Biomol. Chem., 2009, 7, 2176–2181 CAS.
- (a) G. C. Y. Choo, H. Miyamura and S. Kobayashi, Chem. Sci., 2015, 6, 1719–1727 RSC; (b) T. Takanashi, M. Tamura, Y. Nakagawa and K. Tomishige, RSC Adv., 2014, 4, 28664–28672 RSC; (c) Y. M. A. Yamada, H. Ohta, Y. Yuyama and Y. Uozumi, Synthesis, 2013, 2093–2100 CrossRef CAS PubMed; (d) X. Liu, R. S. Ding, L. He, Y. M. Liu, Y. Cao, H. Y. He and K. N. Fan, ChemSusChem, 2013, 6, 604–608 CrossRef CAS PubMed; (e) X. J. Cui, X. C. Dai, Y. Q. Deng and F. Shi, Chem.–Eur. J., 2013, 19, 3665–3675 CrossRef CAS PubMed; (f) W. He, S. B. He, C. L. Sun, K. K. Wu, L. D. Wang and Z. K. Yu, Chin. J. Catal., 2012, 33, 717–722 CAS; (g) L. Wang, W. He, K. Wu, S. He, C. Sun and Z. Yu, Tetrahedron Lett., 2011, 52, 7103–7107 CrossRef CAS PubMed; (h) H. Ohta, Y. Yuyama, Y. Uozumi and Y. M. A. Yamade, Org. Lett., 2011, 13, 3892–3895 CrossRef CAS PubMed; (i) K.-I. Shimizu, K. Shimura, M. Nishimura and A. Satsuma, RSC Adv., 2011, 1, 1310–1317 RSC; (j) L. D. Wang, W. He, K. K. Wu, S. B. He, C. L. Sun and Z. K. Yu, Tetrahedron Lett., 2011, 52, 7103–7107 CrossRef CAS PubMed; (k) W. He, L. D. Wang, C. L. Sun, K. K. Wu, S. B. He, J. P. Chen, P. Wu and Z. K. Yu, Chem.–Eur. J., 2011, 17, 13308–13317 CrossRef CAS PubMed; (l) K. Kim, K. L. Kim and K. S. Shin, J. Phys. Chem. C, 2011, 115, 14844–14851 CrossRef CAS; (m) H. Kimura and H. Taniguchi, Catal. Lett., 1996, 40, 123–130 CrossRef CAS.
- (a) A. C. B. Neves, M. J. F. Calvete, T. Melo and M. M. Pereira, Eur. J. Org. Chem., 2012, 6309–6320 CrossRef CAS PubMed; (b) A. E. C. Collis and I. T. Horvath, Catal. Sci. Technol., 2011, 1, 912–919 RSC; (c) C. Coperet and J. M. Basset, Adv. Synth. Catal., 2007, 349, 78–92 CrossRef CAS PubMed; (d) B. M. L. Dioos, I. F. J. Vankelecom and P. A. Jacobs, Adv. Synth. Catal., 2006, 348, 1413–1446 CrossRef CAS PubMed; (e) A. Corma and H. Garcia, Adv. Synth. Catal., 2006, 348, 1391–1412 CrossRef CAS PubMed; (f) P. McMorn and G. J. Hutchings, Chem. Soc. Rev., 2004, 33, 108–122 RSC.
- (a) N. J. Meehan, A. J. Sandee, N. H. Reek, P. C. J. Kamer, P. W. N. M. van Leeuwen and M. Poliakoff, Chem. Commun., 2000, 1497–1498 RSC; (b) S. Ricken, P. W. Osinski, P. Eilbracht and R. Haag, J. Mol. Catal. A: Chem., 2006, 257, 78–88 CrossRef CAS PubMed.
- A. J. Sandee, D. Dimitrijevic, R. J. van Haaren, J. N. H. Reek, P. C. J. Kamer and P. W. N. M. van Leeuwen, J. Mol. Catal. A: Chem., 2002, 182–183, 309–317 CrossRef CAS.
- (a) Y. Obora, ACS Catal., 2014, 4, 3972–3981 CrossRef CAS; (b) F.-X. Yan, M. Zhang, X.-T. Wang, F. Xie, M.-M. Chen and H. Jiang, Tetrahedron, 2014, 70, 1193–1198 CrossRef CAS PubMed; (c) A. J. A. Watson, R. J. Wakeham, A. C. Maxwell and J. M. J. Williams, Tetrahedron, 2014, 70, 3683–3690 CrossRef CAS PubMed; (d) S. Ogawa and Y. Obora, Chem. Commun., 2014, 50, 2491–2493 RSC; (e) L. K. M. Chan, D. L. Poole, D. Shen, M. P. Healy and T. Donohoe, Angew. Chem., Int. Ed., 2014, 53, 761–765 CrossRef CAS PubMed; (f) J. Ma, N. N. Wang and F. Li, Asian J. Org. Chem., 2014, 3, 940 CrossRef CAS PubMed; (g) C. Chaudhari, S. M. A. H. Siddiki and K.-I. Shimizu, Top. Catal., 2014, 57, 1042–1048 CrossRef CAS; (h) Y. Li, H. Q. Li, H. Junge and M. Beller, Chem. Commun., 2014, 50, 14991–14994 RSC; (i) Y. F. Liang, X. F. Zhou, S. Y. Tang, Y. B. Huang, Y. S. Feng and H. J. Xu, RSC Adv., 2013, 3, 7739–7742 RSC; (j) M. Rueping and V. B. Phapale, Green Chem., 2012, 14, 55–57 RSC; (k) J. Li, W. X. Zhang, F. Wang, M. Jiang, X. C. Dong and W. L. Zhao, Chin. J. Chem., 2012, 30, 2363–2366 CrossRef CAS PubMed; (l) F. Alonso, F. Foubelo, J. C. Gonzalez-Gomez, R. Martinez, D. J. Ramon, P. Riente and M. Yus, Mol. Diversity, 2010, 14, 411–424 CrossRef CAS PubMed; (m) R. Martinez, D. J. Ramon and M. Yus, Tetrahedron, 2006, 62, 8988–9001 CrossRef CAS PubMed; (n) R. Martinez, D. J. Ramon and M. Yus, Tetrahedron, 2006, 62, 8982–8987 CrossRef CAS PubMed; (o) Y. Obora, K. Nakamura and S. Hatanaka, Chem. Commun., 2012, 48, 6720–6722 RSC; (p) Y. Iuchi, Y. Obora and Y. Ishii, J. Am. Chem. Soc., 2010, 132, 2536–2537 CrossRef CAS PubMed.
- O. Kose and S. Saito, Org. Biomol. Chem., 2010, 8, 896–900 CAS.
- M. S. Kwon, N. Kim, S. H. Seo, I. S. Park, R. K. Cheedrala and J. Park, Angew. Chem., Int. Ed., 2005, 44, 6913–6915 CrossRef CAS PubMed.
- Y. M. A. Yamada and Y. Uozumi, Org. Lett., 2006, 8, 1375–1378 CrossRef CAS PubMed.
- G. Xu, Q. Li, J. Feng, Q. Liu, Z. Zhang, X. Wang, X. Zhang and X. Mu, ChemSusChem, 2014, 7, 105–109 CrossRef CAS PubMed.
- See ESI† for details.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra07225e |
| This journal is © The Royal Society of Chemistry 2015 |