Metal-free domino one-pot protocols for quinoline synthesis
Jaideep B. Bharate
ab,
Ram A. Vishwakarma
*ab and
Sandip B. Bharate
*ab
aMedicinal Chemistry Division, CSIR-Indian Institute of Integrative Medicine, Canal Road, Jammu-180001, India. E-mail: sbharate@iiim.ac.in; ram@iiim.ac.in; Fax: +91-191-2586333; Tel: +91-191-2585006
bAcademy of Scientific & Innovative Research (AcSIR), CSIR-Indian Institute of Integrative Medicine, Canal Road, Jammu-180001, India
First published on 30th April 2015
Abstract
Quinoline is one of the most widely investigated scaffolds by synthetic chemists because of its medicinal importance. A wide range of metal-catalyzed, metal-free, multi-step or domino one-pot protocols are reported in the literature for construction of this scaffold. Several reviews have appeared on synthetic aspects of this scaffold, however there is no focused review on metal-free domino one-pot protocols. Domino one-pot protocols offer an opportunity to access highly functionalized final products from simple starting materials. Because of this unique feature of domino protocols, in recent years their utility for generation of molecular libraries has been widely appreciated. In this review, all contributions till March 2015 are surveyed with particular emphasis on metal-free domino reactions for quinoline ring construction and are discussed herein along with mechanistic aspects.
1. Introduction
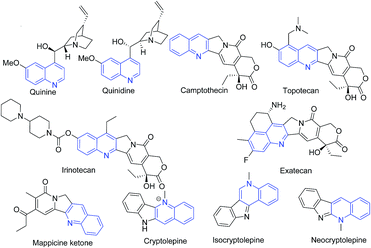
Quinoline (1-aza-napthalene or benzo[b]pyridine) is a weak tertiary base. It was first extracted from coal tar in 1834 by Friedlieb Ferdinand Runge and this source still remains the principal source of commercial quinoline. This scaffold has found many applications in diverse chemical domains. This scaffold has wide occurrence among natural products (alkaloids)1 and is a key structural component of several pharmaceuticals, agrochemicals, dyestuffs, and materials. In coordination chemistry, quinolines are used to chelate metallic ions as N-donor ligands.2 The quinoline scaffold has been reported to possess diverse range of pharmacological activities3–14 including antiprotozoal,15–20 antitubercular,21,22 anticancer,4,23,24 antipsychotics,25 antiinflammatory,26,27 antioxidant,3 anti-HIV,28 antifungal,29 as efflux pump inhibitors,30 and for treatment of neurodegenerative diseases,19 and treatment of lupus,31 etc.The well known antimalarial natural products quinine and quinidine alkaloids isolated from Cinchona bark comprises quinoline scaffold.32,33 Camptothecin is a quinoline alkaloid discovered in 1966 by Wall and Wani through systematic screening of natural products for anticancer drugs. Two camptothecin analogues namely topotecan and irinotecan have been approved for clinical use for cancer chemotherapy,34 and another analog exatecan is under clinical studies. A fused quinoline natural product mappicine ketone is an antiviral lead compound with selective activities against herpes viruses HSV-1 and HSV-2 and human cytomegalovirus (HCMV).35 A fused quinoline alkaloid cryptolepine isolated from Cryptolepis sp. is an antimalarial natural product possessing cytotoxic properties.36 Its structural isomers isocrytolepine and neocryptolepine also possesses antimalarial activity.37 The chemical structures of quinoline class of natural products are shown in Fig. 1.
Quinoline is also part of several clinically used drugs, where their major occurrence is among antimalarial drugs. The aminoquinoline scaffold has been a backbone of antimalarial drugs since 1940s. In this class, chloroquine was the first drug discovered in 1934 by Hans Andersag and coworkers at the Bayer laboratories.38 With the emergence of resistance to chloroquine, a series of its analogs (e.g. amodiaquine, primaquine, mefloquine, tafenoquine, bulaquine, NPC-1161B, AQ-13, IAAQ) were discovered. Other antimalarial quinolines include piperaquine and pyronaridine. Quinoline has also been a part of drugs used for other diseases. This includes fluoroquinolone antibiotic ciprofloxacin (and its analogs), pitavastatin (cholesterol lowering agent), lenvatinib (kinase inhibitor for cancer) and its other structural analogs (such as carbozantinib, bosutinib), tipifarnib (farnesyl transferase inhibitor for leukemia), saquinavir (antiretroviral), bedaquiline (anti-TB), etc. The 2-(2-fluorophenyl)-6,7-methylenedioxy quinolin-4-one monosodium phosphate (CHM-1-P-Na) is a preclinical anticancer agent, showing excellent antitumor activity in a SKOV-3 xenograft nude mice model.39,40 The chemical structures of above discussed representative quinoline based drugs are shown in Fig. 2a. Several quinoline based compounds showed inhibition of kinases involved in cancer progression.4 The chemical structures of representative kinase inhibitors are shown in Fig. 2b.
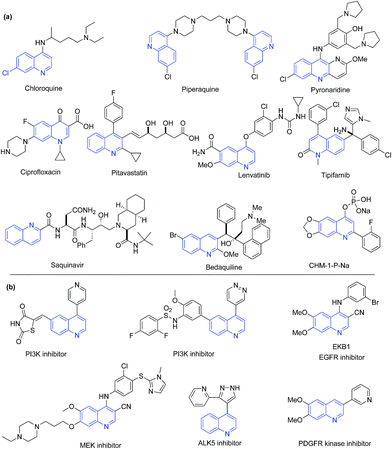 | ||
| Fig. 2 Chemical structures of (a) quinoline containing drugs and clinical candidates; and (b) quinoline-based kinase inhibitors. | ||
As a consequence of their tremendous biological importance, chemists have developed a plethora of methods to elaborate this structure, and most of them have been compiled in a series of reviews.41–47 Recently, Patel's group (2014)41 have reviewed advances in the synthesis of quinolines, which covered very broadly various reports on quinoline synthesis and cited 57 references. Koorbanally's group (2014)42 have reviewed synthesis and anti-cancer activity of 2-substituted quinolines. Nammalwar and Bunce (2014)45 have reviewed recent syntheses of 1,2,3,4-tetrahydroquinolines, 2,3-dihydro-4(1H)-quinolinones and 4(1H)-quinolinones using domino reactions. Alam's group (2013)47 have briefly discussed various synthetic and biological aspects of this scaffold and cited total of 75 references. Hassanin et al. (2012)44 reviewed synthesis and chemical reactivity of pyrano[3,2-c]quinolinones. Mekheimer et al. (2012)46 reviewed recent developments in the chemistry of pyrazolo[4,3-c]quinolines. Barluenga et al. (2009)43 reviewed advances in the synthesis of indole and quinoline derivatives through cascade reactions and cited total of 46 references.
Despite of the fact that large number of metal-free domino one-pot protocols for quinoline synthesis have been published; this has never been reviewed. The metal-free domino protocols provide rapid access to structural diversity, and metal-free nature of the reaction makes these protocols environmentally friendly. Therefore, a critical review on such protocols for synthesis of this medicinally important scaffold is highly desirable. The present review provides a comprehensive compilation of synthetic approaches involving specifically metal-free one-pot domino and multicomponent reactions (MCRs) for quinolines and related fused skeletons.
2. Classical methods for quinoline synthesis
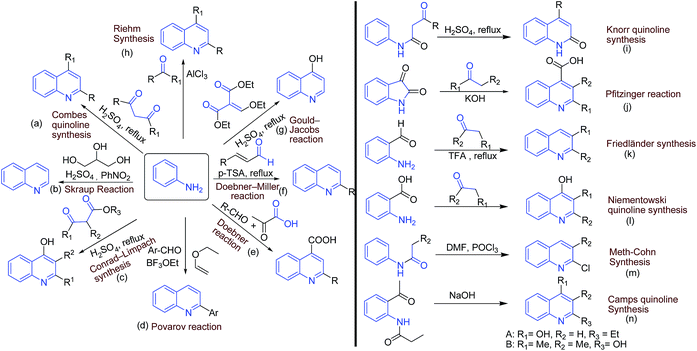
There exist several classical methods (name reactions) for synthesis of quinolines. Most of the methods involve simple arylamines as starting materials. The ‘name reactions’ involving arylamines as one of the starting material includes: (a) Combes quinoline synthesis (from anilines and β-diketones); (b) Skraup synthesis (from ferrous sulfate, glycerol, aniline, nitrobenzene, and sulfuric acid); (c) Conrad–Limpach synthesis (from anilines and β-ketoesters); (d) Povarov reaction (from aniline, benzaldehyde and an activated alkene); (e) Doebner reaction (from anilines, aldehyde and pyruvic acid); (f) Doebner–Miller reaction (from anilines and α,β-unsaturated carbonyl compounds); (g) Gould–Jacobs reaction (from aniline and ethyl ethoxymethylene malonate); and (h) Reihm synthesis (from aniline and acetone).A number of other name reactions exists, which require specifically substituted anilines or related substrates. These includes: (i) Knorr quinoline synthesis (from β-ketoanilide and sulfuric acid); (j) Pfitzinger reaction (from an isatin with base and a carbonyl compound); (k) Friedländer synthesis (from 2-aminobenzaldehyde and carbonyl compounds); (l) Niementowski quinoline synthesis (from anthranilic acid and carbonyl compounds); (m) Meth-Cohn synthesis (from acylanilides and DMF/POCl3); and (n) Camps quinoline synthesis (from an o-acylaminoacetophenone and hydroxide). The synthetic schemes of these classical methods are summarized in Fig. 3.
Despite of the availability of several classical methods for quinoline synthesis, extensive efforts have been made on the development of new metal-free domino protocols for preparation of quinolines, which are described in this review.
3. Metal-free domino one-pot or multicomponent protocols for synthesis of quinolines and related quinoline fused heterocycles
Development of domino reactions for the concise construction of diverse heterocyclic architectures is of a tremendous importance in synthetic organic and medicinal chemistry.48 Extensive amount of efforts have been made in this area towards development of domino one-pot protocols or multicomponent reactions (MCRs) for construction of heterocycles.49–58 Few specific reviews on multicomponent synthesis of particular heterocycles such as pyrroles,50,59 indoles,60 and pyridines61 have been published. Such domino reactions achieve high level of atom-efficiency, and avoids time-consuming isolation and purification of intermediates. Reduction in number of steps is the major advantage with these protocols, thus reduces manpower and avoids waste production.62 This section discusses all reported metal-free one-pot protocols for quinoline synthesis. Most of the metal-free protocols discussed herein, comprises simple acid catalysts, bases, molecular iodine, ionic liquids or organocatalysts, and few methods are catalyst-free. In this section, these protocols have been discussed according to the use of different non-metal reagents/catalysts: (a) acid catalyzed protocols; (b) base catalyzed protocols; (c) molecular iodine catalyzed protocols; (d) ionic liquid mediated quinoline synthesis; (e) organocatalysis for quinoline synthesis; (f) catalyst-free reactions; and (g) miscellaneous reactions.3.1. Acid catalyzed protocols
Acids have been widely used as simple and ecofriendly catalysts and promoters for various organic reactions. Acid catalysts which are routinely used in various organic transformations include trifluoroacetic acid (TFA), formic acid, acetic acid, triflic acid, and pTSA. Acetic acid and formic acid are the two most common reagents available in most of the chemistry laboratories and their use for quinoline synthesis has been well documented. Among various starting materials, arylamines are among most widely used precursors for quinoline synthesis.Many acid catalyzed protocols are based on the traditional name reactions. Povarov reaction is one of the most widely investigated reaction for quinoline synthesis, comprising the aza Diels–Alder cycloaddition as the key step. Recently our group63 have developed an efficient formic acid catalyzed one-pot synthesis of 4-arylquinoline 2-carboxylates 4 in water via three-component Povarov reaction of arylamines 1, glyoxylates 2 and phenylacetylenes 3 (Scheme 1). The reaction mechanism involves a cascade of reactions involving initial condensation of arylamine 1 and ethyl glyoxylate 2 to form imine intermediate I. Next, there is a protonation of the nitrogen of the imine which facilitates the attack by phenylacetylene, resulting in cyclization to produce dihydroquinoline III, which on oxidation produces 4-arylquinoline 2-carboxylates 4. These compounds displayed neuroprotective, antioxidant and Pgp-induction activities.
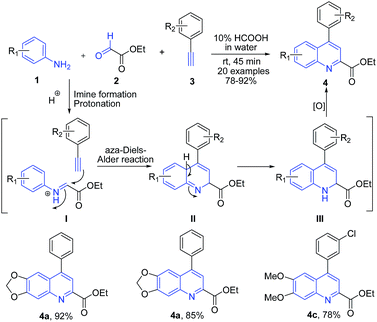 | ||
| Scheme 1 Formic acid-catalyzed synthesis of quinoline-2-carboxylates 4; some representative examples are shown. | ||
Zhang et al.64 reported a three-component Povarov reaction between aryl aldehydes 5, arylamines 1, and alkynes 3 in presence of triflic acid leading to formation of 2,4-disubstituted quinolines 6 (path A of Scheme 2). Interestingly, the use of alkenes 3 instead of alkynes 7 with the increase in reaction time (from 4 h to 8 h) produced same quinoline products 6 (path B of Scheme 2).
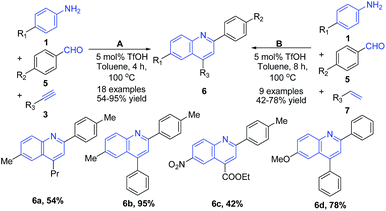 | ||
| Scheme 2 TfOH-catalyzed synthesis of 2,4-disubstituted quinolines 6; some representative examples are shown. | ||
Majumdar et al.65 reported another Povarov-type three-component domino reaction of heterocyclic amines 8, aldehydes 5, and terminal alkynes 3 in the presence of BF3·OEt2, which led to formation of pyrano[3,2-f]quinolines 9a and phenanthrolines 9b. The imine intermediate I undergoes intermolecular concerted type aza-Diels–Alder reaction with an alkyne 3 leading to formation of quinoline skeleton II, which on aromatization produces 9 (Scheme 3).
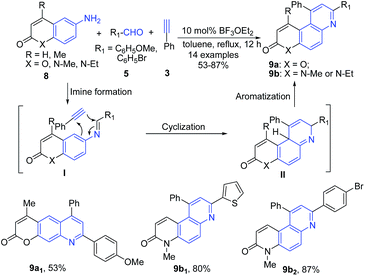 | ||
| Scheme 3 BF3·OEt2-catalyzed synthesis of pyrano[3,2-f]quinolines 9a and phenanthrolines 9b; some representative examples are shown. | ||
Ketene-dithioacetals have been used as important building blocks for construction of heterocycles.66 Ethynyl-S,S-acetals 10 are highly reactive electron-rich dienophiles which undergo regiospecific aza-Diels–Alder (Povarov) reaction with arylimines to produce quinoline skeleton. A triflic acid mediated three-component reaction between ethynyl-S,S-acetals 10, arylamines 1 and aldehydes 5 produced quinoline skeleton 11 via consecutive arylimine I formation, regiospecific aza-Diels–Alder reaction, and reductive amination (Scheme 4).67
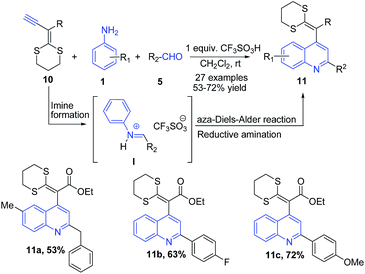 | ||
| Scheme 4 Triflic acid-catalyzed synthesis of 2,4-disubstituted quinolines 11 via three-component aza Diels–Alder reaction; some representative examples are shown. | ||
An interesting utility of Povarov reaction for construction of pentacyclic quinoline based fused heterocycles has been recently reported by Khadem et al.68 This protocol implies the three component reaction between arylamine 1, 2-carboxy benzaldehyde 12 and cyclopentadiene 13 in presence of TFA to furnish isoindolo[2,1-a]quinoline 14 (Scheme 5). The Schiff's base I undergoes a step-wise aza Diels–Alder reaction with cyclopentadiene 13 to produce isoindolo[2,1-a]quinolines 14. Authors mentioned that the concerted [4 + 2] cycloaddition route would afford a mixture of regio-isomeric products due to free N–Ar bond rotation prior to addition.
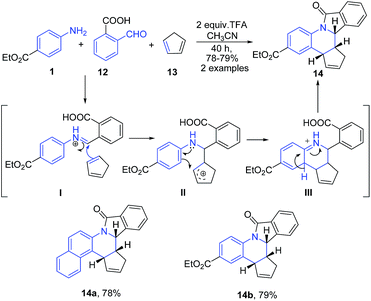 | ||
| Scheme 5 TFA catalyzed synthesis of pentacyclic isoindoloquinolines 14; some representative examples are shown. | ||
Borel et al.69 reported a three-component Povarov reaction of pyridine aldehydes 15 and arylamines 1 with ethyl vinyl ether 16 in presence of boron trifluoride methyl etherate producing 2-(2-pyridyl)quinolines 17 (Scheme 6).
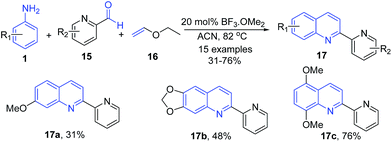 | ||
| Scheme 6 BF3·OMe2-catalyzed synthesis 2-(2-pyridyl)quinolines 17; some representative examples are shown. | ||
Shindoh et al.70 reported triflic imide and triflic acid catalyzed Povarov-Hydrogen-Transfer cascade reaction to produce quinolines 20. The reaction between electron-rich olefins 19 and excess amount of imines 18 in the presence of triflic imide in DCM at 60 °C afforded substituted quinolines 20 in one-pot (Scheme 7).
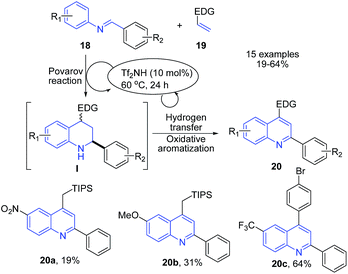 | ||
| Scheme 7 Triflic imide-catalyzed synthesis of imidazopyrrolo-quinolines 20; some representative examples are shown. | ||
para-Toluene sulfonic acid (pTSA) catalyzed condensation of aromatic amines 1 with δ,ε-unsaturated aldehydes 21, followed by intramolecular formal hetero Diels–Alder reaction produced cyclopenta[b]quinolines 22.71 Mechanistically, reaction proceeds through the iminium ion transition state I which further undergoes ring closure via intramolecular Diels–Alder reaction to produce II with a trans-arrangement of allylic cation and an amine. The electrophilic aromatic substitution reaction between allylic cation and aniline moiety then leads to formation of stable cyclopenta[b]quinoline 22 (Scheme 8).
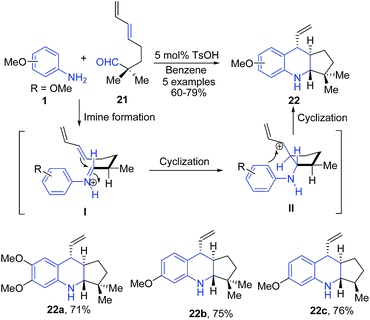 | ||
| Scheme 8 pTSA catalyzed synthesis of cyclopenta[b]quinolines 22 from δ,ε-unsaturated aldehydes 21; some representative examples are shown. | ||
Boron trifluoride etherate is a widely used Lewis acid catalyst in various reactions. Shan et al.72 reported boron trifluoride etherate catalyzed single-step approach toward the regioselective synthesis of 2-alkylquinolines 23 from 3-ethoxycyclobutanones 24 and aromatic amines 1. The imine intermediate I formed from two substrates undergoes intramolecular cyclization followed by aromatization to produce quinoline product 23 (Scheme 9).
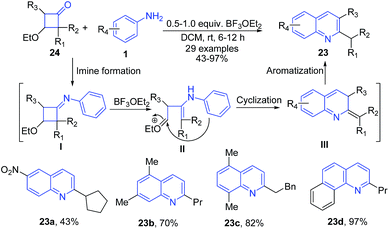 | ||
| Scheme 9 BF3·OEt2-catalyzed synthesis of alkyl quinolines 23 from 3-ethoxycyclobutanones 24 and aromatic amines 1; some representative examples are shown. | ||
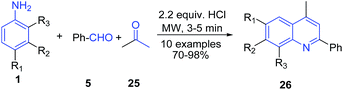
Apart from the Povarov reaction, arylamines are also one of the key precursors in several other protocols. There are several reports on the three-component reaction of arylamines, aryl aldehydes and active methylene compounds leading to formation of a quinoline skeleton. Mirza and Samiei73 reported Doebner type multicomponent reaction of arylamine 1, acetone 25 and benzaldehyde 5 without any solvent under microwave irradiation on the surface of alumina impregnated with hydrochloric acid to produce substituted quinolines 26 (Scheme 10).
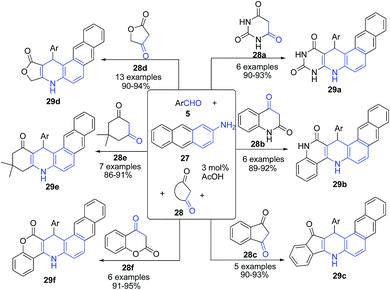
Tu's group74 established a sequential three-component reaction between 2-aminoanthracene 27, aromatic aldehyde 5 and cyclic 1,3-dicarbonyl compounds 28 (such as tetronic acid 28d, 5,5-dimethyl,3-cyclohexanedione 28e, 1,3-indanedione 28c, 3H-chromene-2,4-dione 28f, quinoline-2,4(1H, 3H)-dione 28b and barbituric acid 28a) in acidic medium under microwave irradiation to produce a series of unusual fused heterocyclic compounds, naphtho[2,3-f]quinoline derivatives 29 (Scheme 11). This scaffold exhibited good luminescent properties with emission wavelengths in the blue region.
Khan and Das75 utilized 3-aminocoumarins 30 as the arylamine precursor for synthesis of chromeno[3,4-b]quinolines 31. The pTSA catalyzed one-pot three component reaction between aryl aldehydes 5, 3-aminocoumarins 30, and cyclic 1,3-diketones 28e (Scheme 12) produced chromeno[3,4-b]quinolines 31. The reaction proceeds through the key intermediate I which on cyclization produces chromeno[3,4-b]quinoline 31.
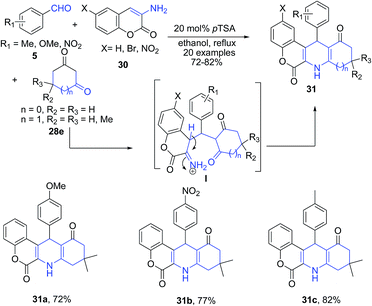 | ||
| Scheme 12 pTSA-catalyzed synthesis of chromeno[3,4-b]quinolines 31; some representative examples are shown. | ||
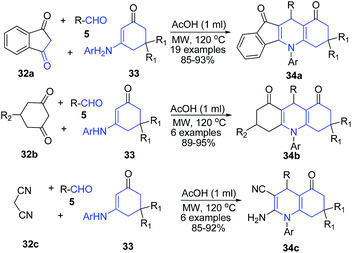
Tu and coworkers76 employed the use of enaminones 33 as the amine precursor and 1,3-indanedione 32a as an active methylene precursor for preparation of quinoline skeleton. The three component one-pot protocol involving treatment of aldehydes 5, 1,3-indanedione 32a and enaminone 33 in presence of acetic acid produced indeno[1,2-b]quinoline-9,11(6H,10H)-diones 34a. Authors also used other active methylene compounds such as 5-substituted-cyclohexane-1,3-dione 32b or malononitrile 32c in this protocol to produce acridine-1,8(2H,5H)-diones 34b or multi-substituted quinolines 34c (Scheme 13). The reaction mechanism involves Michael addition as the key step; and the protocol was found to work both by microwave irradiation and conventional heating.
Azizian et al.77 also utilized enaminones as amine precursors for quinoline synthesis. A three-component reaction between 2-hydroxynaphthalene-1,4-dione 35, 6-amino-uracils 36, and aromatic aldehydes 5 in presence of pTSA in aqueous media produced pyrimido[4,5-b]quinoline-tetraones 37. This reaction has been proposed to proceed by first condensation of 2-hydroxynaphthalene-1,4-dione 35 and aldehyde 5 followed by coupling with 6-amino-uracil 36 and then cyclization of II to yield 37 (Scheme 14).
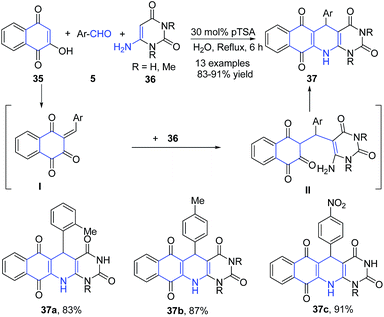 | ||
| Scheme 14 pTSA-catalyzed synthesis pyrimido[4,5-b]quinoline-tetraones 37; some representative examples are shown. | ||
The four-component reaction between naphthylamines 41, phenylhydrazine 39, isatins 40 and 3-ketoesters 38 in presence of pTSA under solvent-free conditions afforded spiro[1H-pyrazolo[3,4-b]benzo[h]dihydroquinolin-4,3-indolin-2-ones] 42a–b (Scheme 15). Authors also employed this 4-CR protocol for anilines instead of naphthylamines, which produced 4-substituted pyrazolo[3,4-b]quinoline derivatives.78
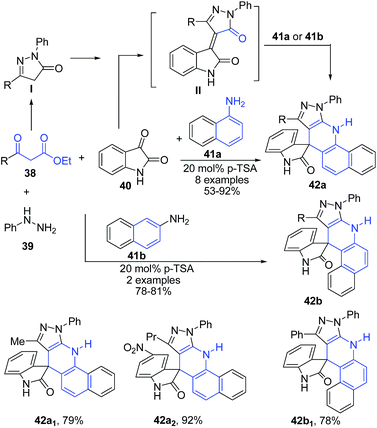 | ||
| Scheme 15 pTSA-catalyzed synthesis of spiro[1H-pyrazolo[3,4-b]benzo[h]dihydroquinolin-4,3-indolin-2-ones] 42 using 4-CR; some representative examples are shown. | ||
Yu et al.79 reported acetic acid catalyzed three-component reaction between aryl aldehyde 5, β-naphthylamine 41b, and 2H-thiopyran-3,5(4H,6H)-dione 44 in the presence of acetic acid leading to formation of benzo[f]thiopyrano[3,4-b]quinolin-11(8H)-ones 43 (Scheme 16).
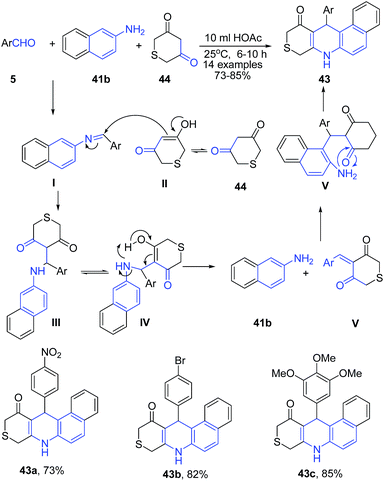 | ||
| Scheme 16 AcOH catalyzed synthesis of benzo[f]thiopyrano[3,4-b]quinolin-11(8H)-ones 43; some representative examples are shown. | ||
Narasimhulu et al.80 used silica supported perchloric acid as a heterogeneous recyclable catalyst for synthesis of various poly-substituted quinolines 45 using Friedlander condensation of 2-aminoarylketones 46 with carbonyl compounds 47 and α-keto esters at ambient temperature (Scheme 17).
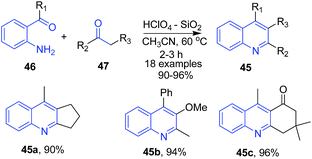 | ||
| Scheme 17 Silica supported perchloric acid-catalyzed synthesis of poly-substituted quinolines 45; some representative examples are shown. | ||
The quinoline synthesis through condensation of benzylideneanilines with active methylene compounds has been reported by two groups. Tanaka et al.81 reported the condensation of benzylideneanilines 48 with carbonyl compounds (aldehydes or ketones or diketones) 47 in presence of catalytic HCl leading to formation of quinolines 49. The enol form of carbonyl compound reacts with imine to form quinoline ring II via intramolecular cyclization, which further on aromatization produces 49 (Scheme 18).
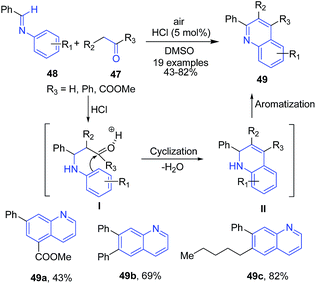 | ||
| Scheme 18 HCl-catalyzed synthesis of substituted quinolines 49; some representative examples are shown. | ||
Bojinov and Grabchev82 reported cyclization of 3-(arylidene-amino)-benzo[de]anthracen-7-ones 50, with 3-oxo-butyric acid ethyl ester 38 in presence of catalytic amount of HCl to produce fluorescent ethyl 3-aryl-1-methyl-8-oxo-8H-anthra[9,1-gh]quinoline-2-carboxylates 51 in 40–43% yields (Scheme 19). The reaction proceeds through key amine intermediate I, which on cyclization produces 51.
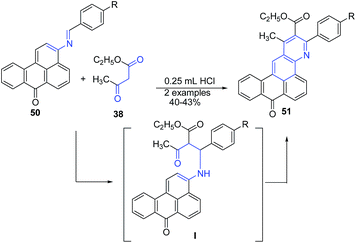 | ||
| Scheme 19 HCl-catalyzed synthesis of anthra[9,1-gh]quinoline-2-carboxylates 51 by cyclization of 3-(arylidene-amino)-benzo[de]anthracen-7-ones 50. | ||
Apart from the above discussed protocols involving either arylamine, enaminone or benzylimine as one of the key precursor, several groups have established protocols involving precursors other than those involved in conventional name reactions. Yu et al.,83 established a domino one-pot protocol for the synthesis of highly substituted imidazopyrroloquinolines 52 by simply refluxing a reaction mixture of different types of isatins 40 and heterocyclic ketene aminals 53 in presence of acetic acid. The reaction mechanism involves cascade of reaction involving first addition of ketene N,N-acetals to the carbonyl group of isatin 40. This was followed by imine-inamine tautomerization, intramolecular cyclization, dehydration and ring opening to produce amino intermediate II. This intermediate on protonation followed by cyclization leads to the formation of imidazopyrroloquinoline 52 (Scheme 20).
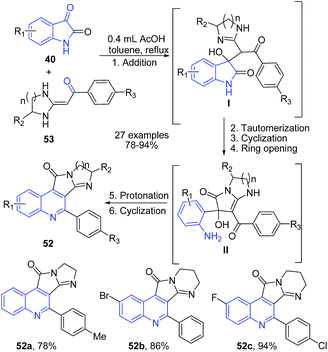 | ||
| Scheme 20 AcOH-catalyzed synthesis of imidazopyrrolo-quinolines 52 via cascade of reactions; some representative examples are shown. | ||
Yu et al.84 described a three-component reaction of enaminones 55, amines 1, and isatin 40 under acidic condition. The reaction proceeds through an unusual hydride transfer from in situ formed dimethylamine to a carbocation intermediate VIII to produce structurally diverse pyrrolo[3,4-c]quinoline-1-ones 54 (Scheme 21).
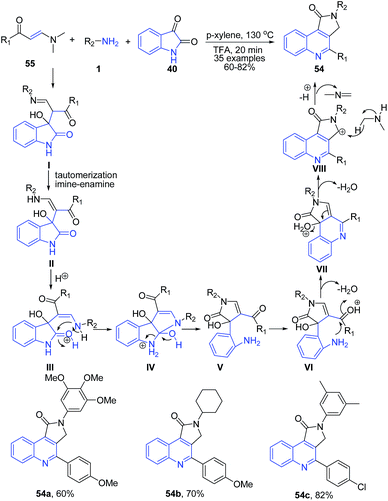 | ||
| Scheme 21 TFA-catalyzed synthesis of pyrrolo[3,4-c]quinoline-1-ones 54; some representative examples are shown. | ||
Quiroga et al.,85 showed that microwave-assisted intramolecular cyclization of N-4-substituted 6-chloropyrimidine-5-carbaldehydes 57 in acetic acid leads to formation of pyrimido[4,5-b]quinolines 56 (deazaflavin analogs), which exhibited excellent fluorescence properties (Scheme 22). The reaction process involves removal of the both Cl and NH2 groups of the starting material, as these groups are not present in the final products.
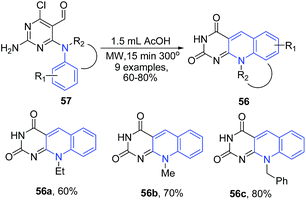 | ||
| Scheme 22 Synthesis of pyrimido[4,5-b]quinolines 56 via intramolecular cyclization of N-4-substituted 6-chloropyrimidine-5-carbaldehydes 57; some representative examples are shown. | ||
The reaction of N-methyl-N-phenylcinnamamides 59 with phenyliodine bis(trifluoroacetate) (PIFA) in the presence of TFA produced 3-arylquinolin-2-one compounds 58. First, the nucleophilic attack on the iodine center by the carbonyl oxygen of the amide moiety in 59 affords 3-azatriene I which undergoes an electrocyclic ring closure and the subsequent proton elimination to give intermediate III. Next, the 1,2-aryl shift followed by the breakage of the O–I bond gives 3-phenylquinolin-2-one 58 (Scheme 23).86
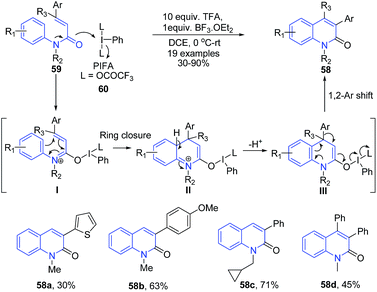 | ||
| Scheme 23 TFA catalyzed synthesis of 3-arylquinolin-2-ones 58 from N-methyl-N-phenylcinnamamides 59; some representative examples are shown. | ||
Arylmethyl azides 62 undergo rearrangement to produce N-aryl iminium ion intermediate I which can be trapped with a variety of nucleophiles.87 Tummatorn et al.88 utilized arylmethyl azides as the precursors to give an N-aryl iminium ion intermediate. Following the addition of ethyl 3-ethoxyacrylate 63, the 2-substituted quinoline products 61 were obtained in moderate to excellent yields (Scheme 24), via a cascade of reactions including intramolecular electrophilic aromatic substitution, elimination and subsequent oxidation.
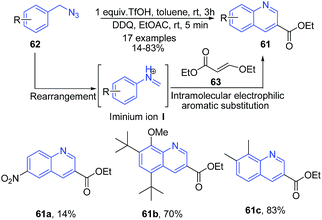 | ||
| Scheme 24 Triflic acid catalyzed domino synthesis of quinolines 57 from arylmethyl azides 62; some representative examples are shown. | ||
A three-component reaction between arylisothiocyanate 65 alkyltriflate 67, and alkynes 66 led to the formation of substituted quinolines 64 in high yields. The reaction undergoes alkyltriflate triggered domino electrophilic activation and avoids the use of a transition-metal catalyst. This transformation consisted of a cascade reaction of the arylisothiocyanate 65 with alkyltriflate 67 to form alkylthiosubstituted carbenium ion I, which followed the reaction with alkyne 66 to form intermediate II and subsequent electrophilic annulation to give quinoline 64 (Scheme 25).89
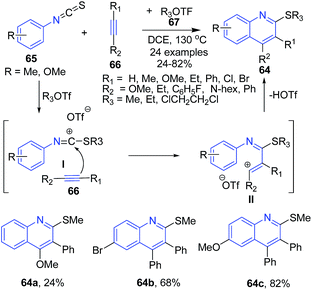 | ||
| Scheme 25 Alkyl triflate triggered-synthesis of quinolines 64 from arylisothiocyanates 65; some representative examples are shown. | ||
The reaction between pyridine-substituted o-alkynylanilines 70 and β-keto esters 38 in presence of pTSA in ethanol produced quinoline-based tetracyclic scaffold 69. Reaction proceeds through sequential hydration-condensation-double cyclization reactions. Interestingly, in the absence of β-keto esters, multisubstituted quinolines 68 were formed via condensation of two molecules of o-alkynylanilines 70 in reasonable yields (Scheme 26).90
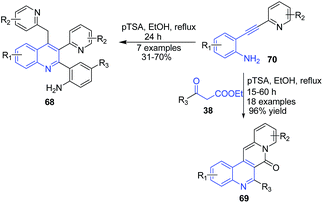 | ||
| Scheme 26 pTSA-catalyzed synthesis of quinoline-based tetracyclic scaffolds 69 and multisubstituted quinolines 68 from o-alkynylanilines. | ||
A cascade reaction of (E)-5-(arylamino)pent-3-en-1-ols 72a and thiols 72b with various aldehydes 5 in the presence of 30 mol% BF3·OEt2 in 1,2-dichloroethane at 80 °C afforded trans-fused hexahydro-1H-pyrano[3,4-c]quinolines 71a and hexahydro-1H-thiopyrano[3,4-c]quinolines 71b in good yields with high selectivity.91 The reaction proceeds via formation of an oxocarbenium ion I from the hemiacetal that is formed in situ from the aldehyde and a homoallylic alcohol likely after activation with BF3·OEt2. The oxocarbenium ion is attacked by an internal olefin resulting in the formation of a carbocation that is simultaneously trapped by a tethered aryl group, leading to the formation of hexahydro-1H-pyrano[3,2-c]quinolines 71 (Scheme 27).
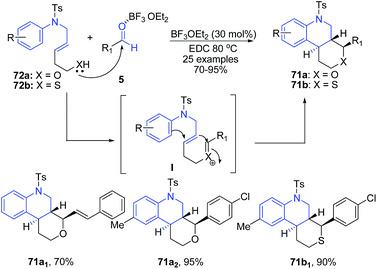 | ||
| Scheme 27 BF3·OEt2-catalyzed synthesis of hexahydro-1H-pyrano[3,4-c]quinolines 71a–b; some representative examples are shown. | ||
Zhang et al.92 developed an efficient synthesis of pyrano[2,3-b]quinolines 74 via the H2SO4-mediated domino cyclization/ring-opening/recyclization reaction of readily available activated cyclopentanes 73. This transformation commences from a H2SO4-mediated Combes-type annulation of cyclopentane to provide an alcohol intermediate I, which on elimination of water, produces a tertiary benzylic cation intermediate II. The elimination of a proton from intermediate II directly provide a terminal alkene intermediate III, which undergoes an intramolecular Markovnikov addition to produce pyrano[2,3-b]quinoline 74 (Scheme 28).
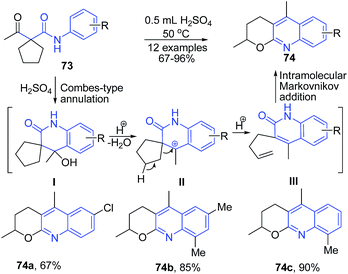 | ||
| Scheme 28 H2SO4-mediated synthesis of pyrano[2,3-b]quinolines 74; some representative examples are shown. | ||
Aksenov et al.93 reported synthesis of 3-aryl-2-quinolones 75 from indoles 76 via a metal-free transannulation reaction of 2-substituted indoles 76 with 2-nitroalkenes 77 in polyphosphoric acid. The conjugation of nitroalkene with indole in presence of PPA produces hydroxamic acid intermediate I. Next the intramolecular nucleophilic attack by the N-hydroxyl moiety at the C-2 of indole followed by tautomerization affords cyclized enamine II. Retro-Diels–Alder reaction followed by migration of acyl group from aniline to the more nucleophilic imine nitrogen produces IV, followed by the nucleophilic attack by the aniline at the acyliminium moiety in IV affords aminoquinoline V, which further on hydrolytic cleavage produces 3-aryl-2-quinolones 75 (Scheme 29).
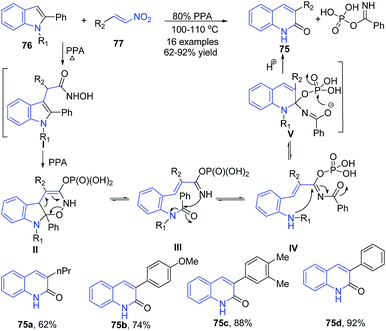 | ||
| Scheme 29 PPA-catalyzed synthesis of 3-aryl-2-quinolones 75 from 2-substituted indoles 76 and nitroalkenes 77; some representative examples are shown. | ||
Aksenov et al.93 also reported a three-component condensation of arylhydrazines 39, 2-nitroalkenes 77 and acetophenone 78 to produce 3-aryl 2-quinolones 75 (Scheme 30).93
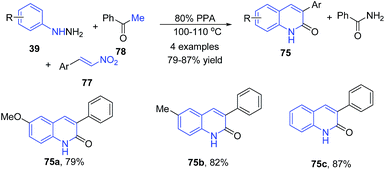 | ||
| Scheme 30 PPA-catalyzed synthesis of 3-aryl-2-quinolones 75 from arylhydrazines 39; some representative examples are shown. | ||
Yamaoka et al.94 reported a Brønsted acid-promoted arene-ynamide cyclization reaction to construct 3H-pyrrolo[2,3-c]quinolines 79. This reaction involves generation of a highly reactive keteniminium intermediate IV from arene-ynamide activated by a Brønsted acid and electrophilic aromatic substitution reaction to give arene-fused quinolines 79 in high yields (Scheme 31).
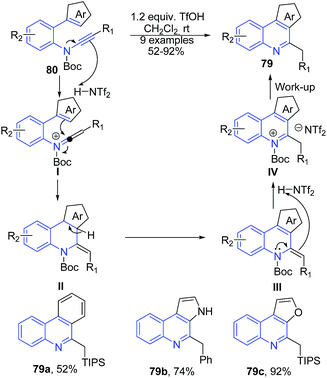 | ||
| Scheme 31 TfOH catalyzed synthesis of 3H-pyrrolo[2,3-c]quinolines 79; some representative examples are shown. | ||
3.2. Base catalyzed protocols
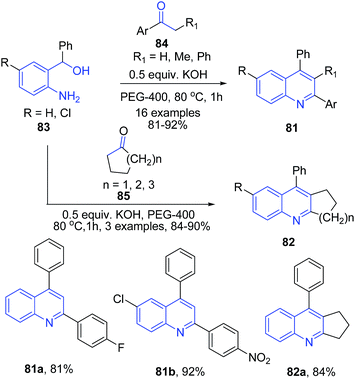
Like acids, various simple and commonly used bases have been employed to catalyze several important organic transformations. This has opened up a way to greener routes for synthesis of heterocyclic structures. Wu et al.95 reported synthesis of substituted quinolines 81 via direct reaction between the corresponding aminoalcohol 83 and ketone 84 using PEG-400 as reaction medium in the presence of a base (Scheme 32). This method was also effective for cyclic ketones 85 such as cyclopentanone, cyclohexanone and cycloheptanone producing corresponding substituted quinolines 82.Zhu and Cai96 reported similar protocol for synthesis of quinolines 86 using N-heterocyclic carbene as a catalyst. The reaction between 2-aminobenzyl alcohol 83 and ketones 84 proceeds via two tandem reactions – alpha-alkylation and indirect Friedländer annulation. The base deprotonates N-heterocyclic carbene salt I to generate a free carbine II. A cross aldol reaction between keto-intermediate IV and deprotonated ketone V, followed by a cyclization leads to formation of quinoline 86 (Scheme 33).
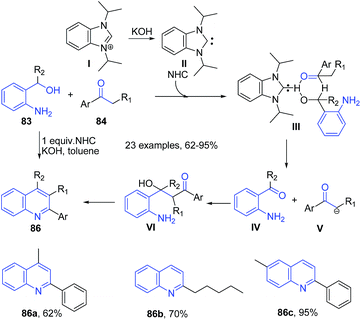 | ||
| Scheme 33 N-heterocyclic carbene and KOH catalyzed synthesis of quinolines 86; some representative examples are shown. | ||
Using similar starting materials (aminobenzylalcohol 83 and ketones 47 or 84), Mierde et al.97 have accomplished the synthesis of 2,3-disubstituted quinolines 87 in the presence of potassium tert-butoxide (Scheme 34). In the same year, Yus and coworkers98 have reported exactly same protocol, with the inclusion of 100 mol% benzophenone as an additive.
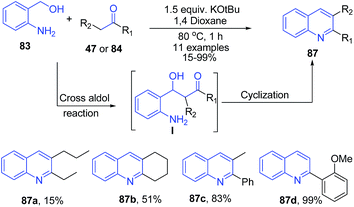 | ||
| Scheme 34 KOtBu-catalyzed synthesis of quinolines 86 from amino benzyl alcohols 83; some representative examples are shown. | ||
Yan et al.99 employed the use of alkyl or aryl nitro olefins 77 and 2-aminobenzaldehydes 90 in the presence of DABCO for synthesis of 2-substituted-3-nitro-1,2-dihydroquinolines 89. The amino group of 90 attacks the aryl nitro olefin 77 to form 1,4-addition intermediate I, which on cyclization followed by dehydration gives product 88. After oxidation with DDQ, high yields of 2-alkyl-3-nitroquinolines 88 were obtained (Scheme 35).
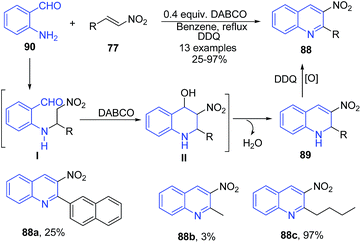 | ||
| Scheme 35 DABCO-catalyzed synthesis of 3-nitro quinolines 88; some representative examples are shown. | ||
Base-catalysed cyclization of β-(2-aminophenyl)-α,β-ynones 92 led to formation of 2,4-disubstituted quinolines 91 through tandem nucleophic addition annulations reactions.100 Interestingly, the exposure of the β-(2-malonylamidophenyl)-α,β-ynone 94 to K2CO3 accomplished the synthesis of fused quinolones 93 through an intramolecular Michael addition/tautomerisation and trans-esterification cascade reaction. Similarly, other fused quinolines 95–97 were also obtained (Scheme 36) from β-(2-aminophenyl)-α,β-ynones.
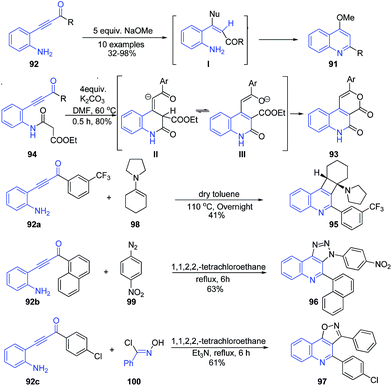 | ||
| Scheme 36 Base-catalyzed synthesis of 2,4-disubstituted quinolines 91 and fused quinolines 93, 95–97 from β-(2-aminophenyl)-α,β-nones. | ||
Wang and coworkers101 reported a three-component reaction between cyanoacetic acid methyl ester 102, substituted secondary amine 103 and 2-aminobenzalehyde 90 in the presence of NaOH in ethanol as a solvent produced 2-aminoquinoline-3-carboxamides 101 in good yields (Scheme 37).
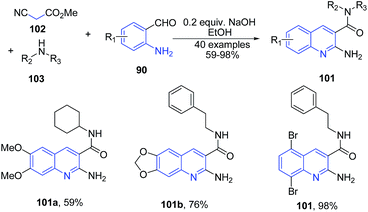 | ||
| Scheme 37 Base catalyzed one-pot three-component synthesis of 2-aminoquinoline-3-carboxamides 101; some representative examples are shown. | ||
Cameron et al.102 described an efficient one-pot procedure for the four-step preparation of 7-hydroxyquinoline 104 from 3-N-tosylaminophenol 105 in presence of diisopropylethylamine in 60% isolated yield. This one-pot procedure has reduced the risk of exposure to acrolein. The 3-N-tosylaminophenol 105 on condensation with acrolein 106 produces intermediate I. In ethanol, this intermediate I is readily converted to the stable acetal II, which further on intramolecular Friedel–Craft reaction, followed by dehydration, oxidation and detosylation produces 7-hydroxyquinoline 104 (Scheme 38).
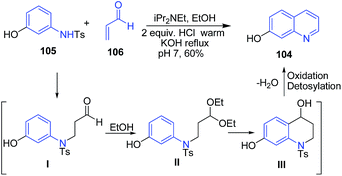 | ||
| Scheme 38 Diisopropylethylamine catalyzed synthesis of 7-hydroxyquinoline 104 from 3-N-tosylaminophenol 105. | ||
The reaction of aminochalcones 108 with tosylmethyl isocyanide 109 in presence of NaOH produced tricyclic pyrrolo[3,4-c]quinolines 107. In this domino process, three new bonds and two rings are successively formed at ambient conditions.103 The overall reaction process involves (i) Michael addition of 109 to aminochalcone 108 under basic conditions that provides the carbanion intermediate I; (ii) intramolecular cyclization of the resulting anion I to form the imidoyl anion intermediate II followed by hydrogen shift and elimination of tosylic acid to give the pyrrole intermediate III; and finally (iii) intramolecular condensation of ketone with amine to furnish pyrrolo[3,4-c]quinoline 107 (Scheme 39).
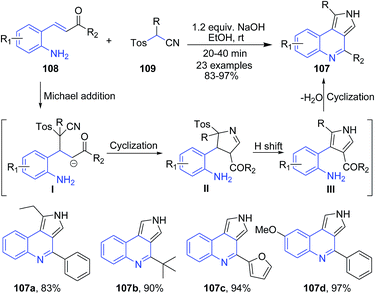 | ||
| Scheme 39 Synthesis of pyrrolo[3,4-c]quinolines 107 in presence of base in ethanol; some representative examples are shown. | ||
Rehan et al.104 reported synthesis of 2-aryl 4-substituted quinolines 110 from O-cinnamylanilines 111 (which are prepared from anilines and cinnamylalcohols). The reaction occurs via a regioselective 6-endo-trig intramolecular oxidative cyclization using KOtBu as a mediator and DMSO as an oxidant at room temperature (Scheme 40).
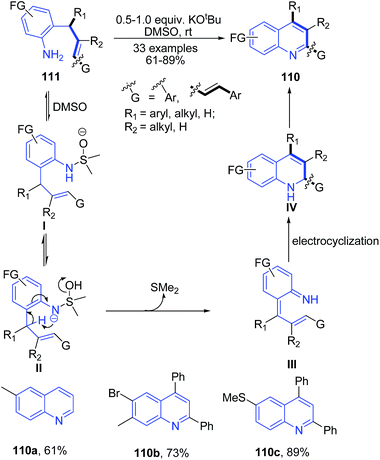 | ||
| Scheme 40 Potassium tert-butoxide catalyzed synthesis of 2-aryl 4-substituted quinolines 110; some representative examples are shown. | ||
The N-protected O-aminobenzaldehydes 113 in presence of K2CO3 in DMSO smoothly react with α,γ-dialkylallenoates 114 under Brønsted basic conditions to yield 2,3-disubstituted quinolines 112. This transformation involves a three-step reaction cascade of Michael addition, aldol condensation, and 1,3-N → C rearrangement (Scheme 41).105
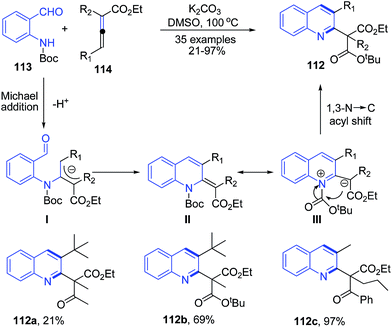 | ||
| Scheme 41 K2CO3 catalyzed synthesis of 2,3-disubstituted quinolines 112; some representative examples are shown. | ||
The treatment of 3-(2-bromophenyl)-3-oxopropanals 115 with amines 1 in presence of K2CO3 in dimethylsulfoxide led to formation of 3-substituted 4-quinolones 114.106 Reaction cascade involves base promoted enamine I – imine II transformation followed by dehydrobromination leading to cyclization to yield quinolone 114 (Scheme 42). In this reaction, weaker bases failed to function in either of the processes and stronger base triggered aldol condensation, however K2CO3 was proved to be the most suitable base.
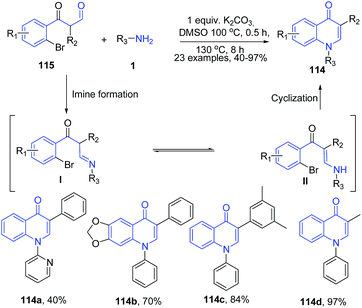 | ||
| Scheme 42 K2CO3-catalyzed synthesis of 3-substituted 4-quinolones 114 in DMSO; some representative examples are shown. | ||
Fu et al.107 have constructed another quinoline ring in the 2-chloroquinoline-3-carbaldehyde structure 117 by treatment with enaminones 33 in presence of Cs2CO3 catalyst, producing 1,8-naphthyridines 116. Initially, the aza-ene addition of enaminones 33 to 2-chloroquinoline-3-carbaldehyde 117 catalyzed by base leads to the formation intermediate I. The intermediate I then undergo an intramolecular cyclization to give intermediate II, which on elimination of water produces 1,8-naphthyridines 116 (Scheme 43).
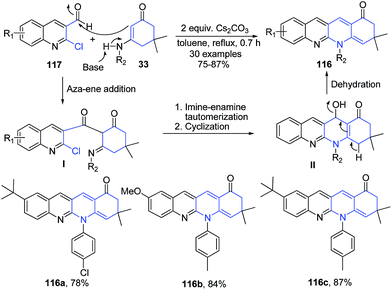 | ||
| Scheme 43 Cs2CO3 catalyzed synthesis of 1,8-naphthyridines 116; some representative examples are shown. | ||
Zhu and coworkers established a facile and efficient method for the preparation of 2-non-substituted quinoline-4-carboxylic acids 118 via the Pfitzinger reaction of isatins 40 with sodium pyruvate 120 following consequent decarboxylation under microwave irradiation (Scheme 44).108
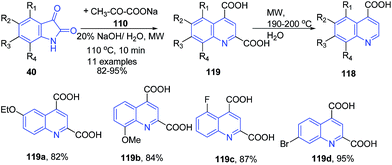 | ||
| Scheme 44 Synthesis of quinoline-4-carboxylic acids 118–119 under basic condition; some representative examples are shown. | ||
Rineh and coworkers109 have established triethylamine mediated protocol for synthesis of quinolines 121 via reaction between ethyl chloropyruvate 123 and activated acetylenic compounds 122 in the presence of nucleophilic form of isatin in water as the solvent. Nucleophilic form of isatin is produced from the reaction of isatin 40 and triethylamine (Scheme 45).
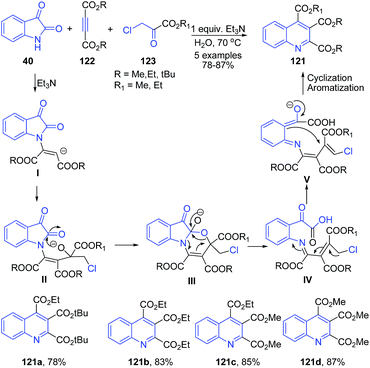 | ||
| Scheme 45 Et3N catalyzed synthesis of substituted quinolines 121 from isatins 40; some representative examples are shown. | ||
Alizadeh et al.110 reported three component reaction of isatins 40, 1-aryl-2-(1,1,1-triphenyl-λ5-phosphanylidene)-1-ethanone 125 and hydrazonoyl chlorides 126 in the presence of Et3N as a catalyst to produce pyrazolo[4,3-c]quinoline 124 (Scheme 46).
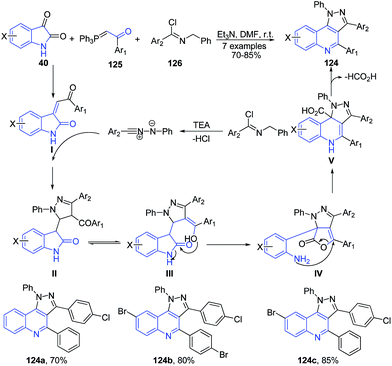 | ||
| Scheme 46 Et3N catalyzed synthesis of pyrazolo[4,3-c]quinolines 124 from isatins 40; some representative examples are shown. | ||
Kato et al.111 developed a domino protocol for synthesis of pyrazolo[1,5-a]quinolines 127 starting from 2-fluorobenzaldehydes 128 and substituted 3,5-dimethyl-1H-pyrazoles 129. In this cascade reaction, the inactivated methyl group of the pyrazoles 129 participates in the Knoevenagel cyclization upon arylating at the nitrogen of the pyrazoles through the SNAr substitution (Scheme 47).
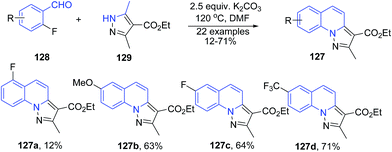 | ||
| Scheme 47 Base catalyzed synthesis of pyrazolo[1,5-a]quinolines 127; some representative examples are shown. | ||
Kato et al.112 reported another similar protocol for synthesis of pyrazolo[1,5-a]quinolines 130 from 2-fluoro aryl aldehydes 128 and pyrazole-3-carboxylic acid ester 131 using cesium carbonate base. This cascade reaction involves a sequential intermolecular aromatic nucleophilic substitution (SNAr) and intramolecular Knoevenagel condensation (Scheme 48).
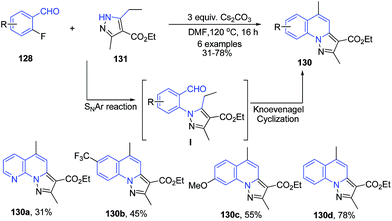 | ||
| Scheme 48 Cs2CO3-catalyzed cascade synthesis of pyrazolo[1,5-a]quinolines 130; some representative examples are shown. | ||
The reaction of 2-methyl benzimidazole 133 with 2-fluorobenzaldehydes 128 in presence of cesium carbonate in DMF produces benzimidazo[1,2-a]quinolines 132 via a cascade reactions involving sequential aromatic nucleophilic substitution and intramolecular Knoevenagel condensation reactions (Scheme 49).113
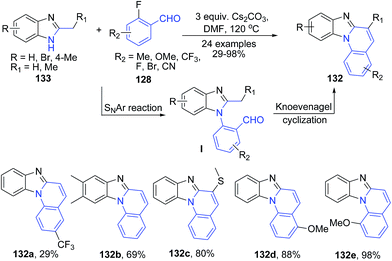 | ||
| Scheme 49 CS2CO3 catalyzed synthesis of benzimidazo[1,2-a]quinolines 132; some representative examples are shown. | ||
Kapoor and coworkers114 have reported synthesis of polyhydroquinolines 134–135 via a four-component one-pot reaction of aldehydes 5, dimedone 28e, active methylene compounds 38 and ammonium acetate 136 under solvent-free conditions at room temperature via grinding. The products of this protocol were obtained simply by recrystallization from ethanol (Scheme 50).
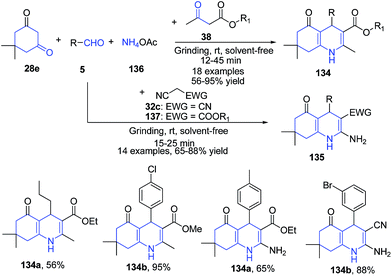 | ||
| Scheme 50 Catalyst and solvent free synthesis of polyhydroquinolines 134–135 using a one pot four component reaction; some representative examples are shown. | ||
Zhu and coworkers115 have developed a transition-metal-free method for the synthesis of C6 phenanthridine derivatives 138 by arylative cyclization of 2-isocyanobiphenyls 139 with arylamines 1 in presence of tert-butyl nitrite (t-BuONO) and using benzoyl peroxide as a promoter and sodium acetate as a base (Scheme 51). Initially, the anilines 1 reacts with t-BuONO to produce aryldiazonium ion which then gets decomposed (releasing N2 and t-BuO˙) in presence of benzoyl peroxide to produce aryl radical. The resulting aryl radical gets added to the terminal divalent carbon of 2-isocyanobiphenyl 139, to produce the N-biphenyl-2-yl imidoyl radical intermediate I. Next, the intramolecular hemolytic aromatic substitution of the imidoyl radical on the pending phenyl ring, forms the cyclohexadienyl radical intermediate III. Finally, deprotonation of the intermediate III produces 138, as depicted in Scheme 51.
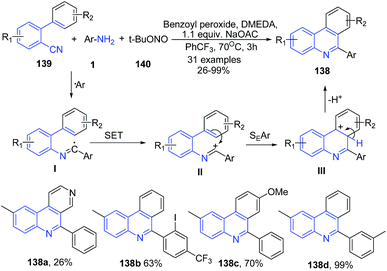 | ||
| Scheme 51 Sodium acetate and benzoyl peroxide mediated synthesis of phenanthridine derivatives 138; some representative examples are shown. | ||
Berteina-Raboin and Guillaumet116 described DBU catalyzed synthesis of pyrido[2′,1′:2,3]imidazo[4,5-b]quinolines 141 from (ethynyl)H-imidazo[1,2-a]pyridin-3-amines 142. The electron-rich secondary amine 142 assists in the hydroarylation of the triple bond after deprotonation by DBU. Next, aromatization leads to formation of pyrido[2′,1′:2,3]imidazo[4,5-b]quinoline 141 (Scheme 52).
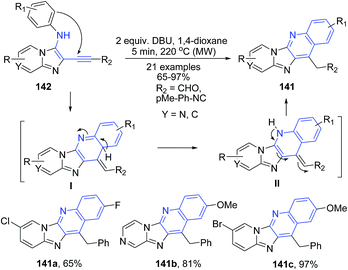 | ||
| Scheme 52 DBU-catalyzed synthesis of pyrido[2′,1′:2,3]imidazo[4,5-b]quinolines 141; some representative examples are shown. | ||
Zhou et al.117 described synthesis of polyfunctionalized quinolines 143 via the sequence of propargyl-allenyl isomerization and aza-electrocyclization from but-2-yn-1-yl-phenylimines 144 (Scheme 53).
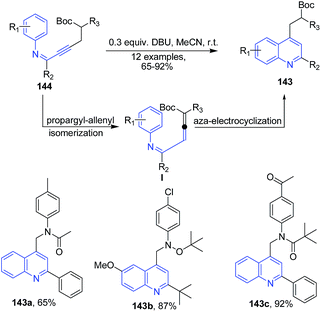 | ||
| Scheme 53 DBU promoted synthesis of polyfunctionalized quinolines 143; some representative examples are shown. | ||
3.3. Molecular iodine catalyzed protocols
Iodine has been very extensively used in organic chemistry to catalyze diverse range of organic transformations including multicomponent reactions.118 Gao et al.119 developed a highly efficient molecular iodine mediated formal [3 + 2 + 1] cycloaddition reaction for the direct synthesis of substituted 2-acyl quinolines 145 from methyl ketones 78, arylamines 1, and styrenes 7. Initial reaction of molecular iodine with acetophenone 78 leads to formation of the α-iodo ketone I, which gets converted to phenylglyoxal II by a subsequent Kornblum oxidation. The reaction of p-toluidine 1 with the aldehyde group of II then gives the C-acyl imine III, which reacts with HI to give the activated C-acyl imine ion IV. This activated C-acylimine species IV then undergoes cycloadition reaction with styrene (Povarov-type reaction) to give intermediate V in the presence of excess or regenerated iodine. Intermediate V then undergoes sequential oxidation and aromatization reactions to give 145 (Scheme 54).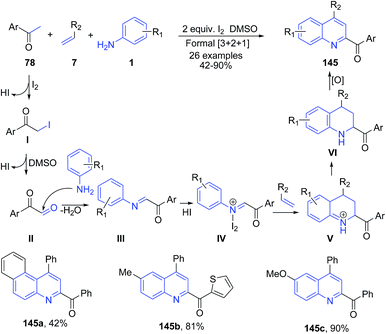 | ||
| Scheme 54 Molecular iodine-catalyzed synthesis of 2-acyl quinolines 145 from a three-component reaction between methyl ketones 78, arylamines 1 and styrenes 7; some representative examples are shown. | ||
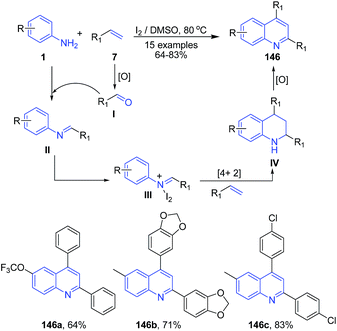
Recently Deshidi et al.120 reported molecular iodine catalyzed tandem reaction between styrenes 7 and anilines 1 producing 2,4-disubstituted quinolines 146. Styrene 7 first gets oxidized to aldehyde I which on condensation with arylamine 1 produces imine II. Imine intermediate II then on coupling with iodine forms iminium ion intermediate III which undergoes aza-Diels–Alder cycloaddition reaction with styrene 7 to form IV. Intermediate IV on oxidation leads to formation of quinoline 146 (Scheme 55).
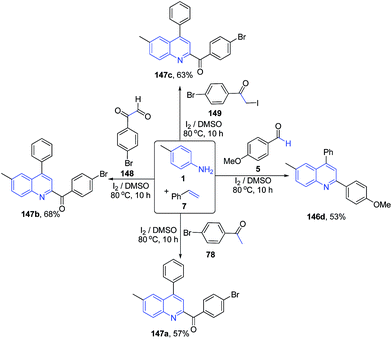
Deshidi et al.120 also reported molecular iodine catalyzed three-component reaction between arylamines 1, styrenes 7 and carbonyl compound 5, 78, 148–149 leading to formation of substituted quinolines 146a, 1147a–c (Scheme 56).
Lin's group have developed molecular iodine catalysed synthesis of quinolines 150–151 from aldehydes, amines, and alkynes at mild reaction conditions.121 The method was also applicable for construction of benzo[f] quinolines 151a (70% yield) and ellipticine 151b (68% yield) (Scheme 57).
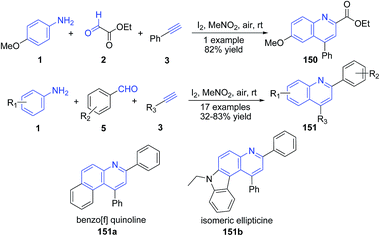 | ||
| Scheme 57 Molecular iodine catalyzed synthesis of 2,4-disubstituted quinolines 150–151; some representative examples are shown. | ||
Wang's group122–124 have developed a mild and efficient method for the synthesis of pyranoquinoline 152, thiopyranoquinoline 153, thienoquinoline 154, chromanoquinolines 155 and naphtho[2,7]naphthyridine 156 derivatives via three-component reaction of aromatic aldehyde 5, naphthalene-2-amines 41b, and heterocycloketones 149 and 161–168 including tetrahydropyran-4-one 161, tetrahydrothiopyran-4-one 162, dihydrothiophen-3(2H)-one 163, chroman-4-one 164 and N-Boc 4-piperidinone 165, using iodine as catalyst (routes A–E).122 On the use of 2-halogenated acetophenones 149 in the place of cyclic ketones, 1,3-diarylbenzo[f]quinolines 157 were obtained (route F).123 Further same group explored the utility of this method for construction of several other fused quinolines viz. benzo[f]furo[3,2-c]quinoline 158 (route G), benzo[f]pyrano[3,2-c]quinoline 159 (route H), and benzo[f]quinolines 160 (route I) using dihydrofuran 166, dihydropyran 167 and n-butylvinyl ether 168 as third coupling partners (Scheme 58).124
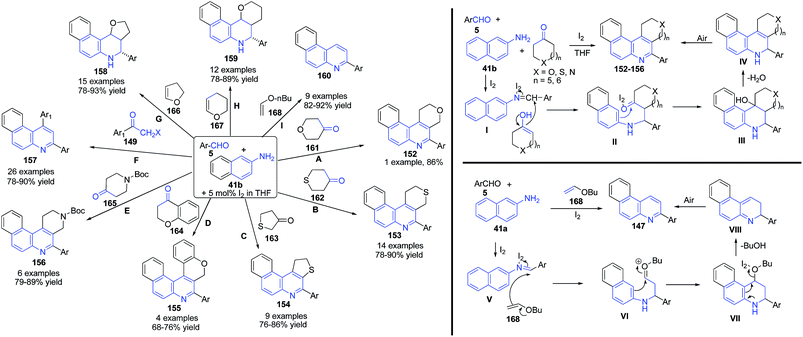 | ||
| Scheme 58 Molecular iodine catalyzed synthesis of pyranoquinoline 152, thiopyranoquinoline 153, thienoquinoline 154, chromanoquinolines 155 and naphtho[2,7]naphthyridine 156 derivatives. | ||
Wang's group125 further investigated this reaction, wherein a three-component reaction of aromatic aldehyde 5, naphthalene-2-amine 41b and tetrahydro-2,5-dimethoxyfuran 170 in methanol catalyzed by iodine, produced 3-aryl-2-(3-arylbenzo[f]quinolin-2-yl)benzo[f]quinoline derivatives 169 via ring opening of furan (Scheme 59).
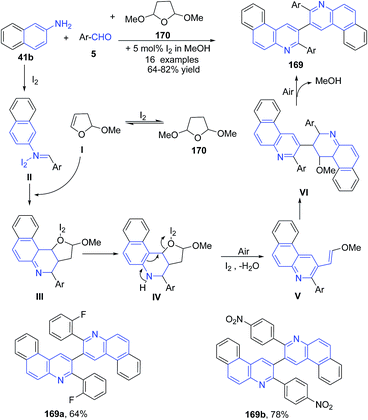 | ||
| Scheme 59 Molecular iodine catalyzed synthesis of bis-benzoquinolines 169; some representative examples are shown. | ||
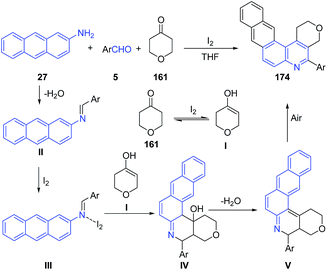
These authors124,126 also investigated the utility of this method for preparation of naphtho[2,3-f]furo[3,2-c]quinolines 171 (route A),124 naphtho[2,3-f]pyrano[3,2-c]quinolines 172 (route B)124 and naphtho[2,3-f]quinolines 173 (route C)124 and naphtho[2,3-f]pyrano[3,4-c]quinolines 174 (route D) (Scheme 60) from anthracen-2-amine 27.126 The mechanism involves the formation of imine II which undergoes covalent bond formation with iodine to produce intermediate III. This intermediate reacts with enol form I of tetrahydropyran-4-one 161 to produce cyclized intermediate IV, which on dehydration produces V. Finally air oxidation of V produces naphthoquinoline 174 (Scheme 61).
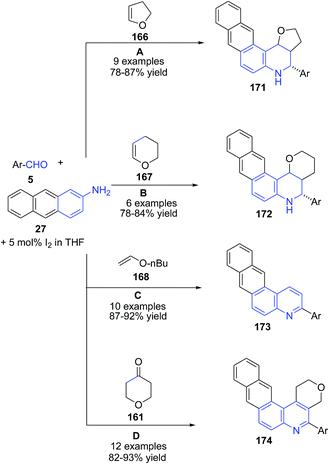 | ||
| Scheme 60 Molecular iodine catalyzed synthesis of naphthoquinolines 171–174 from anthracenyl amines. | ||
Molecular iodine catalyzed three-component imino Diels–Alder reaction of aromatic aldehyde 5, anthracene-2-amine 27 or 1H-indazol-5-amine 177 and cyclopentanone 85 produced cyclopenta[c]naphtho[2,3-f]quinoline 175 and cyclopenta[c]- pyrazolo[4,3-f]quinoline 176 (Scheme 62).127
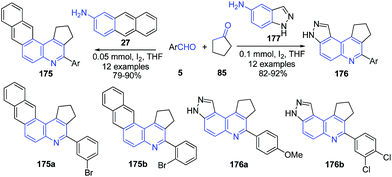 | ||
| Scheme 62 Molecular iodine catalyzed synthesis of cyclopenta[c]naphtho[2,3-f]quinoline 175 and cyclopenta[c] pyrazolo[4,3-f]quinolines 176. | ||
Anionic surfactant sodium dodecyl sulfate has also been employed in heterocycle synthesis.128 Recently Ganguly and Chandra129 have employed the use of molecular iodine and sodium dodecyl sulfate for the construction of quinoline skeleton using a three-component reaction. A three-component coupling of 6-aminocoumarin 8, aromatic aldehyde 5 and an excess of styrene 7 in water in presence of molecular iodine and sodium dodecyl sulfate produced pyrano[3.2-f]quinolin-3-ones 178 and pyrano[2.3-g]quinolin-2-ones 179. The reaction mechanism involves a cascade of two key transformations viz. Povarov reaction and hydrogen transfer (Scheme 63).
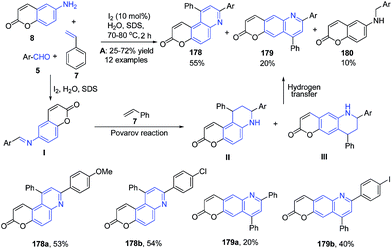 | ||
| Scheme 63 Molecular iodine catalyzed synthesis of synthesis of pyrano[3.2-f]quinolin-3-ones 178 and pyrano[2.3-g]quinolin-2-ones 179; some representative examples are shown. | ||
Wu and coworkers130 reported a mild and efficient route for the synthesis of quinolines 181 and polycyclic quinolines 182–183 via Friedlander annulation utilizing molecular iodine (1 mol%). Treatment of 2-aminoaryl ketone 46 with α-methylene ketones 38 in ethanol in presence of molecular iodine produced quinolines 181. Cyclic ketones such as cyclopentanone 85 and cyclohexadione 28e also underwent smooth condensation with 2-aminoaryl ketones to afford the respective tricyclic quinolines 182–183 (Scheme 64). The synthesis of 181 from 2-aminoaryl ketone 46 with α-methylene ketones 38 has also been reported using (bromodimethyl)sulfonium bromide131 or cyanuric chloride132 as a catalyst.
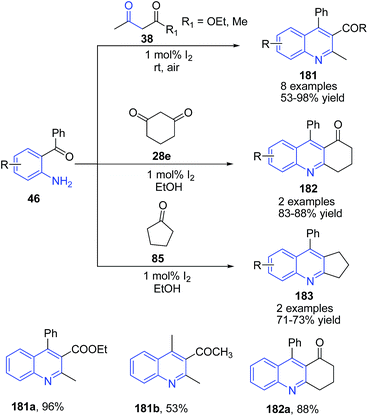 | ||
| Scheme 64 Molecular iodine-catalyzed synthesis of 2,3,4-trisubstituted quinolines 181–183; some representative examples are shown. | ||
Zeng and Cai133 reported a domino protocol for synthesis of benzo[f]quinolinyl acetamides 184 and benzo[h]quinolinyl acetamides 185 from diketene 186, benzyl amines 187, aromatic aldehydes 5 and naphthalene amines 41b using molecular iodine as a catalyst (Scheme 65).
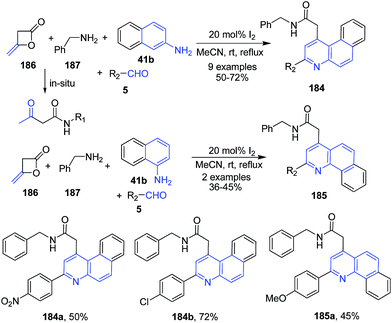 | ||
| Scheme 65 Molecular iodine catalyzed synthesis of benzo[f]quinolinyl and benzo[h]quinolinyl acetamides 184–185; some representative examples are shown. | ||
Fotie et al.134 reported synthesis of a series of unusual 2,3,4,5-tetrahydro-4,4-tetramethylene-1H-cyclopenta[c] quinolines 188 through the Skraup-Doebner–Von Miller quinoline synthesis. The reaction mechanism involves three basic sequences: (a) the formation of a Schiff base I through a reaction between the ketone 85 and the aniline 1 in the first step, followed by (b) a cycloalkenylation at the ortho-position to the amine functional group of the aniline, and (c) an annulations in the final step to close the quinoline ring, leading to a dihydroquinoline derivative 188 as described in Scheme 66.
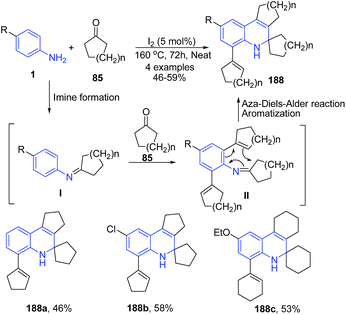 | ||
| Scheme 66 I2-catalyzed synthesis of cyclopenta[c]quinolines 188; some representative examples are shown. | ||
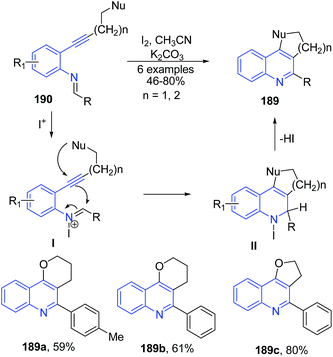
The oxidative cyclization of phenyl-N-(o-alkynylphenyl)imines 190 in presence of molecular iodine produced furanoquinolines 189 (Scheme 67).135 The iodide cation on coupling with imine 190 generated iminium ion I which further undergoes intramolecular cyclization to produce quinoline II, which in elimination of HI produces 189.
Batra's group136 reported molecular iodine catalyzed synthesis of 2-substituted quinolines 191 from substituted primary allylamines 192. Iodine initially activates the carbonyl group, which is then followed by electrophilic cyclization to produce dihydroquinoline II. The intermediate II on the subsequent elimination of two protons in the form of 2 HI molecules produces intermediate V, which finally oxidizes to the quinoline 191 (Scheme 68).
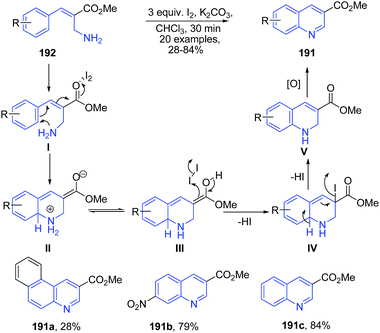 | ||
| Scheme 68 Iodine-catalyzed synthesis of 2-substituted-quinolones 191 from allylamines 192; some representative examples are shown. | ||
2-Tosylaminophenylprop-1-yn-3-ols 194 in the presence of molecular iodine undergoes 6-endo-dig iodocyclization leading to formation of substituted 3-iodoquinolines 193 (Scheme 69).137 The mechanism involves anti-attack of the iodide cation and the nitrogen of the tosylated amino group on the alkyne moiety of 178 to produce an intermediate II, which further undergoes a proton removal by the iodide producing intermediate III. The intermediate III then loses hydroxyl ion to give cation IV, which finally on elimination of tosyl group leads to formation of quinoline 193.
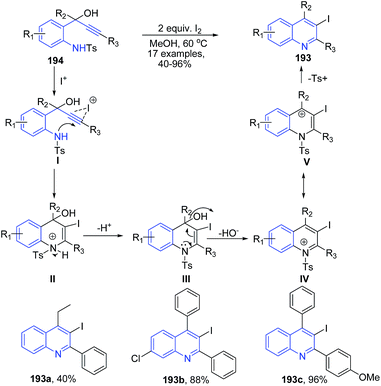 | ||
| Scheme 69 Molecular iodine catalyzed synthesis of 2-aryl-3-iodo-quinolines 193; some representative examples are shown. | ||
Activation of C2 and C3 of indoles 196 by molecular iodine and base followed by in situ reaction with 1-(2-tosylaminophenyl)ketones 197 or 2-tosylaminobenzaldehyde afforded highly substituted indolo(2,3-b)quinolines 195 in moderate to excellent yields.138 This is a domino one-pot protocol involving cascade of three reactions – amination, alkylation and aromatization. The mechanism of this reaction involves electrophilic addition of iodonium to the 3-position of indole 196 to give cation I, which undergoes 2-amination with 197 to afford II. The intermediate II eliminates a molecule of HI in the presence of base to give III. Alkylation and subsequent detosylation of III in the presence of HCl gives 195 (Scheme 70).
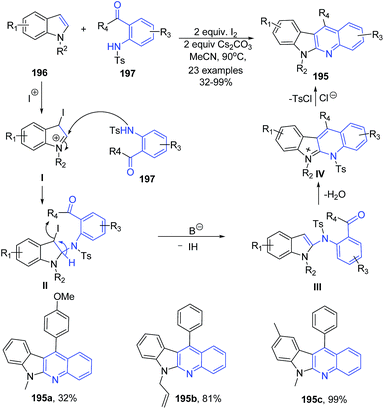 | ||
| Scheme 70 I2-catalyzed synthesis of indolo(2,3-b)quinolines 195; some representative examples are shown. | ||
Isocyanides are one of the promising precursors for the preparation of N-heterocycles such as pyrroles, indoles, and quinolines.139–141 Tu and coworkers142 have established a iodine-promoted domino reaction of 2-aminochromene-3-carbonitriles 199 with various isocyanates 200 for synthesis of polyfunctionalized N-substituted 2-aminoquinoline-3-carbonitriles 198 with high regioselectivity under microwave heating. The reaction of phenyl isocyanate 200 with 2-aminochromene-3-carbonitrile 199 underwent [2 + 2] cyclization to produce β-lactam intermediate I, which then gets hydrolyzed forming a ring-opened intermediate II. Next, the intermediate II releases CO2 to give intermediate III, which undergoes intramolecular cyclization to afford the 1,4-dihydropyridine IV. Finally, the aromarization of IV led to formation of 198 (Scheme 71).
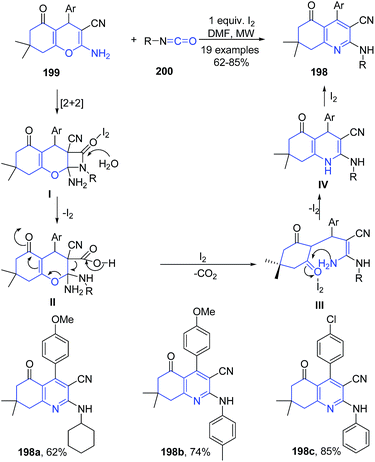 | ||
| Scheme 71 I2-catalyzed synthesis of aminoquinoline-3-carbonitriles 198; some representative examples are shown. | ||
Mitamura and Ogawa143 found that upon photoirradiation of O-alkynylaryl isocyanides 202 in the presence of molecular iodine, it undergoes intramolecular cyclization to afford the corresponding 2,4-diiodoquinolines 201 in good yields. This reaction does not took place in the dark, indicating that the reaction requires photoirradiation (Scheme 72).
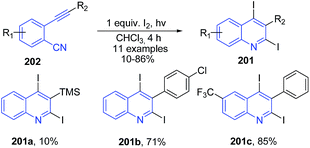 | ||
| Scheme 72 Synthesis of 2,4-diiodoquinolines 201 via the photochemical cyclization of o-alkynylaryl isocyanides 202 with molecular iodine; some representative examples are shown. | ||
3.4. Ionic liquid mediated quinoline synthesis
Ionic liquid have been considered as a green reaction media with recyclability and this has been used as catalyst as well as reaction media.55 Our group144 have developed an expedient and metal-free synthetic protocol for construction of substituted quinolines 203–204 from anilines 1 and phenylacetaldehydes 205 using imidazolium cation-based ionic liquids as the reaction medium. [Bmim]BF4 activates the aldehyde electrophile by interaction with the carbonyl oxygen. The ionic liquid [Bmim]BF4 also enhances the nucleophilicity of the amine through interaction of tetrafluoroborate with N–H bond. The resulting imine intermediate I undergo self-condensation to generate II as a key intermediate. The C-2 benzyl moiety gets cleaved through radical mechanism by release of benzaldehyde, producing 3-substituted quinoline 204 (Scheme 73).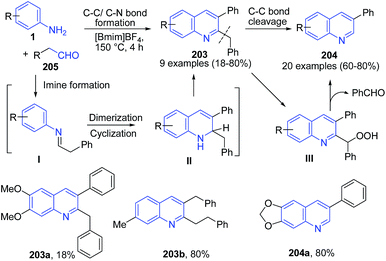 | ||
| Scheme 73 Synthesis of 2,3-disubstituted 203 and 3-substituted quinolines 204 in ionic liquid; some representative examples are shown. | ||
Another ionic liquid mediated synthesis of quinolines is reported, involving a four-component, one-pot reaction of aromatic aldehyde 5, cyclohexanone 85, malononitrile 33c, and amines 1 or 205 in basic ionic liquid [Bmim]OH to produce tetrahydroquinoline-3-carbonitriles 206 (Scheme 74).145
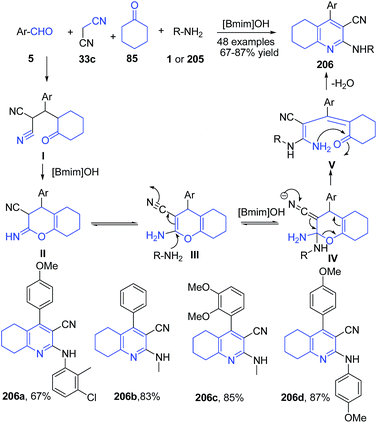 | ||
| Scheme 74 Synthesis of tetrahydroquinoline-3-carbonitriles 206 in ionic liquid; some representative examples are shown. | ||
A two-phase microwave-assisted cascade reaction between isatins 40 and β-ketoamides 208 in [Bmim]BF4/toluene led to the formation of pyrrolo[3,4-c]quinoline-1,3-diones 207 (Scheme 75).146 The recyclability of the ionic liquid for 6 cycles was shown. The prepared pyrrolo[3,4-c]quinoline-1,3-diones displayed antibacterial activity.
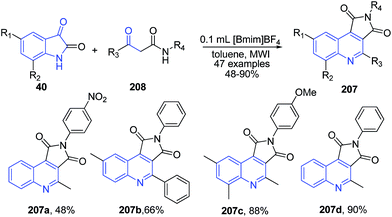 | ||
| Scheme 75 Synthesis of pyrrolo[3,4-c]quinoline-1,3-diones 207 in ionic liquid; some representative examples are shown. | ||
The condensation reaction involving an O-aminoaryl ketones 46 with α-methylene ketones 47 in ionic liquid [Hbim][BF4] as a solvent with methanol as co-solvent at room temperature under ultrasound irradiation afforded the corresponding quinolines derivatives 209 in excellent yields, via tandem addition/annulation reactions.147 The reaction was also applicable to cyclic ketones producing tricyclic compounds (Scheme 76).
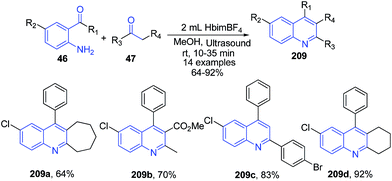 | ||
| Scheme 76 Synthesis of substituted quinolines 209 in ionic liquid under ultra-sound irradiation; some representative examples are shown. | ||
Kowsari and Mallakmohammadi148 described synthesis of quinoline-4-carboxylates 210 by condensation of isatins 40 α-methylene ketones 47 in presence of 0.5 equiv. [Bmim]OH ionic liquid and ultrasonication (Scheme 77). The mechanism of this reaction involves the reaction of isatin 40 with a [Bmim]OH that hydrolyses the amide bond to produce the keto-acid I. The enamine II form on cyclization produces quinoline III which finally on dehydration result in the formation of desired quinoline product 210.
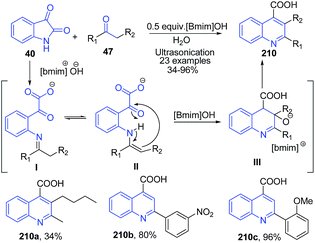 | ||
| Scheme 77 Synthesis of quinoline-4-carboxylates 210 in ionic liquid; some representative examples are shown. | ||
A three-component reaction of aryl aldehyde 5, (E)-3-aminobut-2-enenitrile 212 and 2-hydroxynaphthalene-1,4-dione 35 in ionic liquid produced polysubstituted benzo[h]quinolines 211 (Scheme 78). Another three-component reaction involving condensation of aryl aldehyde 5, (E)-3-aminobut-2-enenitrile 212 and dimedone 28e in ionic liquid produced 1,4,5,6,7,8-hexahydro-2,7,7-trimethyl-5-oxo-4-arylquinoline-3-carbonitriles 213 (Scheme 79).149 The reaction mechanism involves subsequent Knoevenagel condensation, Michael addition, intra-molecular cyclization, and dehydration reaction.
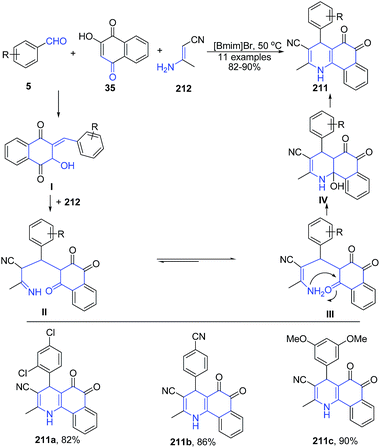 | ||
| Scheme 78 Synthesis of polysubstituted benzo[h]quinolines 211 in ionic liquid; some representative examples are shown. | ||
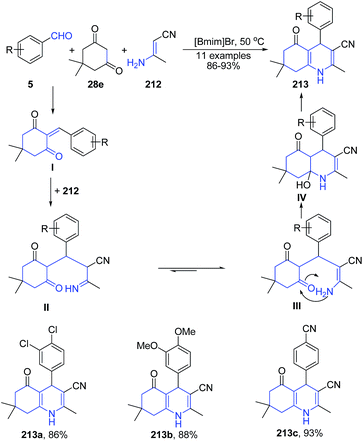 | ||
| Scheme 79 Synthesis of 2,7,7-trimethyl-5-oxo-4-arylquinoline-3-carbonitriles 213 in ionic liquid; some representative examples are shown. | ||
A series of 7-aryl-11,12-dihydrobenzo[h]pyrimido-[4,5-b]quinoline-8,10(7H,9H)-diones 214 were synthesized via three-component reaction of aryl aldehydes 5, 1-naphthylamine 41a and barbituric acid 215 in ionic liquid (Scheme 80).150
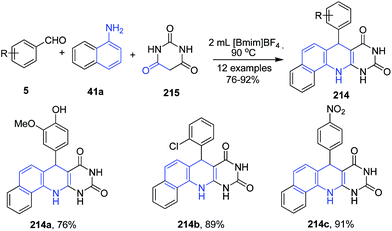 | ||
| Scheme 80 Synthesis of pyrimido-[4,5-b]quinoline-8,10(7H,9H)-diones 214; some representative examples are shown. | ||
3.5. Organocatalysis for quinoline synthesis
The use of small chiral organic molecules as catalysts, has proven to be a valuable and attractive tool for synthesis of enantiomerically enriched molecules and thus it finds wide applications in drug discovery.151 Furthermore, organocatalysis finds tremendous utility in asymmetric C–C bond formation reactions.152,153 The utility of these catalysts in quinoline synthesis has also been well reported. An organocatalytic asymmetric three-component Povarov reaction involving 2-hydroxystyrenes produced structurally diverse cis-disubstituted tetrahydroquinolines 216 in high stereoselectivities of up to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and 97% ee.154 The 2-hydroxystyrene 217 is structurally similar to an dienol species, which participate in a vinylogous Mannich reaction with an aldimine II generated from an aryl aldehyde 5 or aliphatic aldehyde 205 and aniline 1 under the catalysis of a chiral phosphoric acid, forming a transient intermediate III, which principally undergoes an intramolecular Friedel–Crafts reaction (the 1,4-addition of aniline to the enone functionality) to afford enantio-enriched multiply substituted tetrahydroquinolines 216 (Scheme 81).
1 dr and 97% ee.154 The 2-hydroxystyrene 217 is structurally similar to an dienol species, which participate in a vinylogous Mannich reaction with an aldimine II generated from an aryl aldehyde 5 or aliphatic aldehyde 205 and aniline 1 under the catalysis of a chiral phosphoric acid, forming a transient intermediate III, which principally undergoes an intramolecular Friedel–Crafts reaction (the 1,4-addition of aniline to the enone functionality) to afford enantio-enriched multiply substituted tetrahydroquinolines 216 (Scheme 81).
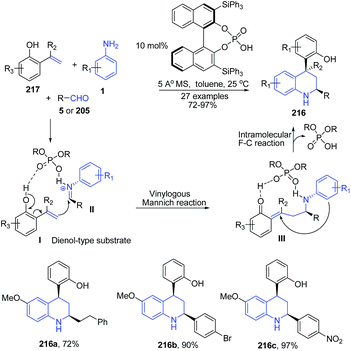 | ||
| Scheme 81 Chiral phosphoric acid-catalyzed synthesis of cis-disubstituted tetrahydroquinolines 216; some representative examples are shown. | ||
A series of 4-aza-podophyllotoxin derivatives 218 have been synthesized regioselectively via the three-component reaction of aldehydes 5, aromatic amines 1, and tetronic acid 28d catalyzed by L-proline.155 L-Proline catalyzes the formation of iminium ion II in a reversible reaction with tetronic acid 28d. The higher reactivity of the iminium ion II compared with the carbonyl species facilitates the addition of aniline 1, via intermediate III, producing intermediate IV. The intermediate IV on elimination of L-proline produces V. The product 218 was then formed by tautomerization of intermediate V (Scheme 82).
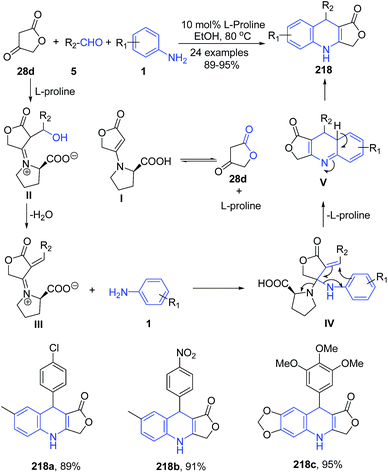 | ||
| Scheme 82 L-Proline-catalyzed synthesis of dihydrofuro[3,4-b]quinolin-1(3H)-ones 218; some representative examples are shown. | ||
Khalafi-Nezhad et al.156 have described a L-proline mediated synthesis of 5-arylpyrimido-[4,5-b]quinoline-diones 219 via a three-component reaction between anilines 1, aldehydes 5 and barbituric acids 215 (or 220) under aqueous conditions. L-Proline activates the aldehyde to produce intermediate I. Similarly, L-proline assists in enolization of the barbituric acid 215 (or 220) to produce II. Coupling of I and II produces adduct III, which further loses a L-proline molecule to generate ortho-quinone methide IV. L-Proline further activates this adduct IV, followed by coupling of aniline produces VI. Intermediate VI subsequently undergoes an intramolecular reaction to give the desired product 219 (Scheme 83).
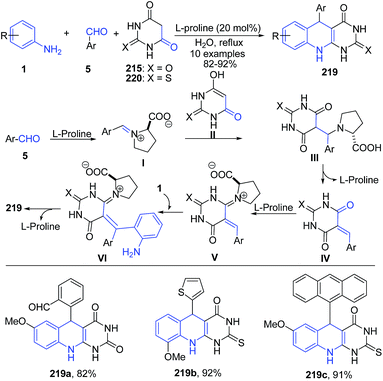 | ||
| Scheme 83 L-Proline-catalyzed synthesis of aryl-pyrimido[4,5-b]quinoline-diones 219; some representative examples are shown. | ||
Khalafi-Nezhad et al.156 also described a L-proline mediated synthesis of 2-amino-4-arylquinoline-3-carbonitriles 221 using a similar three-component reaction between anilines 1, aldehydes 5 and malanonitrile 33c under aqueous conditions (Scheme 84).
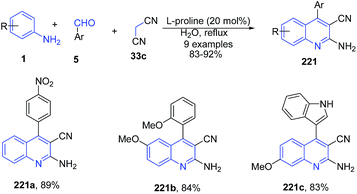 | ||
| Scheme 84 L-Proline-catalyzed synthesis of 2-amino-4-arylquinoline-3-carbonitriles 221; some representative examples are shown. | ||
A series of 2H-benzo[g]pyrazolo[3,4-b]quinoline-5,10(4H,11H)-diones 222 were synthesized using three component reaction of 2-hydroxy-1,4-naphthoquinone 35, aldehydes 5, and aminopyrazoles 223 in the presence of a catalytic amount of L-proline.157 Reaction proceeds via domino Aldol reaction–Michael addition–N-cyclization–tautomerism sequence to give fused quinoline product regioselectively (Scheme 85).
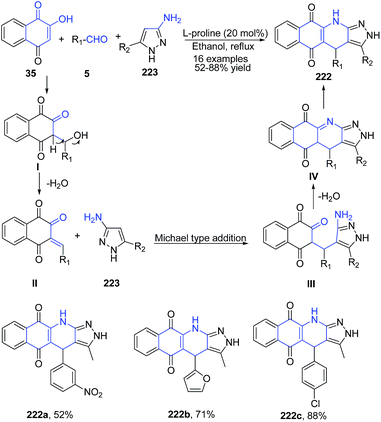 | ||
| Scheme 85 L-Proline-catalyzed synthesis of benzo[g]pyrazolo[3,4-b]quinoline diones 222; some representative examples are shown. | ||
A cascade reaction of ortho-azido-β-nitro-styrenes 225 with various carbonyl compounds 45 furnished substituted quinolines 224 (Scheme 86).158 The Michael reaction of ketone 47 to β-nitroolefins 225 followed by coupling of PPh3 led to formation of iminophosphorane intermediate II via Staudinger reaction. This iminophosphorane II undergoes the intramolecular ring-closure via the aza-Wittig reaction at room temperature to produce quinoline III which on elimination of nitromethane moiety produces 224. The cyclic ketones 85 produced corresponding tricyclic products 226 via intermediate IV.
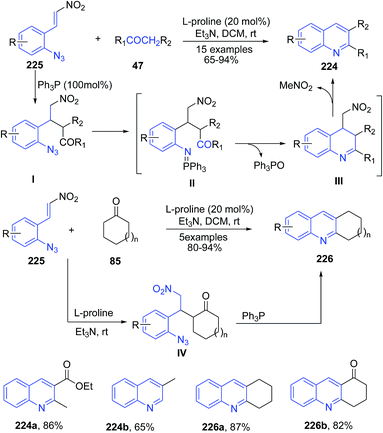 | ||
| Scheme 86 L-Proline-catalyzed synthesis of 2,3-disubstituted quinolines 224, 226; some representative examples are shown. | ||
3.6. Catalyst-free quinoline synthesis
Apart from the use of above discussed simple non-metal catalysts, several reactions proceeds efficiently in suitable solvents without use of any catalyst. Reaction of 2-(aminomethyl)aniline 228 with ketones 47 in presence of oxygen atmosphere produced quinoline products 207.159 Reaction involved condensation of aniline 211 with ketone 47 to form imine I which gets oxidized to aldehyde II by oxygen and high temperature. This was followed by cyclization and dehydration to produce quinolines 227 (Scheme 87).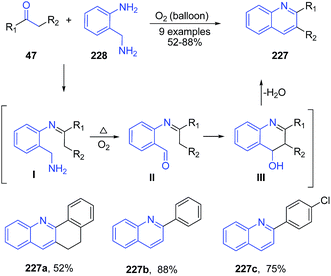 | ||
| Scheme 87 Catalyst-free synthesis of 2-substituted quinolines 227 by treatment of 2-(aminomethyl)aniline 228 with ketones 47; some representative examples are shown. | ||
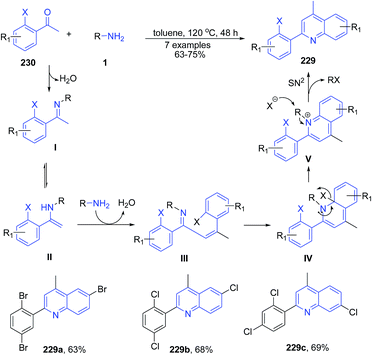
The condensation and cyclization of two molecules of ortho-haloacetophenones 230 with primary amines 1 produced halogen-substituted 2-aryl quinolines 229.160 The mechanism involves first the formation of ketimine I by dehydration of 230 with amine 1. This was then followed by the intermolecular nucleophilic attack of 1 by enamine carbon of II followed by dehydration to give α,β-unsaturated imine III. Next the electrocyclic reaction of III leads to formation of the intermediate IV. Finally the elimination and subsequent SN2 reaction of IV produces 229 (Scheme 88).
A three-component reaction of aromatic aldehyde 5, 1H-indol-5-amine 235, and 1,3-dicarbonyl compounds 28c, 28e, 28g produced pyrrolo[3,2-f]quinoline 231–233 and pyrrolo[3,2-a]acridine 234 derivatives under catalyst-free conditions (Scheme 89).161
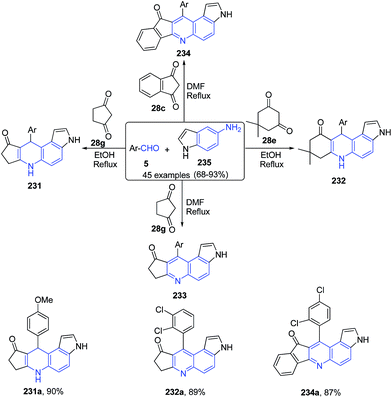 | ||
| Scheme 89 Catalyst free synthesis of pyrrolo[3,2-f]quinoline 231–233 and pyrrolo[3,2-a]acridines 234; some representative examples are shown. | ||
Fayol and Zhu162 have reported synthesis of polysubstituted furo[2,3-c]quinoline 236, simply by mixing an ortho-alkynyl aniline 237, an aldehyde 5, and ammonium chloride in toluene at room temperature, followed by addition of an isocyanoacetamide 238 under heating condition (Scheme 90). The proposed reaction mechanism involves the formation of oxazole III as a key intermediate. Next the intramolecular cycloaddition reaction of an oxazole III as an aza-diene with the properly predisposed triple bond produce an furo[2,3-c]quinolines 236.
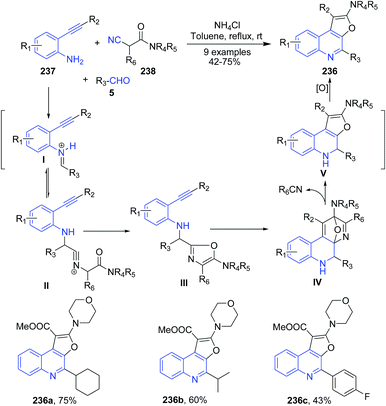 | ||
| Scheme 90 Catalyst-free synthesis of furo[2,3-c]quinolines 236; some representative examples are shown. | ||
The condensation of O-phenylaniline 241 and its homologues with cyclic ketones 85 under hydrothermal conditions led to formation of phenanthridines 239–240.163 The mechanism proposed for this transformation involves aza-triene-type electrocyclization, followed by irreversible cycloalkane ring-fission as crucial steps (Scheme 91).
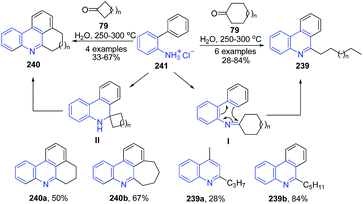 | ||
| Scheme 91 Catalyst free synthesis of phenanthridines 239–240; some representative examples are shown. | ||
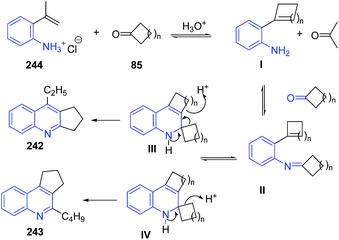
These authors163 further extended this protocol to the reaction of 2-isopropenylanilines HCl 244 to obtain quinoline derivatives 242–243. The reaction pathway has been depicted in Scheme 92.
Yuvaraj et al.164 described a microwave-assisted, chemoselective synthesis of oxazolo[5,4-b]quinoline – fused spirooxindoles 245 via three-component tandem Knoevenagel/Michael addition reaction of 5-amino-3-methylisoxazole 246, β-diketones 28e and isatins 40 in good to excellent yields under catalyst- and solvent- free conditions. A possible mechanism for the established 3CC reaction indicated that the β-diketone 28e initially reacts with isatin 40 to give the Knoevenagel condensation product I which undergoes a Michael-type addition with 5-amino-3-methylisoxazole 246 followed by the cyclocondensation of the intermediate adduct II to give corresponding quinolines 245 (Scheme 93).
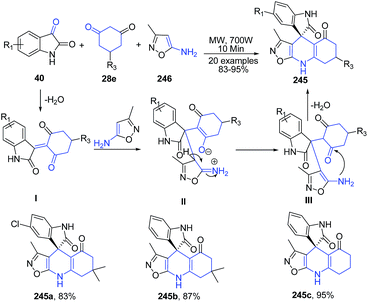 | ||
| Scheme 93 Catalyst free MW assisted synthesis of oxazolo[5,4-b]quinoline-fused spirooxindoles 245; some representative examples are shown. | ||
The four-component domino reaction of 2-hydroxy-1,4-naphthaquinone 35, aromatic aldehydes 5, methyl/ethyl acetoacetate 38 and ammonium acetate in ethanol under microwave irradiation at 100 °C afforded tetrahydrobenzo[g]quinoline-5,10-diones 247 regioselectively in good yields. The mechanism involved first the Mannich reaction between 2-hydroxy-1,4-naphthaquinone 35 with aromatic aldehydes 5 to produce intermediate I which further on release of ammonia produces II. The condensation of II with amine intermediate III leads to formation of a cyclized intermediate IV, which finally on dehydration generated product 247 (Scheme 94).165
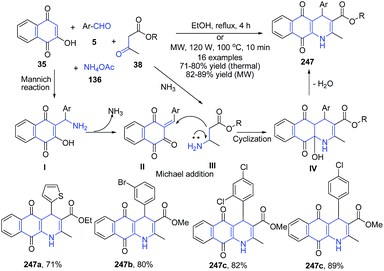 | ||
| Scheme 94 Catalyst free synthesis of tetrahydrobenzo[g]quinolines 247; some representative examples are shown. | ||
Alizadeh and Rezvanian166 reported one-pot, catalyst-free, four-component synthesis of octahydro-imidazo[1,2-a]quinolin-6-ones 248 from aromatic aldehydes 5, cyclic 1,3-diones 28e, diamines 250, and nitro ketene dithioacetal 249 under catalyst and solvent free conditions. Mechanism involves first Knoevenagel condensation between the aldehyde 5 and the cyclic 1,3-dione 28e, resulting in the adduct I. Then the reaction between intermediate I and the ketene aminal II (which is derived from the addition of diamine 250 to nitro ketene dithioacetal 249) gives the Michael adduct III. The Michael adduct III undergoes a cyclocondensation reaction through amino and carbonyl to afford compound 248 (Scheme 95).
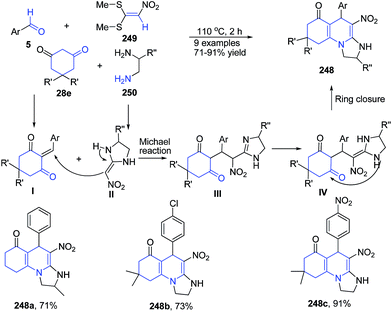 | ||
| Scheme 95 Catalyst-free synthesis of octahydro-imidazo[1,2-a]quinolin-6-ones 248; some representative examples are shown. | ||
Chidurala et al.167 reported one-pot multicomponent atom-efficient, catalyst-free reaction between resorcinol 252, aromatic aldehyde 5, acetoacetanilide 253 and ammonium acetate 136 to produce substituted 1,4-dihydroquinolines 251 (Scheme 96).
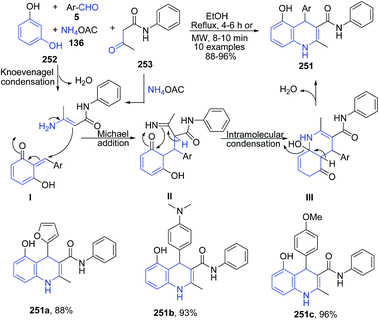 | ||
| Scheme 96 Catalyst-free synthesis of substituted 1,4-dihydroquinolines 251; some representative examples are shown. | ||
Fındık et al.168 reported one-pot four-component condensation of dimedon 28e, α-ionone 255, ammonium acetate 136 and benzaldehyde 5 under reflux condition to produce 7,8-dihydroquinolin-5-(1H,4H,6H)-ones 254 (Scheme 97).
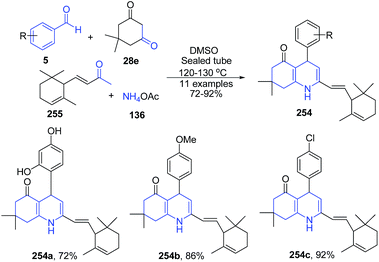 | ||
| Scheme 97 Catalyst free synthesis of substituted 7,8-dihydroquinolin-5-(1H,4H,6H)-ones 254; some representative examples are shown. | ||
3.7. Miscellaneous protocols
Ghorbani-Vaghei and Malaekehpoor169 reported the use of N-bromosuccinimide as a catalyst for synthesis of polycyclic indolo[2,3-b]quinolines 256 from aryl amines 1 with indole-3-carbaldehyde 257 at room temperature. Initially, N-bromosuccinimide catalyzed the formation of an imine I and then a 3-bromo-indolinium cation as intermediate II. After nucleophilic attack by a second mole of aniline, intramolecular cyclization and oxidation lead to indoloquinolines 256. Reaction is shown in Scheme 98.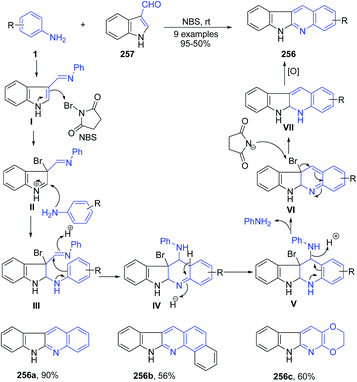 | ||
| Scheme 98 NBS catalyzed synthesis of polycyclic indolo[2,3-b]quinolines 256; some representative examples are shown. | ||
Plaskon et al.170 described synthesis of 7H-chromeno[3,2-c]quinolin-7-ones 258 using TMSCl-mediated recyclization of 3-formylchromone 259 with various anilines 1 (Scheme 99).
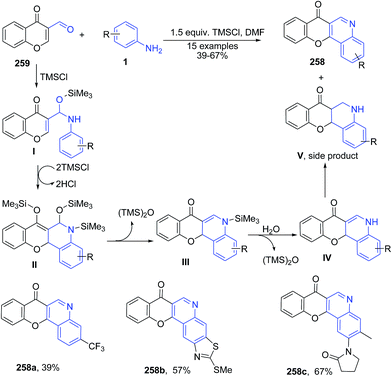 | ||
| Scheme 99 TMSCl-mediated synthesis of quinolines 258 from 3-formyl chromones 259; some representative examples are shown. | ||
Khong and Kwon171 reported phosphine-catalyzed efficient one-pot procedure for preparation of 3-substituted and 3,4-disubstituted quinolines 260 from stable starting materials (activated acetylenes 262 and O-tosylamidobenzaldehydes/O-tosylamidophenones 261, respectively) under mild conditions. Mechanism involves a general base catalysis. Coupling of 261 and 262 in presence of PPh3 produces anion intermediate IV. Nucleophilic addition of the free phosphine to the activated alkyne 262 generated phosphonium allenolate I, which acts as a base to activate the pro-nucleophile IV through deprotonation, resulting in a subsequent general base-catalyzed Michael/aldol reaction to produce V (Scheme 100). Intermediate V on aromatozation produces 260.
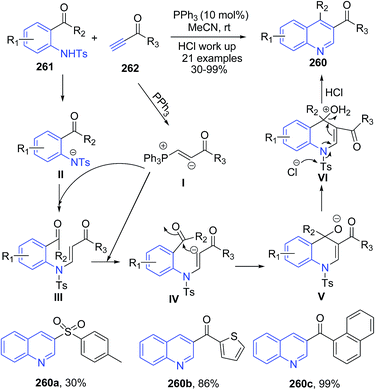 | ||
| Scheme 100 Phosphine-catalyzed synthesis of dihydroquinolines 260; some representative examples are shown. | ||
An efficient and facile one-step synthesis of pyrrolo[3,4-c]quinolinedione derivatives 263 has been developed using ethylenediamine diacetate (EDDA)-catalyzed cascade reactions of isatins 40 and β-ketoamides 208.172 The carbonyl group of isatin 40 gets protonated by EDDA, which facilitates a nucleophilic attack of the enol form of β-ketoamide 208 followed by dehydration and proton transfer to give I. Intermediate I then undergoes intramolecular cyclization by N1 nucleophilic attack of the β-ketoamide group followed by proton transfer to form intermediate II. Ring opening of intermediate II followed by proton transfer gives the free aromatic amine III. Subsequently, the NH2 group of III attacks a carbonyl group by intramolecular cyclization to form intermediate IV, which on elimination of water and deprotonation results in formation of 263 (Scheme 101).
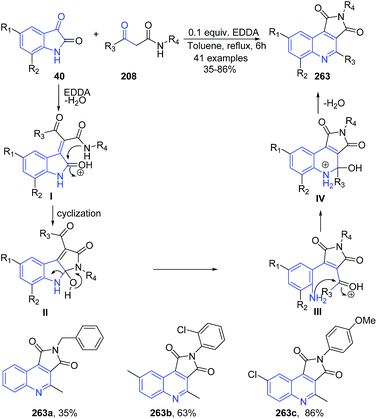 | ||
| Scheme 101 Ethylenediamine diacetate-catalyzed synthesis of pyrrolo[3,4-c]quinolinediones 263; some representative examples are shown. | ||
1,1-Cyclopropane aminoketones 265 on reaction with diethyl azodicarboxylate 266 (DEAD, 2.0 equiv.) in toluene at 80 °C for 8 h produced 2-benzoyl quinolines 264 via oxidation, ring-opening and cyclization.173 The reaction is proposed to proceed via a cascade procedure. 1,1-Cyclopropane aminoketone 265 is first oxidized with DEAD to give cyclopropene intermediate II. Then, ring-opening of II gives N-aza-diene intermediate III, which undergoes an intramolecular [4 + 2] reaction, followed by dehydrogenation to form 264 (Scheme 102).
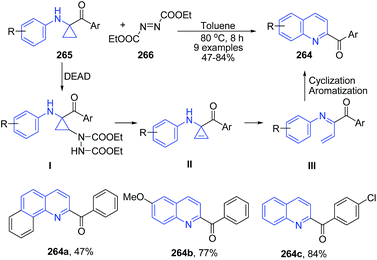 | ||
| Scheme 102 Catalyst free synthesis of 2-benzoyl quinolines 264 from 1,1-cyclopropane aminoketones 265; some representative examples are shown. | ||
The synthesis of highly substituted spiro[indolo-3,10′-indeno[1,2-b]quinolin]-2,4,11′-triones 267 has been developed under CTAB/H2O system to provide spiro-products with excellent yields.174 At first, the nucleophilic addition reaction occurs between the enaminone 33 with the more electrophilic carbonyl centre of isatin 40 in ecofriendly water medium to give an imine species that tautomerizes to yield I (Scheme 103). This intermediate I undergoes intramolecular cyclization to form the intermediate II, which is immediately converted to a more reactive and unstable intermediate III via ring-opening of indoline-2,3-dione. After that, due to the high reactivity, intermediate III instantly undergoes further nucleophilic addition with the other molecule of indane-1,3-dione 28c to produce another imine intermediate IV, which tautomerizes to yield V. Finally, the intramolecular cyclisation of V results in the ultimate spiro compound 267.
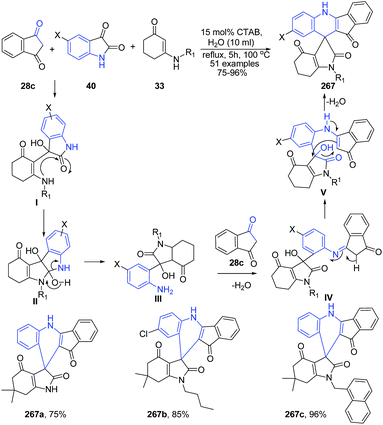 | ||
| Scheme 103 Cetyltrimethyl ammonium bromide (CTAB)-catalyzed synthesis of spiro[indolo-3,100-indeno[1,2-b]quinolin]-2,4,11′-triones 267; some representative examples are shown. | ||
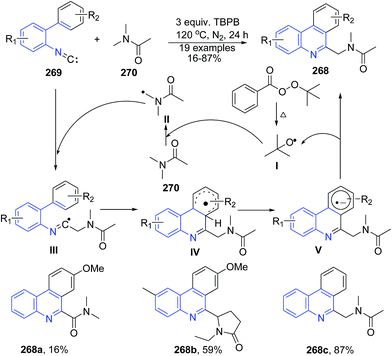
Fang et al.175 reported metal-free cyclization reaction of 2-isocyanobiphenyls 269 with amides 270 by using tert-butyl peroxybenzoate (TBPB) as oxidant, which provided an access to 6-amidophenanthridine 268. The reactions proceeds through a sequence of functionalization of the C(sp3)–H bond adjacent to the nitrogen atom and intramolecular radical aromatic cyclization with good yields (Scheme 104).
4. Summary and future prospects
As illustrated through the comprehensive compilation of role of metal-free domino one-pot reactions for quinoline synthesis, it is clear that these protocols has numerous advantages such as high yields, shorter reaction times, environmentally benign milder reactions and safe operations.Many metal-free domino one-pot protocols have been developed by using inorganic/organic acids, bases, organocatalysts, ionic liquids or molecular iodine. Use of these non-metal reagents certainly makes these protocols an environmentally friendly. Thus, these reagents and solvents have an indispensable role in the development of many new domino one-pot protocols for several other heterocycles. An appropriate use of solvent or reagents as catalysts in such protocols avoids the use of metal-catalyst and allows development of new metal-free methodologies for the efficient synthesis of quinolines. With the great importance of quinoline scaffold in drug discovery, these protocols will have great impact in rapid development of molecular libraries and structure–activity relationship generation.
In summary, metal-free domino one-pot strategies toward quinoline synthesis encompass the vast majority of green chemistry criteria and represent a solid, efficient, experimentally simple, and somehow elegant alternative to other methods. Based on the progress summarized in this review, we feel certain that combined strategy of domino one-pot protocols and metal-free capability of the reaction will find broad applications and will continue to attract much attention in organic synthesis applications.
Acknowledgements
JBB is thankful to CSIR Innovation Center project ITR001 for fellowship. The financial support from DST-SERB is gratefully acknowledged (grant no. SR/FT/CS-168/2011).References and notes
- J. P. Michael, Nat. Prod. Rep., 2008, 25, 166–187 RSC.
- A. Marson, J. E. Ernsting, M. Lutz, A. L. Spek, P. W. N. M. van Leeuwena and P. C. J. Kamer, Dalton Trans., 2009, 621–633 RSC.
- M. O. Püsküllü, B. Tekiner and S. Suzen, Mini-Rev. Med. Chem., 2013, 13, 365–372 Search PubMed.
- V. R. Solomon and H. Lee, Curr. Med. Chem., 2011, 18, 1488–1508 CrossRef CAS.
- J. Lavrado, R. Moreira and A. Paulo, Curr. Med. Chem., 2010, 17, 2348–2370 CrossRef CAS.
- S. Kumar, S. Bawa and H. Gupta, Mini-Rev. Med. Chem., 2009, 9, 1648–1654 CrossRef CAS.
- J. P. Michael, Nat. Prod. Rep., 2002, 19, 742–760 RSC.
- J. P. Michael, Nat. Prod. Rep., 1997, 14, 605–618 RSC.
- R. N. Kharwar, A. Mishra, S. K. Gond, A. Stierle, D. Stierle and S. Bawa, Nat. Prod. Rep., 2011, 28, 1208–1228 RSC.
- I. P. Singh and H. S. Bodiwala, Nat. Prod. Rep., 2010, 27, 1781–1800 RSC.
- P. Williams, A. Sorribas and M.-J. R. Howes, Nat. Prod. Rep., 2011, 28, 48–77 RSC.
- P. M. S. Chauhan and S. K. Srivastava, Curr. Med. Chem., 2001, 8, 1535 CrossRef CAS.
- Y.-L. Chen, K.-G. Fang, J.-Y. Sheu, S.-L. Hsu and C.-C. Tzeng, J. Med. Chem., 2001, 44, 2374 CrossRef CAS PubMed.
- G. Roma, M. D. Braccio, G. Grossi, F. Mattioli and M. Ghia, Eur. J. Med. Chem., 2000, 35, 1021–1035 CrossRef CAS.
- S. Bawa, S. Kumar, S. Drabu and R. Kumar, J. Pharm. BioAllied Sci., 2010, 2, 64–71 CrossRef CAS PubMed.
- B. Gryzło and K. Kulig, Mini-Rev. Med. Chem., 2014, 14, 332–344 CrossRef.
- K. Kaur, M. Jain, R. P. Reddy and R. Jain, Eur. J. Med. Chem., 2010, 45, 3245–3264 CrossRef CAS PubMed.
- A. P. Gorka, A. d. Dios and P. D. Roepe, J. Med. Chem., 2013, 56, 5231–5246 CrossRef CAS PubMed.
- S. Bongarzone and M. L. Bolognesi, Expert Opin. Drug Discovery, 2011, 6, 251–268 CrossRef CAS PubMed.
- K. A. Reynolds, W. A. Loughlin and D. J. Young, Mini-Rev. Med. Chem., 2013, 13, 730–743 CrossRef CAS.
- R. S. Keri and S. A. Patil, Biomed. Pharmacother., 2014, 68, 1161–1175 CrossRef CAS PubMed.
- S. Singh, G. Kaur, V. Mangla and M. K. Gupta, J. Enzyme Inhib. Med. Chem., 2014, 25032745 Search PubMed.
- O. Afzal, S. Kumar, M. R. Haider, M. R. Ali, R. Kumar, M. Jaggi and S. Bawa, Eur. J. Med. Chem., 2014, 25073919 Search PubMed.
- S. Vlahopoulos, E. Critselis, I. F. Voutsas, S. A. Perez, M. Moschovi, C. N. Baxevanis and G. P. Chrousos, Curr. Drug Targets, 2014, 15, 843–851 CrossRef CAS.
- P. Zajdel, A. Partyka, K. Marciniec, A. J. Bojarski, M. Pawlowski and A. Wesolowska, Future Med. Chem., 2014, 6, 57–75 CrossRef CAS PubMed.
- S. Mukherjee and M. Pal, Drug Discovery Today, 2013, 18, 389–398 CrossRef CAS PubMed.
- S. Mukherjee and M. Pal, Curr. Med. Chem., 2013, 20, 4386–4410 CrossRef CAS.
- R. Musiol, Curr. Pharm. Des., 2013, 19, 1835–1849 CrossRef CAS.
- R. Musiol, M. Serda, S. Hensel-Bielowka and J. Polanski, Curr. Med. Chem., 2010, 17, 1960–1973 CrossRef CAS.
- A. Mahamoud, J. Chevalier, A. Davin-Regli, J. Barbe and J. M. Pagès, Curr. Drug Targets, 2006, 7, 843–847 CrossRef CAS.
- N. Costedoat-Chalumeau, B. Dunogué, N. Morel, V. Le Guern and G. Guettrot-Imbert, Presse Med., 2014, 43, e167–180 CrossRef PubMed.
- B. F. Howard, Chem. News, 1931, 142, 129–133 Search PubMed.
- J. Achan, A. O. Talisuna, A. Erhart, A. Yeka, J. K. Tibenderana, F. N. Baliraine, P. J. Rosenthal and U. D'Alessandro, Malar. J., 2011, 10, 144 CrossRef CAS PubMed.
- M. E. Wall, M. C. Wani, C. E. Cook, K. H. Palmer, A. I. McPhail and G. A. Sim, J. Am. Chem. Soc., 1966, 88, 3888–3890 CrossRef CAS.
- I. Pendrak, S. Barney, R. Wittrock, D. M. Lambert and W. D. Kingsbury, J. Org. Chem., 1994, 59, 2623–2625 CrossRef CAS.
- C. W. Wright, J. Addae-Kyereme, A. G. Breen, J. E. Brown, M. F. Cox, S. L. Croft, Y. Gökçek, H. Kendrick, R. M. Phillips and P. L. Pollet, J. Med. Chem., 2001, 44, 3187–3194 CrossRef CAS PubMed.
- P. Grellier, L. Ramiaramanana, V. Millerioux, E. Deharo, J. Schrével, F. Frappier, F. Trigalo, B. Bodo and J.-L. Pousset, Phytother. Res., 1996, 10, 317–321 CrossRef CAS.
- K. Krafts, E. Hempelmann and A. Skórska-Stania, Parasitol. Res., 2012, 11, 1–6 CrossRef PubMed.
- L. C. Chou, C. T. Chen, J. C. Lee, T. D. Way, C. H. Huang, S. M. Huang, C. M. Teng, T. Yamori, T. S. Wu, C. M. Sun, D. S. Chien, K. Qian, S. L. Morris-Natschke, K. H. Lee, L. J. Huang and S. C. Kuo, J. Med. Chem., 2010, 53, 1616–1626 CrossRef CAS PubMed.
- L. C. Chou, M. T. Tsai, M. H. Hsu, S. H. Wang, T. D. Way, C. H. Huang, H. Y. Lin, K. Qian, Y. Dong, K. H. Lee, L. J. Huang and S. C. Kuo, J. Med. Chem., 2010, 53, 8047–8058 CrossRef CAS PubMed.
- S. M. Prajapati, K. D. Patel, R. H. Vekariya, S. N. Panchal and H. D. Patel, RSC Adv., 2014, 4, 24463–24476 RSC.
- K. Gopaul, S. A. Shintre and N. A. Koorbanally, Anticancer Agents Med. Chem., 2014, 25511516 Search PubMed.
- J. Barluenga, F. Rodríguez and F. J. Fañanás, Chem.–Asian J., 2009, 4, 1036–1048 CrossRef CAS PubMed.
- H. M. Hassanin, M. A. Ibrahim, Y. A. Gabr and Y. A. Alnamer, J. Heterocycl. Chem., 2012, 49, 1269–1289 CrossRef CAS PubMed.
- B. Nammalwar and R. A. Bunce, Molecules, 2014, 19, 204–232 CrossRef PubMed.
- R. A. Mekheimer, E. A. Ahmed and K. U. Sadek, Tetrahedron, 2012, 68, 1637–1667 CrossRef CAS PubMed.
- A. Marella, O. P. Tanwar, R. Saha, M. R. Ali, S. Srivastava, M. Akhter, M. Shaquiquzzaman and M. M. Alam, Saudi Pharm. J., 2013, 21, 1–12 CrossRef PubMed.
- L. Q. Lu, J. R. Chen and W. J. Xiao, Acc. Chem. Res., 2012, 45, 1278–1293 CrossRef CAS PubMed.
- B. H. Rotstein, S. Zaretsky, V. Rai and A. K. Yudin, Chem. Rev., 2014, 114, 8323–8359 CrossRef CAS PubMed.
- V. Estevez, M. Villacampa and J. C. Menendez, Chem. Soc. Rev., 2014, 43, 4633–4657 RSC.
- A. Domling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083–3135 CrossRef CAS PubMed.
- M. S. Singh and S. Chowdhury, RSC Adv., 2012, 2, 4547–4592 RSC.
- B. B. Toure and D. G. Hall, Chem. Rev., 2009, 109, 4439–4486 CrossRef CAS PubMed.
- C. de Graaff, E. Ruijter and R. V. A. Orru, Chem. Soc. Rev., 2012, 41, 3969–4009 RSC.
- N. Isambert, M. d. M. S. Duque, J.-C. Plaquevent, Y. Genisson, J. Rodriguez and T. Constantieux, Chem. Soc. Rev., 2011, 40, 1347–1357 RSC.
- K. C. Nicolaou, D. J. Edmonds and P. G. Bulger, Angew. Chem., Int. Ed., 2006, 45, 7134–7186 CrossRef CAS PubMed.
- J. D. Sunderhaus and S. F. Martin, Chem.–Eur. J., 2009, 15, 1300–1308 CrossRef CAS PubMed.
- D. M. D'Souza and T. J. J. Muller, Chem. Soc. Rev., 2007, 36, 1095–1108 RSC.
- V. Estevez, M. Villacampa and J. C. Menendez, Chem. Soc. Rev., 2010, 39, 4402–4421 RSC.
- M. Shiri, Chem. Rev., 2012, 112, 3508–3549 CrossRef CAS PubMed.
- C. Allais, J.-M. Grassot, J. Rodriguez and T. Constantieux, Chem. Rev., 2014, 114, 10829–10868 CrossRef CAS PubMed.
- H. Bienayme, C. Hulme, G. Oddon and P. Schmitt, Chem.–Eur. J., 2000, 6, 3321–3329 CrossRef CAS.
- J. B. Bharate, A. Wani, S. Sharma, S. I. Reja, M. Kumar, R. A. Vishwakarma, A. Kumar and S. B. Bharate, Org. Biomol. Chem., 2014, 12, 6267–6277 CAS.
- X. Zhang, X. Xu, L. Yu and Q. Zhao, Asian J. Org. Chem., 2014, 3, 281–284 CrossRef CAS PubMed.
- K. C. Majumdar, S. Ponra, D. Ghosh and A. Taher, Synlett, 2011, 1, 104–110 CrossRef PubMed.
- L. Pan, X. Bi and Q. Liu, Chem. Soc. Rev., 2013, 42, 1251–1286 RSC.
- Y.-L. Zhao, W. Zhang, S. Wang and Q. Liu, J. Org. Chem., 2007, 72, 4985–4988 CrossRef CAS PubMed.
- S. Khadem, K. A. Udachin, G. D. Enright, M. Prakesch and P. Arya, Tetrahedron Lett., 2009, 50, 6661–6664 CrossRef CAS PubMed.
- C. R. Borel, L. C. A. Barbosa, C. R. A. Maltha and S. A. Fernandes, Tetrahedron Lett., 2015, 56, 662–665 CrossRef CAS PubMed.
- N. Shindoh, H. Tokuyama, Y. Takemoto and K. Takasu, J. Org. Chem., 2008, 73, 7451–7456 CrossRef CAS PubMed.
- N. A. Magomedov, Org. Lett., 2003, 5, 2509–2512 CrossRef CAS PubMed.
- G. Shan, X. Sun, Q. Xia and Y. Rao, Org. Lett., 2011, 13, 5770–5773 CrossRef CAS PubMed.
- B. Mirza and S. S. Samiei, J. Chem. Chem. Eng., 2011, 5, 644–647 CAS.
- S. Tu, S. Wu, S. Yan, W. Hao, X. Zhang, X. Cao, Z. Han, B. Jiang, F. Shi, M. Xia and J. Zhou, J. Comb. Chem., 2009, 11, 239–242 CrossRef CAS PubMed.
- A. T. Khan and D. K. Das, Tetrahedron Lett., 2012, 53, 2345–2351 CrossRef CAS PubMed.
- S.-J. Tu, B. Jiang, R.-H. Jia, J.-Y. Zhang, Y. Zhang, C.-S. Yao and F. Shi, Org. Biomol. Chem., 2006, 4, 3664–3668 CAS.
- J. Azizian, A. S. Delbari and K. Yadollahzadeh, Synth. Commun., 2014, 44, 3277–3286 CrossRef CAS PubMed.
- H. Hosseinjani-Pirdehi, K. Rad-Moghadam and L. Youseftabar-Miri, Tetrahedron, 2014, 70, 1780–1785 CrossRef CAS PubMed.
- C. Yu, H. Zhang, C. Yao, T. Li, B. Qin, J. Lu and D. Wang, J. Heterocycl. Chem., 2014, 51, 702–705 CrossRef CAS PubMed.
- M. Narasimhulu, T. S. Reddy, K. C. Mahesh, P. Prabhakar, C. B. Rao and Y. Venkateswarlu, J. Mol. Catal. A: Chem., 2007, 266, 114–117 CrossRef CAS PubMed.
- S.-y. Tanaka, M. Yasuda and A. Baba, J. Org. Chem., 2006, 71, 800–803 CrossRef CAS PubMed.
- V. B. Bojinov and I. K. Grabchev, Org. Lett., 2003, 5, 2185–2187 CrossRef CAS PubMed.
- F. Yu, S. Yan, L. Hu, Y. Wang and J. Lin, Org. Lett., 2011, 13, 4782–4785 CrossRef CAS PubMed.
- F.-C. Yu, B. Zhou, H. Xu, Y.-M. Li, J. Lin, S.-J. Yan and Y. Shen, Tetrahedron, 2015, 71, 1036–1044 CrossRef CAS PubMed.
- J. Quiroga, J. Trilleras, B. Insuasty, R. Abonía, M. Nogueras, A. Marchal and J. Cobo, Tetrahedron Lett., 2010, 51, 1107–1109 CrossRef CAS PubMed.
- L. Liu, H. Lu, H. Wang, C. Yang, X. Zhang, D. Zhang-Negrerie, Y. Du and K. Zhao, Org. Lett., 2013, 15, 2906–2909 CrossRef CAS PubMed.
- Z. Song, Y.-M. Zhao and H. Zhai, Org. Lett., 2011, 13, 6331–6333 CrossRef CAS PubMed.
- J. Tummatorn, C. Thongsornkleeb, S. Ruchirawat and T. Gettongsong, Org. Biomol. Chem., 2013, 11, 1463–1467 CAS.
- P. Zhao, X. Yan, H. Yin and C. Xi, Org. Lett., 2014, 16, 1120–1123 CrossRef CAS PubMed.
- L. Peng, H. Wang, C. Peng, K. Ding and Q. Zhu, Synthesis, 2011, 11, 1723–1732 Search PubMed.
- B. V. S. Reddy, D. Medaboina, B. Sridhar and K. K. Singarapu, J. Org. Chem., 2013, 78, 8161–8168 CrossRef PubMed.
- Q. Zhang, Z. Zhang, Z. Yan, Q. Liu and T. Wang, Org. Lett., 2007, 9, 3651–3653 CrossRef CAS PubMed.
- A. V. Aksenov, A. N. Smirnov, N. A. Aksenov, I. V. Aksenova, L. V. Frolova, A. Kornienko, I. V. Magedov and M. Rubin, Chem. Commun., 2013, 49, 9305–9307 RSC.
- Y. Yamaoka, T. Yoshida, M. Shinozaki, K.-i. Yamada and K. Takasu, J. Org. Chem., 2015, 80, 957–964 CrossRef CAS PubMed.
- F.-W. Wu, R.-S. Hou, H.-M. Wang, I.-J. Kang and L.-C. Chen, J. Chin. Chem. Soc., 2012, 59, 535–539 CrossRef CAS PubMed.
- Y. Zhu and C. Cai, RSC Adv., 2014, 4, 52911–52914 RSC.
- H. V. Mierde, P. V. D. Voort and F. Verpoort, Tetrahedron Lett., 2008, 49, 6893–6895 CrossRef PubMed.
- R. Martínez, D. J. Ramón and M. Yus, J. Org. Chem., 2008, 73, 9778–9780 CrossRef PubMed.
- M.-C. Yan, Z. Tu, C. Lin, S. Ko, J. Hsu and C.-F. Yao, J. Org. Chem., 2004, 69, 1565–1570 CrossRef CAS PubMed.
- A. Arcadi, F. Marinelli and E. Rossi, Tetrahedron, 1999, 55, 13233–13250 CrossRef CAS.
- K. Wang, E. Herdtweck and A. Domling, ACS Comb. Sci., 2012, 14, 316–322 CrossRef CAS PubMed.
- M. Cameron, R. S. Hoerrner, J. M. McNamara, M. Figus and S. Thomas, Org. Process Res. Dev., 2006, 10, 149–152 CrossRef CAS.
- Z. Hu, Y. Li, L. Pan and X. Xu, Adv. Synth. Catal., 2014, 356, 2974–2978 CrossRef CAS PubMed.
- M. Rehan, G. Hazra and P. Ghorai, Org. Lett., 2015, 17, 1668–1671 CrossRef CAS PubMed.
- P. Selig and W. Raven, Org. Lett., 2014, 16, 5192–5195 CrossRef CAS PubMed.
- Q.-L. Liu, Q.-L. Li, X.-D. Fei and Y.-M. Zhu, ACS Comb. Sci., 2011, 13, 19–23 CrossRef CAS PubMed.
- L. Fu, X. Feng, J.-J. Wang, Z. Xun, J.-D. Hu, J.-J. Zhang, Y.-W. Zhao, Z.-B. Huang and D.-Q. Shi, ACS Comb. Sci., 2015, 17, 24–31 CrossRef CAS PubMed.
- H. Zhu, R. F. Yang, L. H. Yun and J. Li, Chin. Chem. Lett., 2010, 21, 35–38 CrossRef CAS PubMed.
- A. Rineh, M. A. Khalilzadeh, M. M. Hashemi, M. Rajabi and F. Karimi, J. Heterocycl. Chem., 2012, 49, 789–791 CrossRef CAS PubMed.
- A. Alizadeh, L. Moafi, R. Ghanbaripour, M. H. Abadi, Z. Zhu and M. Kubicki, Tetrahedron, 2015, 71, 3495–3499 CrossRef CAS PubMed.
- J.-y. Kato, H. Aoyama and T. Yokomatsu, Org. Biomol. Chem., 2013, 11, 1171–1178 CAS.
- J.-y. Kato, R. Ijuin, H. Aoyama and T. Yokomatsu, Tetrahedron, 2014, 70, 2766–2775 CrossRef CAS PubMed.
- J.-y. Kato, Y. Ito, R. Ijuin, H. Aoyama and T. Yokomatsu, Org. Lett., 2013, 15, 3794–3797 CrossRef CAS PubMed.
- S. Kumar, P. Sharma, K. K. Kapoor and M. S. Hundal, Tetrahedron, 2008, 64, 536–542 CrossRef CAS PubMed.
- Z. Xia, J. Huang, Y. He, J. Zhao, J. Lei and Q. Zhu, Org. Lett., 2014, 16, 2546–2549 CrossRef CAS PubMed.
- M. Arnould, M.-A. Hiebel, S. Massip, J. M. Leger, C. Jarry, S. Berteina-Raboin and G. Guillaumet, Chem.–Eur. J., 2013, 19, 12249–12253 CrossRef CAS PubMed.
- H. Zhou, L. Liu and S. Xu, J. Org. Chem., 2012, 77, 9418–9421 CrossRef CAS PubMed.
- Y.-M. Ren, C. Cai and R.-C. Yang, RSC Adv., 2013, 3, 7182–7204 RSC.
- Q. Gao, S. Liu, X. Wu and A. Wu, Org. Lett., 2014, 16, 4582–4585 CrossRef CAS PubMed.
- R. Deshidi, S. Devari and B. A. Shah, Org. Chem. Front., 2015, 2, 515–519 RSC.
- X. Li, Z. Mao, Y. Wang, W. Chen and X. Lin, Tetrahedron, 2011, 67, 3858–3862 CrossRef CAS PubMed.
- X.-S. Wang, Q. Li, J.-R. Wu and S.-J. Tu, J. Comb. Chem., 2009, 11, 433–437 CrossRef CAS PubMed.
- X.-S. Wang, Q. Li, J. Zhou and S.-J. Tu, J. Heterocycl. Chem., 2009, 46, 1222–1228 CrossRef CAS PubMed.
- X.-S. Wang, J. Zhou, M.-Y. Yin, K. Yang and S.-J. Tu, J. Comb. Chem., 2010, 12, 266–269 CrossRef CAS PubMed.
- D.-S. Chen, Y.-L. Li, Y. Liu and X.-S. Wang, Tetrahedron, 2013, 69, 7045–7050 CrossRef CAS PubMed.
- W. Wang, M. M. Zhang and X. S. Wang, J. Heterocycl. Chem., 2014, 51, 175–178 CrossRef CAS PubMed.
- W. Wang, H. Jiang, M.-M. Zhang and X.-S. Wang, J. Heterocycl. Chem., 2014, 51, 830–834 CrossRef CAS PubMed.
- P. Ghosh and A. Mandal, Catal. Commun., 2011, 12, 744–747 CrossRef CAS PubMed.
- N. C. Ganguly and S. Chandra, Tetrahedron Lett., 2014, 55, 1564–1568 CrossRef CAS PubMed.
- J. Wu, H.-G. Xia and K. Gao, Org. Biomol. Chem., 2006, 4, 126–129 CAS.
- R. Venkatesham, A. Manjula and B. V. Rao, J. Heterocycl. Chem., 2012, 49, 833–838 CrossRef CAS PubMed.
- B. P. Bandgar, P. E. More and V. T. Kamble, J. Chin. Chem. Soc., 2008, 55, 947–951 CrossRef CAS PubMed.
- L.-Y. Zeng and C. Cai, Org. Biomol. Chem., 2010, 8, 4803–4805 CAS.
- J. Fotie, H. V. K. Wangun, F. R. Fronczek, N. Massawe, B. T. Bhattarai, J. L. Rhodus, T. A. Singleton and D. S. Bohle, J. Org. Chem., 2012, 77, 2784–2790 CrossRef CAS PubMed.
- R. Halim, P. J. Scammells and B. L. Flynn, Org. Lett., 2008, 10, 1967–1970 CrossRef CAS PubMed.
- H. Batchu, S. Bhattacharyya and S. Batra, Org. Lett., 2012, 14, 6330–6333 CrossRef CAS PubMed.
- S. Ali, H.-T. Zhu, X.-F. Xia, K.-G. Ji, Y.-F. Yang, X.-R. Song and Y.-M. Liang, Org. Lett., 2011, 13, 2598–2601 CrossRef CAS PubMed.
- S. Ali, Y.-X. Li, S. Anwar, F. Yang, Z.-S. Chen and Y.-M. Liang, J. Org. Chem., 2012, 77, 424–431 CrossRef CAS PubMed.
- S. Sadjadi and M. M. Heravi, Tetrahedron, 2011, 67, 2707–2752 CrossRef CAS PubMed.
- A. Kruithof, E. Ruijter and R. V. Orru, Chem.–Asian J., 2015, 10, 508–520 CrossRef CAS PubMed.
- A. Domling, Chem. Rev., 2006, 106, 17–89 CrossRef PubMed.
- B. Jiang, C. Li, S.-J. Tu and F. Shi, J. Comb. Chem., 2010, 12, 482–487 CrossRef CAS PubMed.
- T. Mitamura and A. Ogawa, J. Org. Chem., 2011, 76, 1163–1166 CrossRef CAS PubMed.
- J. B. Bharate, S. B. Bharate and R. A. Vishwakarma, ACS Comb. Sci., 2014, 16, 624–630 CrossRef CAS PubMed.
- Y. Wan, R. Yuan, F.-R. Zhang, L.-L. Pang, R. Ma, C.-H. Yue, W. Lin, W. Yin, R.-C. Bo and H. Wu, Synth. Commun., 2011, 41, 2997–3015 CrossRef CAS PubMed.
- L. Xia, A. Idhayadhulla, Y. R. Lee, S. H. Kim and Y.-J. Wee, ACS Comb. Sci., 2014, 16, 333–341 CAS.
- M. R. P. Heravi, Ultrason. Sonochem., 2009, 16, 361–366 CrossRef CAS PubMed.
- E. Kowsari and M. Mallakmohammadi, Ultrason. Sonochem., 2011, 18, 447–454 CrossRef CAS PubMed.
- Y. Sun, P.-J. Cai and X.-S. Wang, Res. Chem. Intermed., 2014 DOI:10.1007/s11164-11014-11819-y.
- H. Y. Guo and Y. Yu, Chin. Chem. Lett., 2010, 21, 1435–1438 CrossRef CAS PubMed.
- J. Alemán and S. Cabrera, Chem. Soc. Rev., 2013, 42, 774–793 RSC.
- R. Mahrwald, Drug Discovery Today: Technol., 2013, 10, e29–36 CrossRef PubMed.
- U. Scheffler and R. Mahrwald, Chemistry, 2013, 19, 14346–14396 CrossRef CAS PubMed.
- F. Shi, G.-J. Xing, Z.-L. Tao, S.-W. Luo, S.-J. Tu and L.-Z. Gong, J. Org. Chem., 2012, 71, 6970–6979 CrossRef PubMed.
- C. Shi, J. Wang, H. Chen and D. Shi, J. Comb. Chem., 2010, 12, 430–434 CrossRef CAS PubMed.
- A. Khalafi-Nezhad, S. Sarikhani, E. S. Shahidzadeh and F. Panahi, Green Chem., 2012, 14, 2876–2884 RSC.
- S. Karamthulla, S. Pal, T. Parvin and L. H. Choudhury, RSC Adv., 2014, 4, 15319–15324 RSC.
- Z.-H. Yu, H.-F. Zheng, W. Yuan, Z.-L. Tang, A.-D. Zhang and D.-Q. Shi, Tetrahedron, 2013, 69, 8137–8141 CrossRef CAS PubMed.
- K. Wu, Z. Huang, C. Liu, H. Zhang and A. Lei, Chem. Commun., 2015, 51, 2286–2289 RSC.
- C. Qi, Q. Zheng and R. Hua, Tetrahedron, 2009, 65, 1316–1320 CrossRef CAS PubMed.
- Y.-J. Zhou, D.-S. Chen, Y.-L. Li, Y. Liu and X.-S. Wang, ACS Comb. Sci., 2013, 15, 498–502 CrossRef CAS PubMed.
- A. Fayol and J. Zhu, Angew. Chem., Int. Ed., 2002, 41, 3633–3635 CrossRef CAS.
- B. K. Mehta, K. Yanagisawa, M. Shiro and H. Kotsuki, Org. Lett., 2003, 5, 1605–1608 CrossRef CAS PubMed.
- P. Yuvaraj, K. Manivannan and B. S. R. Reddy, Tetrahedron Lett., 2015, 56, 78–81 CrossRef CAS PubMed.
- B. D. Bala, K. Balamurugan and S. Perumal, Tetrahedron Lett., 2011, 52, 4562–4566 CrossRef PubMed.
- A. Alizadeh and A. Rezvanian, C. R. Chim., 2014, 17, 103–107 CrossRef CAS PubMed.
- P. Chidurala, V. Jetti, R. Pagadala, J. S. Meshram and S. B. Jonnalagadda, J. Heterocycl. Chem., 2014 DOI:10.1002/jhet.2230.
- E. Fındık, M. Ceylan and M. Elmastas, J. Heterocycl. Chem., 2012, 49, 253–260 CrossRef PubMed.
- R. Ghorbani-Vaghei and S. M. Malaekehpoor, Tetrahedron Lett., 2012, 53, 4751–4753 CrossRef CAS PubMed.
- A. S. Plaskon, S. V. Ryabukhin, D. M. Volochnyuk, K. S. Gavrilenko, A. N. Shivanyuk and A. A. Tolmachev, J. Org. Chem., 2008, 73, 6010–6013 CrossRef CAS PubMed.
- S. Khong and O. Kwon, J. Org. Chem., 2012, 77, 8257–8267 CrossRef CAS PubMed.
- L. Xia and Y. R. Lee, Org. Biomol. Chem., 2013, 11, 5254–5263 CAS.
- Z. Mao, H. Qu, Y. Zhao and X. Lin, Chem. Commun., 2012, 48, 9927–9929 RSC.
- A. Mondal, M. Brown and C. Mukhopadhyay, RSC Adv., 2014, 4, 36890–36895 RSC.
- H. Fang, J. Zhao, S. Ni, H. Mei, J. Han and Y. Pan, J. Org. Chem., 2015, 80, 3151–3158 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |