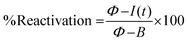Open Access Article
Open Access ArticleElectrochemical sensors cleaned by light: a proof of concept for on site applications towards integrated monitoring systems†
V.
Pifferi‡
ab,
G.
Soliveri‡
ab,
G.
Panzarasa
 c,
S.
Ardizzone
ab,
G.
Cappelletti
ab,
D.
Meroni
ab and
L.
Falciola
*ab
c,
S.
Ardizzone
ab,
G.
Cappelletti
ab,
D.
Meroni
ab and
L.
Falciola
*ab
aDipartimento di Chimica, Università degli Studi di Milano, Via Golgi 19, 20133 Milano, Italy. E-mail: luigi.falciola@unimi.it
bConsorzio Interuniversitario Nazionale per la Scienza e Tecnologia dei Materiali (INSTM), Via Giusti 9, 50121 Firenze, Italy
cDipartimento di Scienze e Innovazione Tecnologica, Università del Piemonte Orientale “Amedeo Avogadro”, Viale T. Michel 11, 15100 Alessandria, Italy
First published on 14th August 2015
Abstract
The potential for on site applications of a SiO2–Ag NPs–TiO2 self-cleaning electrode was demonstrated. Dopamine was used both as the analyte and the fouling agent. Three different UV lamps (a powerful lamp (45 mW cm−2) for photocatalysis, a TLC lamp and a commercial LED torch) were studied. After fouling, total recovery of the electroanalytical performances was achieved upon a short irradiation performed directly in the solution of interest.
The growing demand of more selective and accurate analytical sensors in different fields, ranging from environmental and food quality control to medical diagnosis, has driven scientists to the use of nanostructures.1–3 Their popularity is spreading in almost all classes of sensing platforms (optical, electrical, spectroscopic, electrochemical, etc.) as a consequence of their tremendous performance boost. Nanostructured devices allow us the direct control of the sensing process, rising sensitivity and lowering detection limits. These reach the climax in the field of trace electroanalysis,4,5 where, thanks to the ease of the methodological procedures, standardization of protocols, availability of the needed instrumentations and, of course, the cost per analysis, competitive market devices are shaping. Even so, nowadays, the development of electroanalytical devices is impaired by fouling and passivation issues of the sensing surfaces, which can cause highly irreproducible measurements.1,4,6,7 These phenomena originate from the surface adsorption of interferents and analytes but also of their electrochemical by-products, which can cause a decrease in sensitivity up to the eventual total loss of the analytical signal and the irreversible deactivation of the sensing apparatus, with the consequent need to clean or replace it. On the other hand, the mechanical or electrochemical conventional cleaning procedures of the electrodes, developed to regenerate the surface of a pristine electrode, not only require the removal of the sensor from the cell, but are also tedious, time consuming and generally too invasive, especially for highly engineered nanostructured sensors, causing irreversible physico-chemical modifications of the device. In this context, a tailored device with a reactivation capability that does not affect the sensor morphology and chemistry, or better, which might confer the properties previously discussed to a generic sensing surface, represents a big challenge for the scientific and metrological community.
Notwithstanding its relevance, only few attempts in the literature have been found which proposed to solve such a big issue,8–10 starting from the pioneering work of R. McCreery, in which passivating films of oxide layers on glassy carbon and platinum electrodes could be removed by laser pulses.7,11 However, to the authors' best knowledge, no literature data regard the general applicability and robustness required for an eventual applicative and market impact. Those could permit the creation of an integrated monitoring system to be deployed in the field to register and report data using wireless technology in such a way to enable remote control of the environment. Our group has very recently reported the use of a titanium dioxide thin and robust layer as a self-cleaning surface, paving the way for a general approach toward the realization of self-cleaning electroanalytical sensors.12 We demonstrated that the photocatalytic properties of pure anatase titanium dioxide on the electrode surface can give rise to the self-cleaning of the interface simply by UV irradiation without affecting the morphological structure and the chemical nature of the multilayered sensor.13 As a proof of concept, we tested such a device for the quantification of dopamine,14 a relevant bio-medical analyte15 well known to cause heavy electrode fouling.16,17 Commenting the general impact of that sensor, C.M.A. Brett, in a note recently appeared on Chemistry World,18 congratulated us on the innovative results, but underlined the need to adopt shorter reactivation times and routine labware. On the ground of C.M.A. Brett's suggestion and of the extremely promising results observed in fouling environments, we started a more specific work. On one side, we optimized the self-cleaning conditions in terms of time of treatment, power and wavelength of the UV light; on the other side, we studied the possibility to reactivate our sensor on site, i.e. directly in solution, to yield a system working in continuous, able to be used in an integrated monitoring system.
Moreover, the above mentioned device was developed with the final aim to be used by scientists with different backgrounds, not only in academic research, but also in clinical and industrial laboratories. That requires the implementation of a methodology which uses safer and more commonly available lamps. In our previous work, we obtained the self-cleaning effect by irradiating the device with a UV lamp, ad hoc developed for photocatalytic studies. However, such a lamp is not commonly available in analytical labs and presents significant UV exposure hazard. For this reason, we moved to less powerful UV sources, e.g. a UV LED-torch (online commercially available at an average price of 30 $) and a conventional lamp for the visualization of TLC plates, with the final scope to make such technology portable, simple and user-friendly.
Fig. 1a shows the scheme of the optimized sensor, in which each layer takes a role for the performances of the final device. A conductive support made of fluorine-doped tin oxide (FTO) is covered by a silica layer (800 nm). This layer is functionalized with a monolayer of (3-aminopropyl)triethoxysilane(APTES), which acts as a coulombic binder for the regular and controlled attachment of silver nanoparticles (Ag NPs).12 The silica layer is crucial to control the assembly of the Ag NPs, which are the actual core of the sensor, providing electroanalytical, electrocatalytic and conductive properties.19–21 The nanoparticles are eventually embedded in a protective top titania layer (200 nm), which confers self-cleaning capability. Both silica and titania layers are deposited from non-aqueous sols, following procedures previously reported in literature,22,23 while Ag NPs are synthesized according to a previously published procedure.24
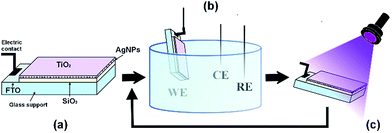 | ||
| Fig. 1 Schematic representation of the device (a), electrochemical cell (b) and cleaning under UV (c). | ||
This self-cleaning device can be conveniently used as the working electrode (WE) in an electrochemical cell, along with a calomel reference electrode (RE) and a platinum wire as the counter electrode (CE) (Fig. 1b), providing excellent performances in the detection of dopamine. Dopamine, a catecholamine neurotransmitter, possesses a great affinity particularly for metal oxide surfaces, on which it readily chemisorbs16 causing irreversible electrode fouling deactivation. This behaviour has so far impaired the development of routine electrochemical detection of catecholamines,15 which is a crucial analytical challenge in everyday diagnosis of neurodegenerative diseases. The fouling problem was solved by irradiating the electrode with near-UV light after having performed the analysis (Fig. 1c). During the irradiation process, the active anatase surface photo-catalytically promotes the formation of electron–hole pairs which in turn react with atmospheric O2, H2O and adsorbed molecules.25–27 The subsequent formation of oxidative radicals degrades dopamine and its by-products adsorbed at the sensor surface, making the device reusable hundreds of times. As shown by the UV-vis transmittance spectrum (Fig. S1, ESI†) of the device, we expect the titania surface to be active in the near-UV region.
The effectiveness of two low-cost, commercially available lamps, compared to a photochemistry layout, was tested. Fig. S2 (ESI†) shows the emission spectra and effective power density of the three adopted setups: (1) UV-LAMP: a powerful (45 mW cm−2) iron-halogenide lamp developed for photocatalysis, with one major emission peak in the UV region at 360 nm and two further peaks in the visible region (400 and 440 nm); (2) TLC-LAMP: a typical mercury lamp for thin layer chromatography (TLC) analysis, commonly found in chemistry and biology laboratories, emitting mostly at 310 and 360 nm (1.7 mW cm−2); (3) TORCH: a commercially available LED torch with a quite sharp emission at 380 nm (14 mW cm−2), without any specific safety requirements.
The self-cleaning efficiency of the sensor with respect to irradiation conditions (time and lamp power) was investigated by an ad hoc fouling/cleaning procedure comprising three steps: (1) background acquisition, (2) fouling (mechanism proposed in Fig. 2, according to the literature28,29) and (3) cleaning. dopamine (1 mM, in 0.1 M phosphate buffer solution at pH = 7.4) was utilized as fouling agent and probe, and Differential Pulse Voltammetry (DPV, range −0.1 V/+0.4 V (SCE), modulation time 0.05 s, interval time 0.5 s, step potential 0.005 V, modulation amplitude 0.05 V) as electroanalytical technique.
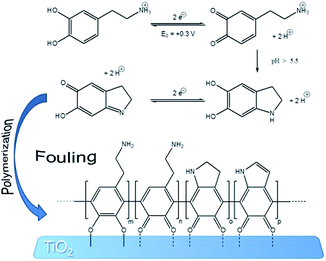 | ||
| Fig. 2 Schematic representation of dopamine fouling.28,29 | ||
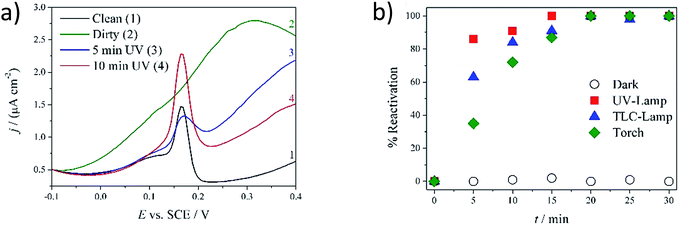
For the background acquisition (Fig. 3a, curve 1), the electrode was dipped in the phosphate buffer solution (in the absence of dopamine). Only the oxidation peak of silver nanoparticles at +0.165 V (SCE) could be detected, representing an indication of the clean and active surface. This peak is that affected by dopamine fouling. The fouling effect is really strong and it is present also at dopamine very low concentration (below 0.03 μM, which is the LOD of our device). However, in order to promote fast deactivation and test the potentialities of our self-cleaning device, a concentrated 1 mM solution of dopamine was specifically adopted. After the addition of dopamine, the voltammetric cycling caused the electrode fouling by the adsorption of dopamine itself and of its by-products onto titania (Fig. 2).28,29
The voltammogram acquired in fresh phosphate buffer (in the absence of dopamine, Fig. 3a, curve 2), after dopamine detection and chemisorptions, clearly shows the disappearance of the Ag NPs oxidation peak and the persistence of the dopamine shoulder at +0.3 V.12 The electrode was removed from the solution, irradiated for a chosen time and tested again in pure phosphate buffer solution (Fig. 3a, curve 3–4). As a function of irradiation time, the dopamine shoulder decreased while the Ag nanoparticles peak increased, as a clear evidence of the successful cleaning process. The time of irradiation was varied from 0 to 30 minutes for all the three lamps tested and also a blank test was acquired, where the electrode was not exposed to UV light source (dark).
In order to describe the fouling and cleaning process of the electrode surface, a %reactivation parameter was defined:
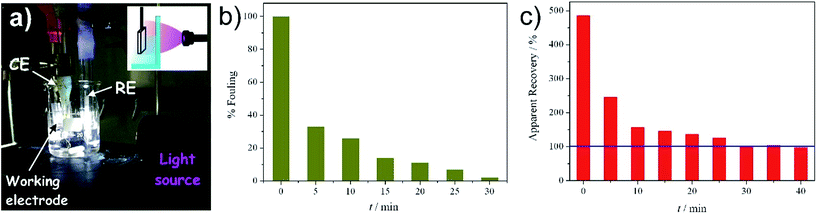
Fig. 3b shows the %reactivation parameter with respect to the irradiation time obtained for the three different tested lamps and for an electrode stored in the dark for the same time (identified as blank). The value measured for the blank was 0% for all the tested times, demonstrating the crucial effect of UV light for electrode regeneration. In fact, complete reactivation (100%) was obtained after at least 20 minutes of irradiation, with different rates, depending on the lamp features. The most efficient lamp was the UV-LAMP, in which the combination of high power and low wavelengths (high energy, Fig. S2†) led to faster degradation of the fouling products. With such a lamp, after only 5 minutes of irradiation, the electrode recovered more than 90% of its initial condition and recovered totally after 10 minutes. Irradiation wavelengths also seem to be a crucial variable to consider for evaluating the lamp performance. TLC-LAMP displays an emission component of 310 nm (higher energetic radiation, Fig. S2†). Notwithstanding its low irradiation power, TLC-LAMP presents an efficient absorption of radiation by the titania layer and consequently an abundance of photo-catalytic events, leading to a high cleaning efficiency. The results obtained with the simple UV LED TORCH are, in our opinion, the most intriguing and valuable. TORCH emits a low energy radiation, near to the visible edge (Fig. S2†), which refers to a safer behaviour with respect to the other two lamps. However, it shows a similar cleaning kinetics compared to UV-LAMP and TLC-LAMP, allowing complete regeneration of the electrode after 20 minutes. In addition, its intrinsic portability makes it perfect for on-field and on-line applications. For this purpose, the development of a procedure that allows direct, on site, cleaning of the device without the need to remove it from the working environment would be highly desirable. As a proof of concept, the device was fouled according to the previously reported procedure and immersed in the electrolyte solution. TORCH was chosen as the UV source and the irradiation of the electrode was performed from outside the reactor (Fig. 4a). Fig. 4b shows the decrease of fouling at the electrode for different irradiation times. The percentages of fouling (%fouling) are calculated as the ratio between the current of the tested device and of the completely fouled electrode, measured at +0.3 V (SCE). Despite the use of the less performing lamp, the intrinsic filter effect of water and of the glass container (borosilicate glass), the fouling species totally disappeared after 30 minutes.
Moreover, in order to further demonstrate the robustness of the method in on-site analytical applications, an on-purpose fouled electrode (soiled after dipping and CV cycling in 1 mM dopamine solution) was immersed in a freshly prepared solution, spiked with dopamine, yielding a final concentration of 1 μM. The sample analysis was performed as previously described, registering the apparent recoveries at fixed time (every 5 minutes) during the cleaning procedure. As expected, see Fig. 4c, apparent recoveries higher than 100% are calculated, indicating heavy fouling and thus overestimation of the analyte. But, after 30 minutes of on-site irradiation, the device was completely cleaned and reactivated, with apparent recovery (IUPAC30) values around 100%. Further, cleaning does not affect the recovery values, demonstrating that only fouling compounds are degraded and no soluble by-products are electrochemically detected, while dopamine in solution is not affected by the cleaning procedure. It is worthwhile noticing that this is not an obvious result: the photocatalytic degradation, only confined to the electrode surface, allows the degradation of adsorbates without altering the very low concentration of the analyte, since photolysis does not occur.
Finally, it is important to underline that the fouling conditions adopted in the present work were selected to be extremely hard. Dopamine was chosen, not only because it is an important neurotransmitter analyte, but also since it is a strong passivating molecule recognized to irreversibly chemisorb and polymerize at the oxide surface.16,17 In this context, to demonstrate the potentialities of the self-cleaning layer, we employed concentrations much higher than those found in body fluids (40 nM).15 Apart from the previously proved robustness, trueness and precision of the device, here, we demonstrated the possibility to use short-time cleaning steps with low energy and low power UV-LED sources. Moreover, the cleaning procedure can be performed directly on site, in the solution of interest. For all the above reasons, the authors forecast on-line/on-field applications: a small UV emitting LED incorporated in the final device, for example, could switch on and off for few minutes between two subsequent analyses. This could permit the continuous regeneration of the electrode surface, eliminating the need of any other maintenance work and allowing the use of this sensor in remote integrated monitoring systems and flow analysis, where shorter detection time are crucial.
Acknowledgements
This work has been supported by Fondazione Cariplo, grant no. 2014-1285.Notes and references
- C. M. A. Brett, Pure Appl. Chem., 2001, 73, 1969–1977 CrossRef CAS.
- L. Rassaei, M. Amiri, C. M. Cirtiu, M. Sillanpaa and F. Marken, TrAC, Trends Anal. Chem., 2011, 30, 1704–1715 CrossRef CAS PubMed.
- X. Ma, W. Hu, C. Guo, L. Yu, L. Gao, J. Xie and C. M. Li, Adv. Funct. Mater., 2014, 24, 5897–5903 CrossRef CAS PubMed.
- C. M. Welch and R. G. Compton, Anal. Bioanal. Chem., 2006, 384, 601–619 CrossRef CAS PubMed.
- F. W. Campbell and R. G. Compton, Anal. Bioanal. Chem., 2010, 396, 241–259 CrossRef CAS PubMed.
- G. Hanrahan, D. G. Patil and J. Wang, J. Environ. Monit., 2004, 6, 657–664 RSC.
- M. Poon and R. L. McCreery, Anal. Chem., 1987, 59, 1615–1620 CrossRef CAS.
- G. K. Mor, M. A. Carvalho, O. K. Varghese, M. V. Pishko and C. A. Grimes, J. Mater. Res., 2011, 19, 628–634 CrossRef.
- Y.-Y. Song, Z. Gao, K. Lee and P. Schmuki, Electrochem. Commun., 2011, 13, 1217–1220 CrossRef CAS PubMed.
- Y.-Y. Song, Z.-D. Gao and P. Schmuki, Electrochem. Commun., 2011, 13, 290–293 CrossRef CAS PubMed.
- M. Poon and R. L. McCreery, Anal. Chem., 1986, 58, 2745–2750 CrossRef CAS.
- G. Soliveri, V. Pifferi, G. Panzarasa, S. Ardizzone, G. Cappelletti, D. Meroni, K. Sparnacci and L. Falciola, Analyst, 2015, 140, 1486–1494 RSC.
- A. Antonello, G. Soliveri, D. Meroni, G. Cappelletti and S. Ardizzone, Catal. Today, 2014, 230, 35–40 CrossRef CAS PubMed.
- K. Jackowska and P. Krysinski, Anal. Bioanal. Chem., 2013, 405, 3753–3771 CrossRef CAS PubMed.
- M. Perry, Q. Li and R. T. Kennedy, Anal. Chim. Acta, 2009, 653, 1–22 CrossRef CAS PubMed.
- R. A. Zangmeister, T. A. Morris and M. J. Tarlov, Langmuir, 2013, 29, 8619–8628 CrossRef CAS PubMed.
- M. C. Henstridge, E. J. F. Dickinson, M. Aslanoglu, C. Batchelor-McAuley and R. G. Compton, Sens. Actuators, B, 2010, 145, 417–427 CrossRef CAS PubMed.
- S. Neil, Chemistry World, Royal Society of Chemistry, 2015, http://www.rsc.org/chemistryworld/2015/01/self-cleaning-electrochemical-sensor-ultraviolet-light Search PubMed.
- V. Pifferi, G. Facchinetti, A. Villa, L. Prati and L. Falciola, Catal. Today, 2015, 249, 265–269 CrossRef CAS PubMed.
- V. Pifferi, V. Marona, M. Longhi and L. Falciola, Electrochim. Acta, 2013, 109, 447–453 CrossRef CAS PubMed.
- L. Falciola, A. Gennaro, A. A. Isse, P. R. Mussini and M. Rossi, J. Electroanal. Chem., 2006, 593, 47–56 CrossRef CAS PubMed.
- X. Wang, R. Xiong and G. Wei, Surf. Coat. Technol., 2010, 204, 2187–2192 CrossRef CAS PubMed.
- G. Maino, D. Meroni, V. Pifferi, L. Falciola, G. Soliveri, G. Cappelletti and S. Ardizzone, J. Nanopart. Res., 2013, 15, 2087 CrossRef.
- G. Panzarasa, J. Chem. Educ., 2014, 91, 696–700 CrossRef CAS.
- A. Fujishima, X. Zhang and D. Tryk, Surf. Sci. Rep., 2008, 63, 515–582 CrossRef CAS PubMed.
- G. Panzarasa, G. Soliveri, K. Sparnacci and S. Ardizzone, Chem. Commun., 2015, 51, 7313–7316 RSC.
- K. Nakata and A. Fujishima, J. Photochem. Photobiol., C, 2012, 13, 169–189 CrossRef CAS PubMed.
- J. Liebscher, R. Mrówczyński, H. A. Scheidt, C. Filip, N. D. Haìdade, R. Turcu, A. Bende and S. Beck, Langmuir, 2013, 29, 10539–10548 CrossRef CAS PubMed.
- W. Harreither, R. Trouillon, P. Poulin, W. Neri, A. G. Ewing and G. Safina, Anal. Chem., 2013, 85, 7447–7453 CrossRef CAS PubMed.
- L. A. Currie, Anal. Chim. Acta, 1999, 391, 105–126 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental section, UV-vis absorption spectrum. See DOI: 10.1039/c5ra12219h |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2015 |