Electrochemical performances of cobalt oxide–carbon nanotubes electrodes via different methods as negative material for alkaline rechargeable batteries†
Yanan Xu,
Yanyin Dong,
Xiaofeng Wang,
Yijing Wang*,
Lifang Jiao,
Huatang Yuan and
Jing Li
College of Chemistry, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Key Laboratory of Advanced Energy Materials Chemistry (MOE), Nankai University, Tianjin 300071, P. R. China. E-mail: wangyj@nankai.edu.cn; Fax: +86 22 23503639; Tel: +86 22 23503639
First published on 24th August 2015
Abstract
Co3O4/CNTs samples are synthesized via different methods and investigated as negative materials for alkaline rechargeable batteries for the first time. The reasons for performance difference of those Co3O4/CNTs electrodes and the impact of CNTs additive amount on cycling properties are explored in detail. CNTs can remarkably enhance the electrochemical activity of Co3O4 materials, leading to a notable improvement of discharge capacity, cycle stability and rate capability. Co3O4/CNTs-C composites via the one-pot reflux method exhibit the desirable electrochemical capability. Co3O4/CNTs-C8 sample (mass ratio of CNTs is 8%) shows the highest discharge capacity of 526.1 mA h g−1. Meanwhile, Co3O4/CNTs-C11 electrode (mass ratio of CNTs is 11%) displays the most outstanding cycle performance with a capacity retention rate of over 97.3% after 200 cycles. A properly electrochemical reaction mechanism of Co3O4/CNTs electrode is also constructed in detail.
1 Introduction
With the rapid development of renewable and clean energy sources, safer and more efficient energy storage devices are urgently required, such as alkaline secondary batteries, lithium-ion batteries, supercapacitors and fuel cells.1–4 Among them, alkaline secondary batteries are regarded as promising energy storage applications due to the powerful and reversible electrochemical redox reactions.5–8 Recently, a new type alkaline rechargeable Ni/Co battery system using Ni(OH)2 as cathode material and Co-based materials as anode materials was systematically proposed, which presented the outstanding discharge capacity and the environmental friendly property. It can be ascribed to the higher utilization, better electrochemical conductivity and multi-electron reaction ability of Co-based materials. The dominant surface faradaic reaction on anode electrode can be expressed as follows:9–14According to the reaction, the utilization of Co is one of the most important factors influencing discharge performance of cobalt-based negative materials, which is largely dependent on the contact area between the electrode and alkaline electrolyte.
Co3O4 has been considered as one of the most promising electrode materials due to high capacities and multi-electron reaction ability during electrochemical reaction process.15–18 But the poor cycle stability and rate capability limit its widespread application. Carbon materials have been proved to enhance electrochemical properties of Co-based materials by improving the dispersion, electroconductibility and utilization of active material.19,20 CNTs have attracted numerous research interests due to high electrical conductivity, good mechanical properties and chemically stable.21–24 We could speculate that CNTs may be a better matrix material to modify the electrochemical performances of Co3O4 materials. To the best of our knowledge, there is no report on the electrochemical properties of Co3O4/CNTs composite as negative electrode material for Ni/Co battery.
In this paper, a series of Co3O4/CNTs samples are prepared by different methods and the reasons for performance difference of those electrodes are investigated in detail. The microstructure and electrochemical characteristics of Co3O4/CNTs composites are also explored in comparison with pure Co3O4 sample. And the additive amount of CNTs may have a great effect on discharge capacities and rate performances of Co-based materials. Moreover, the function mechanism is also proposed in detail.
2 Experimental
2.1. Preparation of Co3O4/CNTs samples
Co3O4 samples were synthesized via a facile reflux condensation route. The typical synthesis process was as follows: 0.14 mmol sodium citrate, 2.5 mmol Co(NO3)2·H2O and 1.2 mmol hexamethylenetetramine were dissolved in 200 mL distilled water to form a homogeneous solution, respectively. Then the solution was heated to 90 °C and refluxed for 6 h. The resultant black solid was centrifuged and washed with water and ethanol, dried in a vacuum oven at 60 °C for 12 h. The sample was heated at 300 °C for 3 h in air atmosphere to obtain the pure Co3O4.Three Co3O4/CNTs samples were synthesized via the following three methods: Co3O4/CNTs-A was prepared by simple grinding Co3O4 with CNTs powder (mass ratio: 8%) for 30 min. Co3O4/CNTs-B was prepared by ball milling Co3O4 and CNTs powders in a same ratio for 10 h. Co3O4/CNTs-C was synthesized using the above one-pot reflux condensation route with the direct addition of CNTs. The different additive content of CNTs under mass ratio of 2%, 5%, 8% and 11% was dispersed in the homogeneous solution with ultrasonication for 2 h during the preparation process. Those Co3O4/CNTs-C composites with different mass ratio were designated as Co3O4/CNTs-C2, Co3O4/CNTs-C5, Co3O4/CNTs-C8, Co3O4/CNTs-C11, respectively. The CNTs content in Co3O4/CNTs-C8 composites is identical to the additive amount of Co3O4/CNTs-A sample and Co3O4/CNTs-B sample.
2.2. Compositional and structural characterization
The crystal structure and surface morphology of materials were characterized by X-ray diffraction (XRD, Rigaku MiniFlex II with Cu Kα radiation), Scanning Electron Microscopy (SEM, JEOL JSM-6700F Field Emission), transmission electron microscope (TEM, JEOL JEM-2100 TEM). The elemental composition was measured by Elemental Analysis (EA, vario EL CUBE) and X-ray photoelectron spectroscopy (XPS, PHI5000 VersaProbe). The specific surface areas and porous nature of materials were further investigated by nitrogen adsorption/desorption measurements on NOVA 2200e.2.3. Electrochemical measurements
Negative electrodes were constructed through mixing as-prepared samples with carbonyl nickel powders at a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The powder mixture was pressed under 20 MPa pressure into a small pellet of 10 mm in diameter and 1.5 mm thickness. Electrochemical measurements were conducted in a three-electrode cell using a Land battery system (CT2001A). The electrolyte was 6 M KOH aqueous solution. Ni(OH)2/NiOOH and Hg/HgO were used as the counter electrode and reference electrode, respectively. The obtained electrodes were charged at a current density of 200 mA g−1 for 3 h. After a 5 min rest, they were discharged at different current densities (100, 200, 500, 1000 mA g−1) to 0.5 V (vs. Hg/HgO). Cyclic voltammetric (CV) measurements were carried out by an electrochemical work station (CHI 660b). Electrochemical impedance spectroscopy (EIS) measurements were carried out by applying an AC voltage with 5 mV amplitude in a frequency range from 0.1 Hz to 100 kHz at open circuit potential.
3. The powder mixture was pressed under 20 MPa pressure into a small pellet of 10 mm in diameter and 1.5 mm thickness. Electrochemical measurements were conducted in a three-electrode cell using a Land battery system (CT2001A). The electrolyte was 6 M KOH aqueous solution. Ni(OH)2/NiOOH and Hg/HgO were used as the counter electrode and reference electrode, respectively. The obtained electrodes were charged at a current density of 200 mA g−1 for 3 h. After a 5 min rest, they were discharged at different current densities (100, 200, 500, 1000 mA g−1) to 0.5 V (vs. Hg/HgO). Cyclic voltammetric (CV) measurements were carried out by an electrochemical work station (CHI 660b). Electrochemical impedance spectroscopy (EIS) measurements were carried out by applying an AC voltage with 5 mV amplitude in a frequency range from 0.1 Hz to 100 kHz at open circuit potential.
3 Results and discussion
XRD patterns of CNTs, Co3O4 and Co3O4/CNTs samples synthesized via different methods were shown in Fig. 1a. For the three Co3O4/CNTs composites, the major diffraction peaks are well indexed with cubic phase of Co3O4 (space group Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, JCPDS no. 42-1467). An additional peak around 27° can be assigned to the characteristic peak of CNTs, and it can be clearly observed in Co3O4/CNTs-C sample. All the peaks of Co3O4 for Co3O4/CNTs composites are relatively broadened with the loading of CNTs, corresponding to the reduced grain size of Co3O4 nanoparticles. Thus the number of electrochemical active sites would be increased, thereby improving the electrochemical activity of electrode materials. X-ray photoelectron spectroscopy measurement of Co3O4/CNTs-C composite was performed in Fig. 1b. The Co 2p spectrum are best fitted by two kinds of Co species, including the Co(III) ions located at 779.5 eV and 795.2 eV, and Co(II) ions at 780.6 eV and 796.7 eV with two satellite peaks, it demonstrates the presence of both Co2+ and Co3+ species in Co3O4/CNTs samples.25,26
m, JCPDS no. 42-1467). An additional peak around 27° can be assigned to the characteristic peak of CNTs, and it can be clearly observed in Co3O4/CNTs-C sample. All the peaks of Co3O4 for Co3O4/CNTs composites are relatively broadened with the loading of CNTs, corresponding to the reduced grain size of Co3O4 nanoparticles. Thus the number of electrochemical active sites would be increased, thereby improving the electrochemical activity of electrode materials. X-ray photoelectron spectroscopy measurement of Co3O4/CNTs-C composite was performed in Fig. 1b. The Co 2p spectrum are best fitted by two kinds of Co species, including the Co(III) ions located at 779.5 eV and 795.2 eV, and Co(II) ions at 780.6 eV and 796.7 eV with two satellite peaks, it demonstrates the presence of both Co2+ and Co3+ species in Co3O4/CNTs samples.25,26
 | ||
| Fig. 1 (a) XRD patterns of CNTs, Co3O4 and Co3O4/CNTs samples synthesized via different methods. (b) XPS spectra of Co 2p for Co3O4/CNTs-C samples. | ||
Typical TEM images in Fig. 2 were carried out to further investigate the microstructure of the obtained samples. Co3O4 samples are composed of numerous nanoparticles with size ranging from 5 to 20 nm in Fig. 2a and b. The nanoparticles are closely packed and held together as a whole, and show good crystalline nature. The starting CNTs samples (Fig. 2c) are formed by numerous microns long nanotubes with uniform diameter size (40–60 nm). For all the Co3O4/CNTs samples in Fig. 2d–f, CNTs can be regarded as a suitable substrate to improve dispersion state of Co3O4 nanoparticles. The agglomerated extent of Co3O4 nanoparticles in Co3O4/CNTs-A sample remains seriously by simply physical mixing processes. And the ball-milling disperse process also cannot alleviate the problem of agglomeration by the root for Co3O4/CNTs-B sample. But for Co3O4/CNTs-C sample, the surfaces of CNTs materials are evenly coated with numerous Co3O4 nanoparticles. It indicates that one-pot reflux method can effectively resolve agglomerated phenomenon and allow better dispersion of Co3O4 nanoparticles, compared with the physical mixture methods. The grain size of nanoparticles is reduced in a certain degree with the loading of CNTs from Fig. 3a and b. Clear lattice fringes for Co3O4 are observed in Fig. 3c, suggesting the crystalline nature of the particles.
 | ||
| Fig. 2 TEM images of Co3O4 (a and b), CNTs (c), Co3O4/CNTs-A (d), Co3O4/CNTs-B (e) and Co3O4/CNTs-C (f) samples. | ||
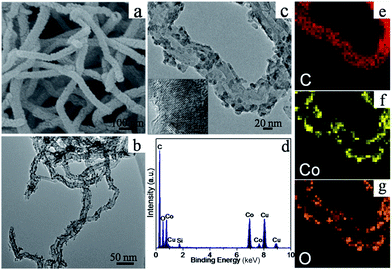 | ||
| Fig. 3 SEM and TEM images (a–c), EDS spectrum (d) and EDS mapping results (e–g) from the Co3O4/CNTs-C samples. | ||
Energy-dispersive X-ray spectrometry (EDS) mapping analysis in Fig. 3e–g also shows that Co3O4 nanoparticles are well-uniform distributing on the surface of CNTs for Co3O4/CNTs-C sample. Furthermore, results from the EDS spectra in Fig. 3d also confirm the elemental composition of Co3O4/CNTs-C determined from atomic absorption spectroscopy. The presence of Cu and Si is from the carbon-coated Cu grid.
Specific surface areas and porous nature of as-synthesized Co3O4 and Co3O4/CNTs-C sample in Fig. 4 were further investigated by Brunauer–Emmett–Teller (BET) nitrogen adsorption/desorption measurements. Based on the BET equation, the specific surface areas of Co3O4, Co3O4/CNTs-A, Co3O4/CNTs-B and Co3O4/CNTs-C samples are 82.0, 91.2, 107.4 (as shown in Fig. S1†) and 164.9 m2 g−1, respectively. And pore size distributions of Co3O4 sample show a sharp peak at ∼3.8 nm and a wide major peak at ∼17.5, while only one sharp peak at ∼3.8 nm is observed for Co3O4/CNTs-C samples. It shows that CNTs introduced by one-pot reflux method can effectively enhance the dispersion extent of Co3O4 nanoparticles with the reduced particles size. The unique features can make Co3O4/CNTs-C hybrid accessible to electrolytes to a larger extent, which promotes the hybrid nanocomposites as potential electrode candidates for alkaline rechargeable Ni/Co battery with high specific capacities and excellent cycling stability.27,28
 | ||
| Fig. 4 Nitrogen adsorption and desorption isotherms and the pore size distribution curves of as-prepared Co3O4 (a) and Co3O4/CNTs-C (b) samples. | ||
Cycle performances of the CNTs, pure Co3O4 and Co3O4/CNTs composites were shown in Fig. 5a at a current density of 100 mA g−1. CNTs can be regarded as an inactive matrix without the contribution for discharge capacities. All the electrodes need a long activation process before the intrinsic capacities are realized. Pure Co3O4 electrode shows the relatively low discharge capacity with the poor capacity retention rate of 47.5% after 200 cycles (S200 = C200/Cmax × 100%). It's worth mentioning that specific capacities and cycling stability of the three Co3O4/CNTs electrodes have been remarkably enhanced. It is mainly because the addition of CNTs can successfully allow good conductivity and well dispersion of Co3O4 nanoparticles. Meanwhile, Co3O4/CNTs-C electrode displays the desirable electrochemical properties as we expected. The maximum discharge capacity can reach 487 mA h g−1 with the capacity retention rate of 86.2%. It is ascribed to the high specific surface areas of Co3O4/CNTs-C samples with the reduced particles size. The unique nanostructure can improve the dispersion of active material and enlarge the contact area between the electrode and alkaline electrolyte, compared with the physical mixture methods. Coulombic efficiency of Co3O4/CNTs-C materials can maintain at 76% after 30 cycles, which is significantly improved than that of Co3O4/CNTs-A and Co3O4/CNTs-B materials.
 | ||
| Fig. 5 Discharge capacities at a current density of 100 mA g−1 (a) and rate performances (b) of the CNTs, pure Co3O4 and Co3O4/CNTs samples. | ||
Rate capabilities of Co3O4/CNTs composites synthesized via different methods were measured in Fig. 5b. The delivered discharge capacities of Co3O4/CNTs-C electrode are 503, 482, 464, 438, 411 and 453 mA h g−1 at the different current densities of 50, 100, 200, 500, 1000 and 50 mA g−1 respectively, which are superior than the values of Co3O4/CNTs-A (416, 375, 320, 296, 244 and 314 mA h g−1) and Co3O4/CNTs-B materials (430, 409, 378, 336, 298 and 341 mA h g−1) under the same condition. These excellent data demonstrate that the Co3O4/CNTs-C composites are the promising alternative anode materials for alkaline secondary batteries.
To explore the influence of CNTs additive amount on cycle performance of Co3O4/CNTs electrodes, the related tests were also conducted in Fig. 6a. Determined with an Element Analyzer, the measured content of CNTs in Co3O4/CNTs-C2, Co3O4/CNTs-C5, Co3O4/CNTs-C8 and Co3O4/CNTs-C11 composites is 2.15%, 5.24%, 7.69% and 10.7%, respectively. And these values are consistent with the actual mass ratios of additive CNTs in the experiments. Discharge capacities of Co3O4/CNTs electrodes firstly increase with the increasing content of CNTs and then decrease. These results illustrate that the appropriate amount of CNTs can lead to a remarkable enhancement of discharge capacity and cycle stability. But an excess of CNTs is not helpful to enhance the cycling properties of Co-based materials, which leads to the density decrease of active material for rechargeable Ni/Co batteries. Co3O4/CNTs-C8 sample shows the highest discharge capacity of 526.1 mA h g−1 at a current density of 100 mA g−1. Meanwhile, Co3O4/CNTs-C11 electrode shows the most outstanding cycle performance with a capacity retention rate of over 97.3% after 200 cycles. Results of rate performance tests also indicate that Co3O4/CNTs-C8 displays the most prominent discharge capacities (corresponding values are 549, 526, 519, 510, 499 and 526 mA h g−1), while Co3O4/CNTs-C11 electrode possesses the best cycling stability.
 | ||
| Fig. 6 Discharge capacities at a current density of 100 mA g−1 (a) and rate performances (b) of Co3O4/CNTs-C composites (different mass ratio of additive CNTs). | ||
In order to explain the different phenomenon of electrochemical properties, CV curves of Co3O4/CNT-C8 electrode for different cycles were studied in Fig. 7a. Only one pair of remarkable reduction–oxidation peak appears, demonstrating that most of the discharge capacities are arising from faradaic reactions occurring on the electrode. The curve shape and peak voltage is consistent with the other Co-based materials.11,29–31 The reversible reaction between Co and Co(OH)2 can be confirmed occurring on Co3O4/CNT-C electrode, suggesting that CNTs is used as a conductive substrate without electrochemical reactions. The integral area of redox peaks gradually increase, implying that discharge capacity gradually increases during charge–discharge process. The result is consistent with the measurements of cycling performance. XRD patterns of Co3O4/CNTs-C8 electrode at different charged/discharged state were shown in Fig. 7b. At fully charged state of the 1st cycle, the weakening of Co3O4 and the appearing of Co and Co(OH)2 are clearly observed. It indicates that Co3O4 gradually transforms into Co and Co(OH)2 during the initial charging processes. When discharged to −0.5 V, the diffraction peaks of Co(OH)2 become apparent and Co3O4 gradually disappears. Only peaks of Co and Co(OH)2 are detected after ten cycles. Compared with the charged state, the peak intensity of Co at the 10th discharged state weakens while peak intensity of Co(OH)2 strengthens. It illustrates that some Co transforms to Co(OH)2 during the discharge process. These results indicate that the electrochemical reaction on Co3O4/CNTs-C8 electrode at activation process can be summarized as follows:
 | ||
| Fig. 7 CV curves (a), XRD patterns (b), charge–discharge curves at 100 mA g−1 (c) and different current densities (d) of Co3O4/CNTs-C8 composite. | ||
Charge:
| Co3O4 + 4H2O + 2e− → 3Co(OH)2 + 2OH− | (1) |
| Co(OH)2 + 2e− → Co + 2OH− | (2) |
Discharge:
| Co + 2OH− → Co(OH)2 + 2e− | (3) |
After activation process, charge–discharge reaction occurring on electrodes transforms into reversible redox reaction:
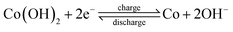 | (4) |
Charge–discharge curves of Co3O4/CNTs-C8 electrode at the current density of 100 mA g−1 further confirm the proposed mechanism in Fig. 7c. The charge–discharge curves of the 1st and 2nd cycle at activation process are different from other curves. It can be ascribed that Co3O4 gradually transforms into Co and Co(OH)2 during the initial charging processes. For the 1st charged state, only a long platform at −0.98 V can be observed and it illustrates that Co3O4 transforms to Co(OH)2 (eqn (1)).27,31 But for the following cycles, two potential charge platforms in curves are observed. The first charge platform at about −0.89 V ascribes to the change from Co(OH)2 to Co, corresponding to eqn (2). The other one at −1.1 V assigns to the electrolysis reaction of water. Meanwhile, only one potential platform at around −0.79 V (vs. Hg/HgO) occurs during the discharge process corresponding to eqn (3), and no changes appear in the following cycles. This fact once again reinforces that the electrochemical reaction occurring on the Co3O4 electrode is only reversible conversion between Co and Co(OH)2 (eqn (4)) at reversible redox process.
CNTs in Co3O4/CNTs electrodes show two functions during charge–discharge processes. The addition of CNTs leads to well dispersion of active Co3O4 nanoparticles. Meanwhile, Co3O4 nanoparticles decorating by CNTs are impregnated in alkaline solution during the charge–discharge process, which can provide unobstructed pathways for OH− transport and increase the contact area between active materials and alkaline solution. The two aspects are in favor of the electrochemical redox to enhance the capacity of Co3O4/CNTs electrode.
4 Conclusions
In summary, a series of Co3O4/CNTs composites are prepared by different methods for alkaline rechargeable battery and the reasons for performance difference of those electrodes are explored in detail. The one-pot reflux method (method C) can allow better dispersion of Co3O4 nanoparticles with the reduced size and the larger specific surface area of Co3O4/CNTs composites, compared with the physical mixture methods (method A, B). Electrochemical investigations also indicate that Co3O4/CNTs-C composites exhibit the desirable electrochemical capability. The additive amount of CNTs also has an important influence on cycling performances of Co3O4/CNTs-C composites. Co3O4/CNTs-C8 sample (mass ratio of CNTs is 8%) shows the highest discharge capacity of 526.1 mA h g−1. Meanwhile, Co3O4/CNTs-C11 electrode (mass ratio of CNTs is 11%) displays the most outstanding cycle performance with a capacity retention rate of over 97.3% after 200 cycles. High discharge capacities and excellent rate capability demonstrate that Co3O4/CNTs-C composites through one-pot reflux route are the suitable negative materials for high power applications. In addition, the faradic reaction between Co and Co(OH)2 for Co3O4/CNTs electrode has been further investigated.Acknowledgements
This work was financially supported by 973 (2011CB935900), NSFC (51471089), MOE (IRT13R30), 111 Project (B12015), Research Fund for the Doctoral Program of Higher Education of China (20120031110001), Tianjin Sci & Tech Project (10SYSYJC27600).Notes and references
- F. Y. Cheng, J. Liang, Z. L. Tao and J. Chen, Adv. Mater., 2011, 23, 1695–1715 CrossRef CAS PubMed.
- V. Etacheri, R. Marom, R. Elazari, G. Salitra and D. Aurbach, Energy Environ. Sci., 2011, 4, 3243–3262 CAS.
- P. Leung, X. H. Li, C. P. de Leon, L. Berlouis, C. T. J. Low and F. C. Walsh, RSC Adv., 2012, 2, 10125–10156 RSC.
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245–4270 CrossRef CAS.
- R. J. Wang and Z. H. Yang, RSC Adv., 2013, 3, 19924–19928 RSC.
- K. Vijayamohanan, T. S. Balasubramanian and A. K. Shukla, J. Power Sources, 1991, 34, 269–285 CrossRef CAS.
- J. H. Huang and Z. H. Yang, RSC Adv., 2015, 5, 33814–33817 RSC.
- X. P. Gao, S. M. Yao, T. Y. Yan and Z. Zhou, Energy Environ. Sci., 2009, 2, 502–505 CAS.
- D. S. Lu, W. S. Li, F. M. Xiao and R. H. Tang, Electrochem. Commun., 2010, 12, 362–366 CrossRef CAS PubMed.
- Y. Wang, J. M. Lee and X. Wang, Int. J. Hydrogen Energy, 2010, 35, 1669–1673 CrossRef CAS PubMed.
- X. Y. Zhao, L. Q. Ma, Y. Yao, M. Yang, Y. Ding and X. D. Shen, Electrochim. Acta, 2010, 55, 1169–1174 CrossRef CAS PubMed.
- S. M. Yao, K. Xi, G. R. Li and X. P. Gao, J. Power Sources, 2008, 184, 657–662 CrossRef CAS PubMed.
- X. Y. Zhao, L. Q. Ma and X. D. Shen, J. Mater. Chem., 2012, 22, 277–285 RSC.
- L. L. Jin, X. W. Li, H. Ming, H. H. Wang, Z. Y. Jia, Y. Fu, J. Adkins, Q. Zhou and J. W. Zheng, RSC Adv., 2014, 4, 6083–6089 RSC.
- L. Wang, Z. H. Dong, Z. G. Wang, F. X. Zhang and J. Jin, Adv. Funct. Mater., 2013, 23, 2758–2764 CrossRef CAS PubMed.
- C. Z. Yuan, L. Yang, L. R. Hou, L. F. Shen, X. G. Zhang and X. W. Lou, Energy Environ. Sci., 2012, 5, 7883–7887 CAS.
- W. Sugimoto, H. Iwata, Y. Yasunaga, Y. Murakami and Y. Takasu, Angew. Chem., Int. Ed., 2003, 42, 4092–4096 CrossRef CAS PubMed.
- L. Li, Y. N. Xu, C. H. An, Y. J. Wang, L. F. Jiao and H. T. Yuan, J. Power Sources, 2013, 238, 117–122 CrossRef CAS PubMed.
- Y. N. Xu, X. F. Wang, C. H. An, Y. J. Wang, L. F. Jiao and H. T. Yuan, J. Power Sources, 2014, 272, 328–334 CrossRef CAS PubMed.
- F. Cai, Y. R. Kang, H. Y. Chen, M. H. Chen and Q. W. Li, J. Mater. Chem. A, 2014, 2, 11509–11515 CAS.
- Y. Liu, C. Y. Nie, X. J. Liu, X. T. Xu, Z. Sun and L. K. Pan, RSC Adv., 2015, 5, 15205–15225 RSC.
- J. X. Li, M. Z. Zou, W. W. Wen, Y. Zhao, Y. B. Lin, L. Z. Chen, H. Lai, L. H. Guan and Z. G. Huang, J. Mater. Chem. A, 2014, 2, 10257–10262 CAS.
- H. Zhang, H. Qiao, H. Y. Wang, N. Zhou, J. J. Chen, Y. G. Tang, J. S. Li and C. H. Huang, Nanoscale, 2014, 6, 10235–10242 RSC.
- X. M. Zhai, W. Yang, M. Y. Li, G. Q. Lv, J. P. Liu and X. L. Zhang, Carbon, 2013, 65, 277–286 CrossRef CAS PubMed.
- S. L. Xiong, J. S. Chen, X. W. Lou and H. C. Zeng, Adv. Funct. Mater., 2012, 22, 861–871 CrossRef CAS PubMed.
- H. M. Du, L. F. Jiao, Q. H. Wang, Q. N. Huan, L. J. Guo, Y. C. Si, Y. J. Wang and H. T. Yuan, CrystEngComm, 2013, 15, 6101–6109 RSC.
- D. S. Lu, W. S. Li, C. L. Tan and R. H. Zeng, Electrochim. Acta, 2009, 55, 171–177 CrossRef CAS PubMed.
- Q. H. Wang, L. F. Jiao, H. M. Du, Q. T. Huan, W. X. Peng, D. W. Song, Y. J. Wang and H. T. Yuan, Electrochim. Acta, 2011, 56, 4992–4995 CrossRef CAS PubMed.
- Z. W. Lu, S. M. Yao, G. R. Li, T. Y. Yan and X. P. Gao, Electrochim. Acta, 2008, 53, 2369–2375 CrossRef CAS PubMed.
- L. Li, Y. P. Wang, Y. J. Wang, Y. Han, F. Y. Qiu, G. Liu, C. Yan, D. W. Song, L. F. Jiao and H. T. Yuan, J. Power Sources, 2011, 196, 10758–10761 CrossRef CAS PubMed.
- S. Baskar, D. Meyrick, K. S. Ramakrishnan and M. Minakshi, Chem. Eng. J., 2014, 253, 502–507 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra12721a |
| This journal is © The Royal Society of Chemistry 2015 |

