Effect of L-tyrosine on aerobic sludge granulation and its stability†
Jinghai Luoa,
Li Weia,
Tianwei Hao*a,
Weiqi Xuea,
Hamish R. Mackeyc and
Guang-Hao Chenab
aDepartment of Civil and Environmental Engineering, The Hong Kong University of Science and Technology, Clear Water Bay, Hong Kong, China. E-mail: thao@connect.ust.hk
bSYSU-HKUST Research Centre for Innovative Environmental Technology, Sun Yat-sen University, Guangzhou, China
cCollege of Science and Engineering, Hamad Bin Khalifa University, Education City, Doha, Qatar
First published on 5th October 2015
Abstract
Aerobic sludge granulation and its stability remain challenging in applications. Tyrosine, a compound in extracellular polymeric substances (EPSs) extracted from sludge, is reported to be closely associated with sludge granulation and its stability. In order to confirm this, this study investigated the effect of L-tyrosine on granulation and disintegration of granular sludge in two identical sequencing airlift bioreactors (SABRs): one dosed with L-tyrosine (6 mg L−1) and the other without dosing. Changes in the physiochemical and biological properties of the aerobic granular sludge (AGS) and organic and nitrogen removal in both reactors operated under different ratios of chemical oxygen demand (COD) to nitrogen were closely monitored for 120 days. The L-tyrosine dosing shortened full granulation of AGS by 1 month. Disintegration of the granules and deterioration in the COD and nitrogen removal capability were not observed in the L-tyrosine dosed reactor even when the ratio of COD/N in the influent was reduced from 4 to 1 unlike the control reactor. This clearly confirmed the contribution of L-tyrosine in promoting AGS granulation and its stability. Both the enrichment of quorum sensing auto-inducer relating bacteria genera (21%) and the stable production of EPS were suggested as main reasons for the positive effect of L-tyrosine on the granulation and stability of AGS.
1. Introduction
As a special form of biofilm, aerobic granular sludge (AGS) has been recognized as a low energy and small footprint technology for substituting activated sludge processes for municipal and industrial wastewater treatment.1 The merits of AGS, including improved settleability, high biomass retention, and high flexibility against changes in pollution and environmental conditions, have been intensively demonstrated.2 These advantages ensure a high application potential of the AGS technology in wastewater treatment. Nonetheless, efficient start-up and stability of AGS process still remain critical issues.3Extracellular polymeric substance (EPS) are known to affect the granulation process of AGS.4 The main components of EPS, proteins (PN) and polysaccharides (PS), are further found to be important in maintaining the properties of AGS.5 The granular structure is supported by a backbone mainly composed of PS1,6 and the PN may improve AGS granulation and structure integrity due to enhanced surface hydrophobicity and the reduced surface negative charge.4,7 Furthermore, alginate-like exopolysaccharides (ALE) extracted from the EPS of AGS developed with synthetic wastewater have been shown to improve the formation of AGS.8 In addition, the EPSs also contain other components, such as lipids and humic, fulvic, amino acids, etc.1,4 Their contribution to granulation and structural stability of AGS are less well known. Therefore, further investigation on possible contributing components of EPSs in AGS is deemed necessary to resolve critical issues.
Our recent study discovered a significant decrease in tyrosine-like compounds in the EPS extracted from the AGS that disintegrated when it was exposed to a low COD/N ratio (=1) medium in a sequencing airlift bioreactor (SABR).9 The effect of tyrosine has been investigated on formation of biofilm, and mainly focused on L- and D-tyrosine. The L-tyrosine was found to be a precursor to N-acyl-tyrosine which is involved in bacteria signaling and control of biofilm formation.10 On the contrary, Kolodkin-Gal et al.11 reported that D-amino acids including D-tyrosine could prevent biofilm formation. Mixed effects were also observed in “L” isomers of various amino acids, including L-tyrosine; some promoted while some inhibited biofilm formation.12 An exploratory study on the role of L-tyrosine in aerobic sludge granulation while maintaining the structure integrity needs to be carried out. Aerobic sludge granulation and stability in a SABR dosed with L-tyrosine and that without L-tyrosine dosing as the control system were tested in parallel. Close monitoring of the physical, chemical and biological characterization of both AGS granules were performed. Findings from this study would shed light on the role of EPS in the AGS formation and stability.
2. Materials and methods
2.1 Reactor and operation
Aerobic sludge granulation was conducted in two identical SABRs (100 cm in height and 5 cm in diameter with a working volume of 1.1 L in each reactor). The SABRs were operated in parallel under the same conditions, one serving as the control reactor (Ra) free of L-tyrosine, while the other (Rb) tested the effect of L-tyrosine (Sigma Aldrich, St. Louis, MO, USA) dosed via its influent at a constant rate of 6 mg L−1, as adopted from a biofilm formation control study.11,13 A 2 L min−1 air flow rate was applied in each reactor, maintaining a superficial up-flow air velocity of 1.2 cm s.14 Both reactors had a 2.4 h operation cycle comprised of 6 min feeding, 120 min aeration, 5 min settling, 5 min decanting and 8 min idling. The volumetric exchange ratio of each reactor was set at 50%, corresponding to a hydraulic retention time (HRT) of 4.8 h. Details of the operation strategies are provided in Table A.1 in the ESI.†2.2 Feeding
Both reactors were inoculated with 2 g L−1 of activated sludge taken from a local sewage treatment plant in Hong Kong. Synthetic wastewater was prepared by diluting the synthetic stock solution (Table A.2 in ESI†) with tap water to set the influent COD concentration at a typical level, i.e. 400 mg L−1. Acetate, glucose and yeast comprised the source of organics. In order to decrease the COD/N ratio in the influent from 4 (phase I) to 1 (phase II), ammonium chloride was added from day 60 accordingly, in order to increase the influent ammonium concentration from 100 to 400 mg N per L in phase I and II respectively (Table A.3 in ESI†). The ratio of bicarbonate (NaHCO3) to ammonium-nitrogen (N) in the influent was fixed at 4 to maintain pH at 7.9. The ambient temperature in the laboratory was 23 ± 2 °C.2.3 Analytical methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230 and 250 s−1 were applied. The specific area and total pore volume of sludge were also examined by using an automatic instrument (ASAP 2020, Micrometrics) and then determined from the Brunauer–Emmett–Teller (BET) method.17
230 and 250 s−1 were applied. The specific area and total pore volume of sludge were also examined by using an automatic instrument (ASAP 2020, Micrometrics) and then determined from the Brunauer–Emmett–Teller (BET) method.17EPS content and component. EPS was first extracted from the sludge using a formaldehyde-NaOH method.18 The carbohydrate or polysaccharide (PS) in EPS was then quantified by using the phenol-sulfuric acid (PSA) method with glucose as the standard.19 The protein (PN) in EPS was determined from the modified Lowry colorimetric method with bovine albumin serum as the standard.20 A luminescence spectrometer (F-4500 FL Spectrophotometer, Hitachi) was used to examine the EEM spectra of extracted EPS for components identification with methodology applied as previously described.9
Low quality sequences were removed from raw sequence data by trimming the barcode tags and primer sequences. FASTA files were generated from the resulting sequences according to the barcodes of individual samples. The sequences were then aligned using the software Mothur ver. 1.17.0 (ref. 21) and the distance matrix was produced. Operational taxonomic units (OTU) were determined at 90, 95 and 97% similarities (Mothur v. 1.17.0). Rarefaction curves and diversity indices (ACE and Chao1) were determined from the calculated OTUs using the same software. In the taxonomy-based analysis, the representative sequences from each OTU were subjected to the RDP-II Classifier of the Ribosomal Database Project (RDP),22 the National Centre for Biotechnology Information (NCBI) BLAST,23 and the Greengenes Databases.24 The relative abundance and occurrence of the tags assigned into these three samples were visualized as a heat map using the Multi Experiment Viewer (MeV) v 4.8.1 software.
3. Results
3.1 Formation of aerobic granules
Disintegration of AGS induced by low COD/N ratio (i.e., COD/N = 1) has been confirmed in our previous work.9 Thus, to eliminate unexpected effects imposed by factors other from the L-tyrosine, both reactors were started up under an appropriate COD/N ratio of 4 in the formation phase (phase I). The seeding sludge was aerated for 2 days in both reactors prior to the inoculation. A short settling time (5 min) was then applied in both reactors. MLSS was intensively monitored during the start-up period. In the control reactor, a decrease of MLSS was observed within the first 18 days (from 2 to 1.4 g L−1), but the sludge in the test reactor showed excellent settling properties, enabling a continuous increase in MLSS from initially 2 to 6.4 g L−1 at day 76 (see Fig. 1a). | ||
| Fig. 1 Profile of sludge indices under granulation period (phase I) with COD/N ratio of 4 for: (a) MLSS, (b) mean diameter of granules, and (c) SVI5 and SVI5/SVI30. | ||
Fig. 1b shows the mean particle diameter of the sludge in each reactor. The mean particle size in the test reactor reached 200 μm after 7 days and then became stable at 2150 ± 20 μm after 32 days. The SVI5 of both reactors was initially 100 mL g−1. After 11 days' operation, SVI5 of the test reactor significantly decreased to 35 mL g−1 and subsequently remained at around 47 mL g−1 throughout the entire period. Conversely, the SVI5 of the control reactor stayed unchanged until day 39, and then gradually reduced to 76 mL g−1 after day 46. Apparently, granulation in the control reactor was much slower than that in the test reactor, though its sludge floc size was measured to be 200 μm or above from day 14. The ratio of SVI5 to SVI30 is often used to evaluate the extent of granulation, and when the value is close to unity full granulation is recognized.25 Fig. 1c illustrates that the ratio of SVI5/SVI30 was close to unity in the test reactor after 11 days, and 39 days were required for the control reactor, i.e. 28 days longer than the test reactor.
3.2 Stability of aerobic granular sludge
In our previous stability study of AGS, the granules underwent a condition of stepwise decrease in COD/N ratio from 4 to 1, and eventually disintegration occurred at a ratio of 1.9 Accordingly, after full granulation was achieved in these two reactors, the COD/N ratio was decreased from 4 at day 60 to 1 at day 120 to investigate the effect of L-tyrosine on the stability of aerobic granules, during which the key physical, chemical and biological characteristics of both AGSs were monitored closely. The respective results are summarized below. | ||
| Fig. 2 Profile of sludge indices in phase II with COD/N = 1: (a) mean diameter of granules, (b) SVI5 and SVI5/SVI30, and (c) MLSS. | ||
Meanwhile, the SVI5 of the control reactor increased from 55 ± 5 mL g−1 to 110 ± 10 mL g−1. However, that of the test reactor maintained at 50 ± 5 mL g−1 till the end of the experiment. According to the ratio of SVI5/SVI30, AGS in the control reactor disintegrated after day 88, while the test reactor maintained the extent of granulation during the whole operation in phase II with COD/N equal to 1 (see Fig. 2b). Due to the deterioration of the settling ability of AGS in the control reactor, half of the sludge was washed out, i.e. the MLSS concentration decreased from 6 g L−1 at day 94 to 3 g L−1 at the end of the experiment. Comparatively, the MLSS in the test reactor increased from 5.9 to 7.6 g L−1 (see Fig. 2c).
 | ||
| Fig. 3 Profile of the removal efficiencies in both reactors during the whole operation for (a) ammonia and (b) TN. | ||
The maximum oxidation rate of the ammonium and nitrite in both reactors in phases I (COD/N = 4) and II (COD/N = 1) were determined from the cycle tests conducted with a dissolved oxygen (DO) concentration fixed at 8.2 mg L−1. As shown in Table 1, the rates of the test reactor were determined to be 8.4 and 3.0 mg N per g VSS per h, 50 and 43% higher than that of the control reactor (5.6 and 2.1 mg N per g VSS per h) in phase I (COD/N = 4). The maximum oxidation rate of ammonia increased to 19.4 in the control reactor and 21.8 mg N per g VSS per h in the test reactor while the ratio of COD/N decreased to 1 in phase II. The nitrite oxidation rate in the test reactor maintained at 2.9 mg N per g VSS per h, but in the control reactor, it increased threefold to 6.1 mg N per g VSS per h due to the disintegration of AGS in control reactor after the COD/N ratio decreased to 1.
| Phase I, COD/N = 4 | Phase II, COD/N = 1 | |||
|---|---|---|---|---|
| Control reactor | Testing reactor | Control reactor | Testing reactor | |
| Max. NH4+ oxidation rate | 5.6 ± 0.2 | 8.4 ± 0.3 | 19.4 ± 0.5 | 21.8 ± 0.5 |
| Max. NO2− oxidation rate | 2.1 ± 0.1 | 3.0 ± 0.2 | 6.1 ± 0.2 | 2.9 ± 0.1 |
In phase I, TN loss in the control reactor (35 ± 5%) was greater than that (30 ± 5%) in the test reactor (see Fig. 3b), which could be ascribed to higher porosity of AGS in the testing reactor (Table 2) decreasing anaerobic zones in granules. When the COD/N ratio decreased from 4 to 1, the TN loss gradually reduced in this reactor due to disintegration of granules, however the TN loss in the test reactor still remained at between 20 and 30% when the ratio reached 1, with the stable porosity and surface area features of AGS maintained (see Table 2). The reasons for such stable properties of the granules is further discussed in Section 3.2.3. The COD removal efficiency of both reactors was maintained at more than 85% throughout the operation.
| COD/N | BET surface area (m2 g−1) | Total pore volume (mL per kg VSS) | ||
|---|---|---|---|---|
| Control reactor | Testing reactor | Control reactor | Testing reactor | |
| 4 | 1.9 ± 0.2 | 3.6 ± 0.4 | 9.0 ± 0.6 | 13.4 ± 1.2 |
| 1 | 4.5 ± 0.4 | 3.4 ± 0.3 | 18.1 ± 1.1 | 12.6 ± 0.9 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230 s−1 and 250 s−1 successively. This confirmed that the sludge in this reactor was of pure granular structure.16 However, when the same G-values were applied to the test reactor, break-up and re-coagulation were observed (see Fig. 4a) though the granules remained intact, indicating that flocculent sludge-like properties were incorporated in the granules in the test reactor. The same sludge behavior in the test reactor was observed in phase II (COD/N = 1), with a complete dispersion occurring in the control reactor (see Fig. 4b).
230 s−1 and 250 s−1 successively. This confirmed that the sludge in this reactor was of pure granular structure.16 However, when the same G-values were applied to the test reactor, break-up and re-coagulation were observed (see Fig. 4a) though the granules remained intact, indicating that flocculent sludge-like properties were incorporated in the granules in the test reactor. The same sludge behavior in the test reactor was observed in phase II (COD/N = 1), with a complete dispersion occurring in the control reactor (see Fig. 4b).
 | ||
| Fig. 4 Changes in the mean particle size of the granular sludge during the cohesion tests: (a) phase I (COD/N = 4), (b) phase II (COD/N = 1). | ||
The physical strength of the granules is usually negatively correlated with its porosity,26 which determines substrate transport and oxygen diffusion within the granules. Hence the porosities of the granules in both reactors under different COD/N ratios were examined, as shown in Table 2. With the COD/N ratio decreased from 4 to 1, the surface area (BET) and total pore volume of the granules in the control reactor clearly rose from 1.9 to 4.5 m2 g−1 and 9.0 to 18.1 mL per kg VSS respectively, in line with the results of the cohesion tests. Accordingly, the surface area and total pore volume of the granules in the test reactor were maintained at 3.5 m2 g−1 and 13.0 mL per kg VSS, respectively.
4. Discussion
4.1 Effect of L-tyrosine on formation and stability of AGS
The production of EPS, especially the PS and PN compounds, plays a key role in aerobic granulation and structural integrity via altering the physical–chemical properties of the cellular surface of AGS, such as hydrophobicity and charge.5,27 The change of certain components in EPS, e.g., tyrosine-like compounds fading out in the disintegrating AGS was revealed by Luo et al.9 In the present study, the effect of L-tyrosine on AGS is to: accelerate granulation (stage I) with L-tyrosine at 6 mg L−1, acquiring AGS formation within 11 days, i.e. 5 days shorter than the fastest granulation period ever reported (see Table 3); provide stable reactor performance; and provide structural integrity of AGS in terms of the changes in diameter and physical strength (phase II).4.2 The production of EPS
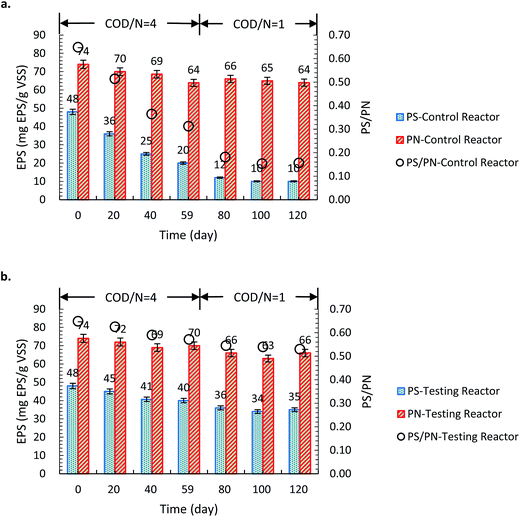
As shown in Fig. 5, in phase I (COD/N = 4) the PS content of the granules in the test reactor reached 38 mg per g VSS, two times greater than that in the control reactor. When the COD/N ratio reduced to 1 at day 60, the PS extracted from the granules in the test reactor slightly decreased to 35 mg per g VSS; comparatively it significantly decreased by 50% to 10 mg per g VSS in the control reactor, which in turn disintegrated the granules (Fig. 2). This indicated the importance of EPS in stability maintaining of the AGS. Stable secretion of EPS in the test reactor can be ascribed to three reasons. Firstly, the porous structure of the L-tyrosine-promoted granules. Higher porosity of the aerobic granules in the test reactor could relieve the limitation of mass transfer and thus maintained PS secretion in the center of the granules.1,4 Secondly, the L-tyrosine as substrate can be utilized by bacteria to synthesize N-acyl-tyrosine which promotes the production of EPS.10 Thirdly, the L-tyrosine is the main compound of the tyrosine kinase and tyrosine phosphatases which are vital to cell regulatory enzymes for a number of microbial processes, including EPS secretion.32At the end of experiment, a 3D-EEM analysis was conducted to identify the different tyrosine–EPS in both reactors. The results indicate that the tyrosine protein-like (region A) substances were observed in granules of both reactors in phase I. Subsequently the tyrosine protein-like contours faded out in the granules of the control reactor after the COD/N ratio decreasing from 4 to 1, but can be detected in the testing reactor throughout the reactor operation (see Fig. A.1 in ESI†). In our pervious study, the tyrosine protein-like compound in EPS correlated with the disintegration of aerobic granules under extremely low COD/N ratio. Therefore, the accumulation of tyrosine protein-like would be one of the reasons for the integrity structure of aerobic granules in testing reactor under phase II.
4.3 The improvement of microbial community
The microbial communities of the granules in both reactors were analyzed and compared by 16S rDNA pyrosequencing at the end of the experiment. Approximately 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 50
000 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 effective sequence tags were retrieved from the two types of granules (control and L-tyrosine-promoted). Sufficient sequencing was confirmed by the rarefaction curves (see Fig. A.2 in ESI†).
000 effective sequence tags were retrieved from the two types of granules (control and L-tyrosine-promoted). Sufficient sequencing was confirmed by the rarefaction curves (see Fig. A.2 in ESI†).
At the phylum level, Proteobacteria in L-tyrosine-promoted granules accounted for 80% much higher than the control granules (57%). This phylum is reported to associate with the production of N-acyl-tyrosine which can promote production of EPS.10 The abundance of Bacteroidetes (27%), which are responsible for secreting lectin-specific EPS (glycoconjugates) for cell attachment,33 was 3.5 times higher in the L-tyrosine-promoted granules than in the control granules (8%).
At the genus level (Fig. 6), the abundance of Flavobacterium (3.3%), Nitrosomonas (3.0%) and Thauera (15%) in the L-tyrosine-promoted granules were much higher than those of the control granules (0.2, 1.6 and 0.7%, respectively). Meanwhile, all of the aforesaid genera have been shown to contain species that positively correlate with the quorum sensing (QS) auto-inducers during granulation.34 The QS auto-inducers, especially N-acyl-homoserine-lactone (AHL), have been recently recognized as signaling molecules for biofilm development.34 These genera could be driving or accelerating the granulation when L-tyrosine is dosed. In addition, chemical similarities between AHL and N-acyl-tyrosine has been reported previously10 and they both can enhance the formation of biofilm. However connections between L-tyrosine, AHL and these genera are not clear yet, and further study is deserved.
 | ||
| Fig. 6 Taxonomic classification of bacterial 16s rDNA reads retrieved from sludge of the control and testing reactors at genera level. | ||
The portion of Flavihumibacter was larger in the L-tyrosine-promoted granules (1.9%) than the control granules (0.1%). Flavihumibacter belongs to filamentous bacteria which can accelerate aerobic granulation with high porosity by offering a structural network to aggregate the cells together.35 Therefore, the richness of Flavihumibacter with L-tyrosine dosing could be another cause for the high porosity of AGS in the test reactor.
AOB Nitrosomonas in the L-tyrosine-promoted and control granules accounted for 3 and 1.5% respectively. NOB Nitrospira and Nitrobacter were rare in both granules (<0.3%), and such low abundance of NOB reflects the accumulation of nitrite in both reactors. The minimum sludge retention times (SRTs) were calculated for both AOB and NOB, as shown in Table A.5 in ESI.† The mean SRT of both reactors was 8 days longer than the minimum SRT for AOB and NOB, i.e. 4.4 and 1.8 days. Tarre et al., (2007) indicated an optimum pH range for AOB and NOB growth of 7.0 to 8.5 and 7.3 to 7.5, respectively. The low NOB abundance is most likely caused by the influent pH of 7.5–8.0 in the reactors. Additionally, the highly porous structure of L-tyrosine-promoted granules is beneficial to the development of AOB abundance from which oxygen can easily penetrate into core area of the granules.
5. Conclusions
With dosing of L-tyrosine, test reactor achieved full granulation after 11 days' operation, and maintained structural integrity when decreased COD/N ratio from 4 to 1. Granules in test reactor were determined more porous than that in the control reactor, and this property potentially mitigates the mass transfer limitation in the granules. The stable secretion of EPS and the enrichment of genera related to the secretion of QS auto-inducers and filamentous in the test reactor were suggested as the main reasons for the positive effect of tyrosine on the granulation and stability of AGS.Acknowledgements
This work is partly supported by China Natural Science Foundation (38000-41030553).References
- S. S. Adav and D. J. Lee, J. Hazard. Mater., 2008, 154, 1120–1126 CrossRef CAS PubMed.
- M. K. de Kreuk, N. Kishida and M. C. M. van Loosdrecht, Water Sci. Technol., 2007, 55, 75–81 CrossRef CAS.
- K. Y. Show, D. J. Lee and J. H. Tay, Appl. Biochem. Biotechnol., 2012, 167, 1622–1640 CrossRef CAS PubMed.
- B. S. McSwain, R. L. Irvine, M. Hausner and P. A. Wilderer, Appl. Environ. Microbiol., 2005, 71, 1051–1057 CrossRef CAS PubMed.
- J. E. Schmidt and B. K. Ahring, Appl. Microbiol. Biotechnol., 1994, 42, 457–462 CAS.
- B. Long, C. Z. Yang, W. H. Pu, J. K. Yang, Y. F. Shi, J. Wang, J. Bai, X. Y. Zhou, G. S. Jiang, C. Y. Li and F. B. Li, Bioresour. Technol., 2014, 169, 244–250 CrossRef CAS PubMed.
- L. Zhu, M. L. Lv, X. Dai, Y. W. Yu, H. Y. Qi and X. Y. Xu, Bioresour. Technol., 2012, 107, 46–54 CrossRef CAS PubMed.
- Y. M. Lin, M. de Kreuk, M. C. M. van Loosdrecht and A. Adin, Water Res., 2010, 44, 3355–3364 CrossRef CAS PubMed.
- J. H. Luo, T. W. Hao, L. Wei, H. R. Mackey, Z. Q. Lin and G. H. Chen, Water Res., 2014, 62, 127–135 CrossRef CAS PubMed.
- J. W. Craig, M. A. Cherry and S. F. Brady, J. Bacteriol., 2011, 193, 5707–5715 CrossRef CAS PubMed.
- I. Kolodkin-Gal, D. Romero, S. Cao, J. Clardy, R. Kolter and R. Losick, Science, 2010, 328, 627–629 CrossRef CAS PubMed.
- S. N. Goh, A. Fernandez, S. Z. Ang, W. Y. Lau, D. L. Ng and E. S. G. Cheah, Journal of Biology and Life Science, 2013, 4, 103–115 CrossRef.
- H. J. Xu and Y. Liu, Water Res., 2011, 45, 5796–5804 CrossRef CAS PubMed.
- J. H. Tay, Q. S. Liu and Y. Liu, Water Sci. Technol., 2004, 49, 35–40 CAS.
- APHA, AWWA and WEF, Washington, D.C, 21st edn, 2005.
- J. F. Wan, I. Mozo, A. Filali, A. Line, Y. Bessiere and M. Sperandio, Biochem. Eng. J., 2011, 58–59, 69–78 CrossRef CAS PubMed.
- V. Fierro, F. V. Torne, D. Montane and A. Celzard, Microporous Mesoporous Mater., 2008, 111, 276–284 CrossRef CAS PubMed.
- H. Liu and H. H. Fang, J. Biotechnol., 2002, 95, 249–256 CrossRef CAS.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. A. Rebers and F. Smith, Anal. Chem., 1956, 28, 350–356 CrossRef CAS.
- B. Frølund, T. Griebe and P. H. Nielsen, Appl. Microbiol. Biotechnol., 1995, 43, 755–761 CrossRef.
- P. D. Schloss, S. L. Westcott, T. Ryabin, J. R. Hall, M. Hartmann, E. B. Hollister, R. A. Lesniewski, B. B. Oakley, D. H. Parks, C. J. Robinson, J. W. Sahl, B. Stres, G. G. Thallinger, D. J. van Horn and C. F. Weber, Appl. Environ. Microbiol., 2009, 75, 7537–7541 CrossRef CAS PubMed.
- J. R. Cole, Q. Wang, E. Cardenas, J. Fish, B. Chai, R. J. Farris, A. S. Kulam-Syed-Mohideen, D. M. McGarrell, T. Marsh, G. M. Garrity and J. M. Tiedje, Nucleic Acids Res., 2009, 37, 141–145 CrossRef PubMed.
- M. Johnson, I. Zaretskaya, Y. Raytselis, Y. Merezhuk, S. McGinnis and T. L. Madden, Nucleic Acids Res., 2008, 36, W5–W9 CrossRef CAS PubMed.
- T. Z. de Santis, P. Hugenholtz, N. Larsen, M. Rojas, E. L. Brodie, K. Keller, T. Huber, D. Dalevi, P. Hu and G. L. Andersen, Appl. Environ. Microbiol., 2006, 72, 5069–5072 CrossRef CAS PubMed.
- M. K. de Kreuk, J. J. Heijnen and M. C. M. van Loosdrecht, Biotechnol. Bioeng., 2005, 90, 761–769 CrossRef CAS PubMed.
- R. Lemaire, R. I. Webb and Z. G. Yuan, ISME J., 2008, 2, 528–541 CrossRef CAS PubMed.
- Y. Liu, S. Y. Yang, J. H. Tay, Q. S. Liu, L. Qin and Y. Li, Enzyme Microb. Technol., 2004, 34, 371–379 CrossRef CAS PubMed.
- X. H. Wang, M. H. Diao, Y. Yang, Y. J. Shi, M. M. Gao and S. G. Wang, Bioresour. Technol., 2012, 110, 105–110 CrossRef CAS PubMed.
- M. Pijuan, U. Werner and Z. G. Yuan, Water Res., 2011, 45, 5075–5083 CrossRef CAS PubMed.
- L. Liu, D. W. Gao, M. Zhang and F. Yuan, J. Hazard. Mater., 2010, 181, 382–387 CrossRef CAS PubMed.
- X. M. Li, Q. Q. Liu, Q. Yang, L. Gao, G. M. Zeng, J. M. Hu and W. Zheng, Bioresour. Technol., 2009, 100, 64–67 CrossRef CAS PubMed.
- A. J. Standish, A. A. Salim, H. Zhang, R. J. Capon and R. Morona, PLoS One, 2012, 7, 5 Search PubMed.
- M. B. Chiristin, R. N. Thomas, M. F. Bernhard and A. Rudolf, Syst. Appl. Microbiol., 2013, 36, 417–425 CrossRef PubMed.
- C. H. Tan, K. S. Koh, C. Xie, M. Tay, Y. Zhou, R. Williams, W. J. Ng, S. A. Rice and S. Kjelleberg, ISME J., 2014, 8, 1186–1197 CrossRef CAS PubMed.
- Y. M. Zheng, H. Q. Yu and S. J. Liu, Chemosphere, 2006, 63, 1791–1800 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra14596a |
| This journal is © The Royal Society of Chemistry 2015 |