Preparation of glucose-responsive and fluorescent micelles via a combination of RAFT polymerization and chemoenzymatic transesterification for controlled release of insulin
Lei Lia,
Guohua Jiang*abc,
Xiangxiang Dua,
Hua Chena,
Yongkun Liua,
Qin Huanga,
Xiangdong Kongd and
Juming Yaoabc
aDepartment of Materials Engineering, Zhejiang Sci Tech University, Hangzhou 310018, China. E-mail: ghjiang_cn@zstu.edu.cn; Tel: +86 571 86843527
bNational Engineering Laboratory for Textile Fiber Materials and Processing Technology (Zhejiang), Hangzhou 310018, China
cKey Laboratory of Advanced Textile Materials and Manufacturing Technology (ATMT), Ministry of Education, Hangzhou 310018, China
dCollege of Life Science, Zhejiang Sci Tech University, Hangzhou 310018, China
First published on 28th August 2015
Abstract
A glucose-responsive and fluorescent copolymer was prepared via a one-pot method that combines RAFT polymerization and enzymatic transesterification. The as-prepared copolymer tended to self-assemble into spherical micelles that were confirmed using 1H NMR, FT-IR and GPC, and transmission electron microscopy (TEM) and dynamic light scattering (DLS) analysis. Due to the attachment of fluorescent side groups, the optical properties of the micelles were analyzed using a UV-Vis spectrometer, fluorescence spectrophotometer and confocal laser scanning microscopy (CLSM). Owing to the amphiphilic and glucose-responsive properties, the micelles could be used as carriers for loaded insulin. The insulin release behaviour was evaluated when triggered by glucose in vitro. In addition, the resultant micelles exhibited relatively lower cytotoxicity and excellent stability against a protein solution. The insulin retained its structure stability after release, investigated through circular dichroism (CD) spectra analysis. These results showed that the obtained glucose-responsive and fluorescent polymer micelles with a good biological imaging performance and biocompatibility could be one of the effective candidate carriers for controllable release of insulin.
Introduction
Diabetes mellitus causes a series of clinical illnesses because of an absolute or relative deficiency of insulin, characterized by an accumulation of glucose in the blood.1–3 To maintain glucose homeostasis, diabetes requires multiple daily injections of exogenous insulin to control the concentration of glucose in blood, which often causes chronic pain and suffering.4,5 Therefore, it is imperative to develop self-regulated insulin delivery systems that can automatically release insulin in response to fluctuation of the blood glucose concentration.6 There are two kinds of typical glucose-responsive materials that are used for the controllable release of insulin: one method is to utilize protein-based biological components, such as glucose oxidase7–11 and concanavalin A,12 which can recognize or bind with glucose molecules. However, protein-based biological components may have problems such as instability, biotoxicity, and immunogenicity, which may hinder their functionality in glucose-triggered insulin delivery systems.13,14 The other is to use non-protein-based candidates, such as phenylboronic acid (PBA) and its derivatives, to form linkages with glucose.15 Due to their various chemical structures and stability, they have been widely investigated for applications in insulin delivery systems. Developing a vehicle based on PBA that can realize self-regulated release of insulin is of great interest for drug delivery systems.Various self-regulated insulin delivery systems based on PBA and its derivatives, such as modified insulin,16 micelles,17,18 gels,19 and films,20 have been investigated previously. Among them, the most commonly applied are smart micelles fabricated using PBA or its derivatives which are sensitive to glucose. For example, Chen et al.21 prepared phenylboronic acid functionalized block copolymers, monomethoxy poly(ethylene glycol)-b-poly(L-glutamic acid-co-N-3-L-glutamylamidophenylboronic acid) (mPEG-b-P(GA-co-GPBA)), by modifying mPEG-b-PGA with 3-aminophenylboronic acid (APBA). The resultant diblock copolymers could be self-assembled into micelles in phosphate buffer at physiological pH (pH 7.4). More interestingly, at pH 7.4, the hydrodynamic radii (Rh) of the micelles increased with an increase in glucose concentration by formation of hydrophilic PBA–glucose complexes. The in vitro release profiles revealed that the release of insulin from the micelles could be triggered by glucose, i.e. less insulin was released under a healthy blood glucose level (1 mg mL−1 glucose), while quick release occurred under diabetic blood glucose levels (above 2 mg mL−1 glucose). Our group prepared glucose-responsive amphiphilic copolymer micelles as drug carriers using 3-phenylpyruvic acid (3-PPA) or 2-ketobutyric acid (2-KBA) as a photoinitiator. The as-prepared polymeric micelles exhibited excellent glucose sensitivity. Loaded insulin could be released from micelles triggered by the regulation of temperature and glucose concentration in the environment.22–25 Polymeric micelles based on the copolymerization of poly (methoxypolyethylene glycol acrylamide) (MePEGA, macroinitiator), 3-acrylamide phenylboronic acid (APBA) and 2-nitrobenzyl acrylate (NBA) using a surfactant-free miniemulsion reversible addition fragmentation chain transfer (RAFT) polymerization method have been prepared to encapsulate insulin. The loaded insulin could be released from the micelles triggered by regulation of the UV light irradiation and different glucose concentrations. The insulin retained its structure stability after release.25
Although significant advances have been achieved for glucose-responsive polymeric micelles for controlled release of insulin over the past decade,26–29 multi-step reactions and time-consuming post-treatment activities need to be adopted for preparation of the micelles which limits their applications in biological systems. The combining of different (catalytic) reactions into a one-pot system should not only avoid tedious intermediate purification steps, but also provide a powerful and exquisite strategy for sophisticated polymer synthesis and modification.30–35 Herein, novel glucose-sensitive and fluorescent polymer micelles with a good biological imaging performance and biocompatibility were synthesized via a one-pot combination of surfactant-free microemulsion RAFT polymerization and enzymatic transesterification for the first time. The influence of glucose in solution on the morphology and hydrodynamic radius (Rh) of the micelles was studied. Insulin, a model drug, was loaded into the micelles, and its release can be triggered by the presence of glucose.
Experimental
Materials
Methoxypolyethylene glycol (PEG400, Aladdin Reagent Ltd. Co.), 2,2,2-trifluoroethyl methacrylate (TFEMA, J&K Chemical, 98%), 9-anthracenemethanol (AMOH, Aladdin Reagent Ltd. Co.), 3-aminobenzeneboronic acid (APBA, Aladdin Reagent Ltd. Co.) and candida antarctica lipase B (CALB, Beijing Cliscent Science and Technology Co., LTD) were used as purchased. Azobisisobutyronitrile (AIBN, 98%) was supplied by the East China Chemical Co., Ltd. (Shanghai, China) and purified by recrystallization twice from ethanol and dried in vacuum prior to use. The chain transfer agent S-1-dodecyl-S′-(α,α′-dimethyl-α′′-acetic acid) trithiocarbonate (DMP) were synthesized according to preciously published procedures.36 MilliQ water was used in all experiments.Synthesis of the polymeric micelles
The typical polymerization procedure is described as follows: TFEMA (0.21 g, 1.20 mmol), APBA (0.073 g, 0.53 mmol), AMOH (0.025 g, 0.12 mmol), PEG400 (0.22 g, 0.55 mmol), DMP (0.01 g, 0.0439 mmol), AIBN (0.01 g, 0.0439 mmol) and CALB (0.01 g) were added to a Schlenk tube with 10 mL of cyclohexane/water (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). The mixture was firstly stirred for 10 min at room temperature. Then, the mixture was degassed three times using a freeze–pump–thaw procedure. Finally, the tube was flame-sealed under vacuum and placed in a pre-heated oil-bath at 40 °C for 24 h. The final polymeric nanoparticles were obtained through dialysis (MWCO = 3500 Da) and freeze-drying with a yield of over 80%. The drug-loaded polymeric micelles were prepared using a similar route except that a certain amount of insulin (0.01 g, 0.0017 mmol) was added into the mixture before polymerization.
10). The mixture was firstly stirred for 10 min at room temperature. Then, the mixture was degassed three times using a freeze–pump–thaw procedure. Finally, the tube was flame-sealed under vacuum and placed in a pre-heated oil-bath at 40 °C for 24 h. The final polymeric nanoparticles were obtained through dialysis (MWCO = 3500 Da) and freeze-drying with a yield of over 80%. The drug-loaded polymeric micelles were prepared using a similar route except that a certain amount of insulin (0.01 g, 0.0017 mmol) was added into the mixture before polymerization.
Characterization
1H NMR spectroscopy was performed on an AVANCE AV 400 MHz Digital FT-NMR operating at 400 MHz using deuterated chloroform (CDCl3) as the solvent, and tetramethylsilane (TMS) as an internal standard. Fourier transform infrared (FT-IR) spectra were recorded on a Nicolet 5700 spectrophotometer using an ATR cell or KBr pellets for the samples. Dynamic light scattering (DLS) measurements were performed in aqueous solution using HORIBA Zetasizer LB-550V apparatus at 25 °C. Transmission election microscopy (TEM) was performed using a JEM-2100 TEM operated at an accelerating voltage of 200 kV, whereby a small drop of solution was deposited onto a copper EM grid and dried at 40 °C under atmospheric pressure. Gel permeation chromatography (GPC) analysis was carried out using a Waters 1525 pumping system (USA) at the flow rate of 0.5 mL min−1 with an Ultrahydrogel 500 column (Waters). The eluent was THF. UV-visible spectra were obtained using a Hitachi U-3010 spectrophotometer. Fluorescence spectra at room temperature were measured with a LS-45 fluorescence spectrophotometer. The morphology of the cells was observed by using an optical microscope (Leica, Germany) with an overall magnification of 100×. The cell uptake of the AM-APBA-PEG polymeric micelles was evaluated using confocal microscopic imaging. Briefly, the MC3T3 cells were seeded in a glass bottom dish with a density of 1 × 105 cells per dish. On the day of treatment, the MC3T3 cells were incubated with AM-APBA-PEG at a final concentration of 80 μg mL−1 for 3 h at 37 °C. Afterwards, the cells were washed three times with PBS in order to remove the AM-APBA-PEG and then fixed with 4% paraformaldehyde for 10 min at room temperature.In vitro drug release tests and the circular dichroism spectroscopy
Insulin-loaded polymer was injected into a dialysis bag with MWCO = 6000 Da, and then the dialysis bag was placed in PBS with different glucose concentrations (0, 1, 2, 3, 4, 5 mg mL−1). At certain time intervals, 1 mL of the buffer solution was taken to measure the insulin concentration, and then replaced by the same volume of release medium. The concentration was detected by measuring the UV-Vis absorbance at 235 nm. The activity and the structure stability of the released insulin were analysed using circular dichroism (CD), and the resulting spectrum was compared to that of standard free insulin. The free insulin was dissolved in 0.05 N HCl. The micelles containing insulin were prepared and allowed to release insulin for 36 h at 37 °C. CD measurements were carried out on a Jasco J-810 CD spectropolarimeter at 25 °C with a cell length of 0.1 cm. For the far-UV CD spectra, the samples were scanned from 190 to 250 nm and the results accumulated five times, at a resolution of 0.2 nm and scanning speed of 700 nm min−1.Cytotoxicity of the micelles
Cytotoxicity can be monitored using the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. The cytotoxicity experiments were carried out following a similar procedure reported previously. MC3T3 cells were selected and seeded onto 96-well plates at a density of 1 × 105 cells per well with 100 μL of growth medium containing 10% FBS. After 24 h of cell attachment, the cells were incubated with 10, 20, 40, 80, 120 or 160 μg mL−1 AM-APBA-PEG for 24 and 48 h, respectively. Three replicate wells were used per microplate, and the experiment was repeated three times. Cell viability (%) = (Ni/Nc) × 100, where Ni and Nc are the absorbance of the surviving cells treated with or without the AM-APBA-PEG polymeric micelles, respectively. The results are presented as the mean ± the standard deviation (SD).Results and discussion
Herein, a glucose-responsive and fluorescent copolymer, AM-APBA-PEG, is synthesized via a one-pot synthetic strategy that combines surfactant-free microemulsion reversible additional-fragmentation chain transfer (RAFT) polymerization and enzymatic transesterification. The synthetic strategy in the current report is schematically illustrated in Scheme 1. CALB lipase as an enzymatic catalyst was employed to catalyze the transesterification between the 2,2,2-trifluoroethyl methacrylate monomer (TFEMA) and primary alcohols 9-anthracene methanol (9-AMOH), hydrophilic polyethylene glycol (PEG-400) and amino-phenyl boronic acid (3-APBA), to form the respective target monomers R1 (AM)-methacrylate (AM-MA), R2 (APBA)-methacrylate (APBA-MA) and R3 (PEG)-methacrylate (PEG-MA). The new as-prepared monomers subsequently participated in microemulsion RAFT polymerization using S-1-dodecyl-S′′-(α,α′′-dimethyl-α′′-acetic acid) trithiocarbonate (DMP) as a RAFT agent and surfactant.37,38 The introduction of 9-AMOH endowed the obtained polymer micelles with fluorescence, while they also had good water solubility due to the hydrophilic PEG chains. Due to the hydrophobic properties of the side groups modified by 3-APBA and 9-AMOH, the obtained glucose-sensitive and fluorescent polymer is expected to self-assemble into polymeric micelles. During the process of the synthesis, the hydrophobic drug insulin also can be loaded into the self-assembled polymeric micelles.The formation and structure of the resultant copolymer was firstly verified using 1H NMR, as shown in Fig. 1A. The absence of the peak at 4.32 ppm from the methylene (–O–CH2–CF3) of TFEMA indicates that the transesterification reaction is nearly complete.39 The peaks at about 3.6 and 5.8 ppm are contributed by the protons from methylene (–CH2–) groups of the PEG and AM block, respectively. The weak peaks at about 7.0–8.0 ppm are the phenyl protons in the PBA and AM segments. Furthermore, the DMP and TFE segments also can be found due to swelling in the CDCl3, which indicates the AM-APBA-PEG polymeric micelles were successfully synthesized. Considering the integral values of the PEG segment protons (–CH2CH2–) at 3.65–3.74 ppm, the methylene (–CH2–) protons at 5.70 ppm, and the phenyl and anthracene protons at 7.00–8.51 ppm, the calculated ratio of the degree of polymerization of the AM-APBA-PEG copolymer is m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) p = 1.2
p = 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.0
5.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.8, which is close to the feed ratio. The number average molecular weight (Mn) of the final obtained polymer was about 1.6 × 104 g mol−1 with a narrow PDI (∼1.26) by gel permeation chromatography (GPC) measurement (Fig. 1B). The FT-IR spectra of the AM-APBA-PEG copolymer and AMOH are shown as Fig. 1C. The stretching vibrations (C–H) of the methyl and methylene groups are observed at 2919 cm−1 and 2873 cm−1.40 A series of absorbance bands are observed for AMOH located between 1400 cm−1 and 1500 cm−1, which can be assigned to the stretching vibrations of the polycyclic aromatic rings. After the reaction, an obvious –OH band of the AMOH dye located at 3400 cm−1 decreases. Meanwhile, some new bands appear within the range 1600–1000 cm−1, as shown in Fig. 1D. The AM-APBA-PEG copolymer displays a strong amide I band at 1542 cm−1 and a weak amide II band at 1261 cm−1 (in-plane N–H bending and C–N stretching).41 The peak at 1347 cm−1 can be assigned to B–O stretching.42 On the other hand, one characteristic peak located at 1097 cm−1 (the stretching vibration of C–O) emerged in the spectrum of the AM-APBA-PEG copolymer, further confirming the successful incorporation of PEG into the polymer. Besides, the carbonyl (C
5.8, which is close to the feed ratio. The number average molecular weight (Mn) of the final obtained polymer was about 1.6 × 104 g mol−1 with a narrow PDI (∼1.26) by gel permeation chromatography (GPC) measurement (Fig. 1B). The FT-IR spectra of the AM-APBA-PEG copolymer and AMOH are shown as Fig. 1C. The stretching vibrations (C–H) of the methyl and methylene groups are observed at 2919 cm−1 and 2873 cm−1.40 A series of absorbance bands are observed for AMOH located between 1400 cm−1 and 1500 cm−1, which can be assigned to the stretching vibrations of the polycyclic aromatic rings. After the reaction, an obvious –OH band of the AMOH dye located at 3400 cm−1 decreases. Meanwhile, some new bands appear within the range 1600–1000 cm−1, as shown in Fig. 1D. The AM-APBA-PEG copolymer displays a strong amide I band at 1542 cm−1 and a weak amide II band at 1261 cm−1 (in-plane N–H bending and C–N stretching).41 The peak at 1347 cm−1 can be assigned to B–O stretching.42 On the other hand, one characteristic peak located at 1097 cm−1 (the stretching vibration of C–O) emerged in the spectrum of the AM-APBA-PEG copolymer, further confirming the successful incorporation of PEG into the polymer. Besides, the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching vibration of the TFE segment is found at 1712 cm−1. These results suggest that the functional side groups were successfully introduced into the chain of the AM-APBA-PEG copolymer.
O) stretching vibration of the TFE segment is found at 1712 cm−1. These results suggest that the functional side groups were successfully introduced into the chain of the AM-APBA-PEG copolymer.
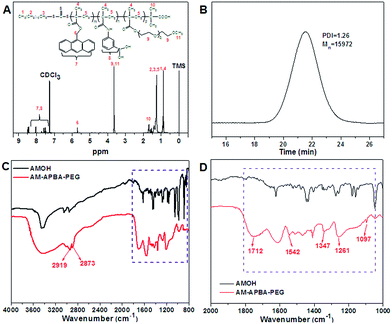 | ||
| Fig. 1 The 1H NMR spectrum (CDCl3) (A) and GPC trace (THF) (B) of the AM-APBA-PEG copolymer; FT-IR spectra of the AM-APBA-PEG copolymer and AMOH (C and D). | ||
Due to the amphiphilic properties, the copolymer in aqueous solution can be self-assembled into micelles with hydrophobic APBA and AMOH segments as the core, and the hydrophilic PEG segment as the shell. The diameters of the AM-APBA-PEG polymeric micelles are observed with a range from 70 to 90 nm, as determined using TEM analysis (Fig. 2A). This shows that these polymeric micelles have a core–shell structure spherical morphology (Fig. 2B). The relatively smaller size of the micelles is beneficial to their applications in biology, since micelles with a size under 200 nm are more likely to be taken up by cells.43,44 The micelles were found to have a hydrodynamic diameter around 68 nm, which was observed by DLS measurements at pH = 7.4 and a concentration of glucose of 0 mg mL−1, as shown in Fig. 2C. The diameter value is close to that of the TEM measurements. Inspired by the delicate composition and structure of most outer-cell membranes,45–47 the protein-adsorption-resistance properties of the as-prepared micelles have been investigated and utilized in creating a biomimetic surface/interface. The stability of the as-prepared micelles against 10% FBS solution was investigated by measuring the hydrodynamic diameter change as shown in Fig. 2D. The as-prepared micelles almost retained their original size even after incubating for 48 h in 10% FBS, due to the great stability against the protein solution. Thus this supports their potential stability when applied in vivo. To further confirm the glucose-response of the as-prepared micelles, the effect of glucose concentration on the hydrodynamic diameter was evaluated. As shown in Fig. 2C, when the concentration of glucose was increased to 1 mg mL−1, an average size of the micelles of around 106 nm can be observed. Further DLS studies of the micelles indicated that glucose-induced swelling occurred with increasing the glucose concentration. In particular, a diameter of ∼524 nm can be observed when the concentration of glucose increased to 5 mg mL−1. The general swelling process of the polymeric micelles in glucose aqueous solution is that when the glucose molecules permeate into the core of the polymeric micelles, the negatively charged APBA can combine with the 1,2-diols of glucose and form stable and soluble phenyl borates, which drives the equilibrium to the side for forming more combinations and results in the swelling of the polymeric micelles. This augurs well for the potential application of such micelles as nanosized drug delivery vehicles, since the abrupt change in the hydrophilicity of the cores is expected to allow “triggered release” of hydrophobic drugs.48
As shown in Fig. 3A, an AM-APBA-PEG sample in aqueous solution (0.02 wt%) shows a strong absorption band located at 252 nm. The AM-APBA-PEG micelles have a π–π conjugated structure so the absorption peak at 252 nm may be attributable to the electron transition of π → π*. It is noteworthy that the entire spectrum shows no absorption except for at the absorption wavelength of 252 nm, which indicates the excellent dispersibility of the AM-APBA-PEG micelles in water. Fig. 3B shows the fluorescence excitation and emission spectra of the AM-APBA-PEG micelles. The micelles in aqueous solution have an excitation band at 252 nm. Under light irradiation at 252 nm, three emission bands at 281, 391 and 412 nm can be observed. The intensity of the emission band at 412 nm is stronger than that of the peaks at 281 and 391 nm. For a more intuitive observation of the fluorescence properties of the as-prepared micelles, illumination photographs of the dispersed aqueous solution of micelles irradiated at 365 nm were recorded with a digital camera. A blue fluorescence phenomenon (left bottle) can be observed when the concentration is controlled at 0.5 wt%, as shown in the inset photograph of Fig. 3B-b compared to its aqueous solution and water (right bottle) as contrasts under a daylight lamp in Fig. 3B-a. We speculated that the fluorescence properties of the resultant polymers had a close relationship with their architecture.
The potential cell imaging applications of the AM-APBA-PEG polymeric micelles were further investigated based on the cell uptake behavior of AM-APBA-PEG by CLSM as shown in Fig. 3C. Strong fluorescence in the cytoplasm could be clearly observed after the MC3T3 cells were incubated with 80 μg mL−1 of AM-APBA-PEG for 3 h. Furthermore, the areas with relatively weak fluorescence intensity appear to be in the location of the cell nuclei. These preliminary results suggested that AM-APBA-PEG could be facilely taken up by cells and is mainly located in the cytoplasm. So we believe that AM-APBA-PEG could not enter into the cell nuclei directly. The cell morphology was observed in order to examine the morphological effects of the polymeric micelles on the MC3T3 cells. Thus, the MC3T3 cells were incubated with the AM-APBA-PEG micelle culture medium. When the concentration of AM-APBA-PEG was as high as 80 μg mL−1, optical microscopy observation demonstrated that the cells maintained their normal morphology (Fig. 3D). These preliminary results imply that AM-APBA-PEG has good biocompatibility.
The relative cytotoxicity of the micelles was estimated using a MTT viability assay against the MC3T3 cells. Fig. 4 shows the cell viability after 24 h and 48 h of incubation with the micelles at different concentrations of the polymeric micelles. When the tested concentration of micelles reached 160 μg mL−1, the viability of the cells still remained above 90%, indicating a low toxicity of the as-prepared micelles. Such low cytotoxicity of the AM-APBA-PEG polymeric micelles is partly due to limited organic solvents being involved during the surfactant-free miniemulsion RAFT polymerization and the excellent biocompatibility of the PEG chains at the surface of the micelles.49–51
 | ||
| Fig. 4 Viability of the MC3T3 cells against the AM-APBA-PEG polymeric micelles for 24 and 48 h using a MTT assay, data are presented as average standard deviation (n = 5). | ||
Insulin is hydrophilic in acid solution (pH = 2.0–3.0) and basic solution (pH = 8.0–8.5), but it is hydrophobic in the range of pH = 5.3–5.4 (the isoelectric point of insulin).52–54 When the pH is increased to 7.4, the majority of insulin is hydrophobic, and thus, insulin can be entrapped in the core of the micelles due to the hydrophobic interactions between PBA and insulin. The insulin loading capacity is 18.5% as determined using UV-Vis spectroscopy. The loaded insulin can be released under certain external conditions. To further confirm the controlled release behaviour of these polymeric micelles, we investigated the effect of glucose concentration on the stimuli-responsiveness of AM-APBA-PEG in PBS solution at 37 °C. As shown in Fig. 5A, the insulin release profiles exhibit glucose responsive characteristics. The higher the glucose concentration, the greater the degree of release observed for detection over 36 h. The insulin release percentage at the glucose concentration of 5 mg mL−1 is much higher than that at 0–4 mg mL−1. Only about 14% of the loaded insulin can be released from the polymeric micelles with a concentration of glucose of 0 mg mL−1. This is mainly caused by the permeation of insulin. With increasing the concentration of glucose, the release rate of insulin is increased as well. More than 55.3% of the loaded insulin can be released with a concentration of glucose of 2 mg mL−1. The highest cumulative amount of insulin released is ∼82% in the buffer solution after 36 h incubation with the glucose concentration of 5 mg mL−1. To test the bioactivity of the released insulin, CD spectroscopy was used to determine the conformation of the insulin after release from the micelles.55 CD spectroscopy is an efficient technique to evaluate the conformational changes in insulin. Free insulin shows two negative bands at 208 and 222 nm in the far-UV region (Fig. 5C), which matches with previous observations.56,57 Based on the principle that the ratio of the band at 208 nm arising from the α-helix structure to that at 222 nm from the β-structure ([Φ]208/[Φ]222) can be used to qualitatively measure the overall conformational structure of insulin, the standard insulin and the released insulin suspended in the aqueous solution were analyzed using a far UV-CD spectropolarimeter, respectively. No significant conformational change was detected for the insulin released from the polymeric micelles at pH 7.4 in comparison with the standard insulin, and the [Φ]208/[Φ]222 ratio for the standard insulin and released insulin is 1.12 and 1.23, respectively. Furthermore, no significant difference in the CD spectra was observed between the released insulin and standard insulin, indicating an undistorted secondary structure of insulin after release. In other words, the insulin released from the AM-APBA-PEG micelles could stay bioactive.
Conclusions
In summary, we have developed a facile one-pot method for preparation of an AM-APBA-PEG copolymer through controllable RAFT polymerization and enzymatic transesterification of APBA, a functional fluorescent dye (AMOH), and a widely used biomedical material (PEG). The as-prepared AM-APBA-PEG copolymer tended to self-assemble into spherical micelles with a core–shell structure due to its amphiphilic features. Owing to the micelle surface being covered with PEG, the obtained micelles showed excellent water dispersibility, great stability against FBS proteins and low toxicity against cells. Due to the conjugation of APBA side groups onto the chain of the copolymer, which are sensitive to glucose, the micelles could be further used as carriers for loaded insulin. The insulin release behaviour was evaluated when triggered by glucose in vitro. This strategy of combining RAFT polymerization and enzymatic transesterification technology provides an economical and efficient avenue for the fabrication of multi-functional polymeric materials by a one-pot route, which are promising as a potential candidate for controlled drug release in vivo.Conflict of interest
The authors declare no competing financial interest.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51373155) and the “521 Talents Training Plan” in Zhejiang Sci-Tech University (ZSTU).Notes and references
- P. K. Carmines, Curr. Opin. Nephrol. Hypertens., 2010, 19, 85 CrossRef CAS PubMed.
- C. George, A. Lochner and B. Huisamen, J. Ethnopharmacol., 2011, 137, 298 CrossRef CAS PubMed.
- W. Tai, R. Mo, J. Di, V. Subramanian, X. Gu, J. B. Buse and Z. Gu, Biomacromolecules, 2014, 15, 3495 CrossRef CAS PubMed.
- P. Buchwald, S. R. Cechin, J. D. Weaver and C. L. Stabler, Biomed. Eng. Online, 2015, 14, 28 CrossRef PubMed.
- Y. Yao, L. Zhao, J. Yang and J. Yang, Biomacromolecules, 2012, 13, 1837 CrossRef CAS PubMed.
- L. Li, G. Jiang, X. Du, T. Jiang, H. Chen and Y. Liu, J. Polym. Mater., 2015, 32, 79–87 Search PubMed.
- J. Liang, Y. Ma, S. Sims and L. Wu, J. Mater. Chem. B, 2015, 3, 1281 RSC.
- D. Meadows and J. Schultz, Anal. Chim. Acta, 1993, 280, 21 CrossRef CAS.
- R. Russell and M. Pishko, Anal. Chem., 1999, 71, 3126 CrossRef CAS.
- R. Rounds, B. Ibey, H. Beier, M. Pishko and G. Coté, J. Fluoresc., 2007, 17, 57 CrossRef CAS PubMed.
- S. Kang and Y. H. Bae, J. Controlled Release, 2003, 86, 115 CrossRef CAS.
- J. J. Kim and K. Park, J. Controlled Release, 2001, 77, 39 CrossRef CAS.
- J. Lakowicz and B. Maliwal, Anal. Chim. Acta, 1993, 271, 155 CrossRef.
- T. Koschinsky and L. Heinemann, Diabetes/Metab. Res. Rev., 2001, 17, 113 CrossRef CAS PubMed.
- V. Lapeyre, I. G. S. Chevreux and V. Ravaine, Biomacromolecules, 2006, 7, 3356 CrossRef CAS PubMed.
- H. Asada, T. Douen, M. Waki, S. Adachi, T. Fujita, A. Yamamoto and S. Muranishi, J. Pharm. Sci., 1995, 84, 682 CrossRef CAS PubMed.
- Y. Yao, X. Wang, T. Tan and J. Yang, Soft Matter, 2011, 7, 7948 RSC.
- Y. Yao, L. Zhao, J. Yang and J. Yang, Biomacromolecules, 2012, 13, 1837 CrossRef CAS PubMed.
- T. Hoare and R. Pelton, Biomacromolecules, 2008, 9, 733 CrossRef CAS PubMed.
- Z. Gan, H. Abe and Y. Doi, Macromol. Chem. Phys., 2002, 203, 2369 CrossRef CAS PubMed.
- L. Zhao, J. Ding, C. Xiao, P. He, Z. Tang, X. Pang, X. Zhuang and X. Chen, J. Mater. Chem., 2012, 22, 12319 RSC.
- G. Jiang, T. Jiang, Y. Wang, X. Du, Z. We and H. Zhou, RSC Adv., 2014, 4, 33658 RSC.
- G. Jiang, X. Du, Z. Wei, T. Jiang, H. Zhou and Z. Qiu, Eur. Polym. J., 2014, 60, 33 CrossRef CAS PubMed.
- X. Du, G. Jiang, L. Li, Y. Liu, H. Chen and Q. Huang, Colloid Polym. Sci., 2015, 293, 2129 CAS.
- G. Jiang, T. Jiang, H. Chen, L. Li, Y. Liu, H. Zhou, Y. Feng and J. Zhou, Colloid Polym. Sci., 2015, 293, 209 CAS.
- L. Sun, X. Zhang, C. Zheng, Z. Wu, X. Xia and C. Li, RSC Adv., 2012, 2, 9904 RSC.
- Y. Wang, X. Zhang, Y. Han, C. Cheng and C. Li, Carbohydr. Polym., 2012, 89, 124 CrossRef CAS PubMed.
- L. Zhao, X. Zhang, Y. Yao, C. Yu and J. Yang, Macromol. Chem. Phys., 2014, 215, 1609 CrossRef CAS PubMed.
- L. Zhao, C. Xiao, J. Ding, P. He, Z. Tang, X. Pang, X. Zhuang and X. Chen, Acta Biomater., 2013, 9, 6535 CrossRef CAS PubMed.
- C. Fu, C. Zhu, S. Wang, H. Liu, Y. Zhang, H. Guo, L. Tao and Y. Wei, Polym. Chem., 2013, 4, 264 RSC.
- S. Wang, C. Fu, Y. Zhang, L. Tao, S. Li and Y. Wei, ACS Macro Lett., 2012, 1, 1224 CrossRef CAS.
- L. Mespouille, M. Vachaudez, F. Suriano, P. Gerbaux, O. Coulembier, P. Degée, R. Flammang and P. Dubois, Macromol. Rapid Commun., 2007, 28, 2151 CrossRef CAS PubMed.
- F. F. Wolf, N. Friedemann and H. Frey, Macromolecules, 2009, 42, 5622 CrossRef CAS.
- Z. Huang, X. Zhang, X. Zhang, C. Fu, K. Wang, J. Yuan, L. Tao and Y. We, Polym. Chem., 2015, 6, 607 RSC.
- Z. Huang, C. Fu, S. Wang, B. Yang, X. Wang, Q. Zhang, J. Yuan, L. Tao and Y. Wei, Macromol. Chem. Phys., 2015, 216, 1483 CrossRef CAS PubMed.
- J. T. Lai, D. Filla and R. Shea, Macromolecules, 2002, 35, 6754 CrossRef CAS.
- X. Wang, G. Jiang, X. Li, B. Tang, Z. Wei and C. Mai, Polym. Chem., 2013, 4, 4574 RSC.
- G. Jiang, Y. Wang, R. Zhang, R. Wang, X. Wang, M. Zhang, X. Sun, S. Bao, T. Wang and S. Wang, ACS Macro Lett., 2012, 1, 489 CrossRef CAS.
- C. Fu, C. Zhu, S. Wang, H. Liu, Y. Zhang, H. Guo, L. Tao and Y. Wei, Polym. Chem., 2013, 4, 264 RSC.
- B. Sun, Y. Lin and P. Wu, Appl. Spectrosc., 2007, 61, 765 CrossRef CAS PubMed.
- S. H. Brewer, A. M. Allen, S. E. Lappi, T. L. Chasse, K. A. Briggman, C. B. Gorman and S. Franzen, Langmuir, 2004, 20, 5512 CrossRef CAS.
- R. M. Sankar, K. M. S. Meera and D. Samanta, Colloids Surf., B, 2013, 112, 120 CrossRef CAS PubMed.
- K. Y. Win and S.-S. Feng, Biomaterials, 2005, 26, 2713 CrossRef CAS PubMed.
- X. Wang, X. Sun, G. Jiang, R. Wang, R. Hu, X. Xi, Y. Zhou, S. Wang and T. Wang, J. Appl. Polym. Sci., 2013, 128, 3289 CrossRef CAS PubMed.
- T. Trongsatitkul and B. M. Budhlall, Colloids Surf., B, 2013, 103, 244 CrossRef CAS PubMed.
- K.-H. Hwangbo, Y.-J. Kim and K. Y. Cho, Appl. Surf. Sci., 2012, 263, 291 CrossRef CAS PubMed.
- M. J. Felipe, P. Dutta, R. Pernites, R. Ponnapati and R. C. Advincula, Polymer, 2012, 53, 427 CrossRef CAS PubMed.
- S. Liu, J. V. M. Weaver, M. Save and S. P. Armes, Langmuir, 2002, 18, 8350 CrossRef CAS.
- B. Ozcelik, A. Blencowe, J. Palmer, K. Ladewig, G. W. Stevens, K. M. Abberton, W. A. Morrison and G. G. Qiao, Acta Biomater., 2014, 10, 2769 CrossRef CAS PubMed.
- Y. Ikeda and Y. Nagasaki, J. Appl. Polym. Sci., 2014, 131, 40293 CrossRef PubMed.
- G. Jiang, J. Sun and F. Ding, J. Biomater. Sci., Polym. Ed., 2014, 25, 241 CrossRef CAS PubMed.
- M.-J. Zhang, W. Wang, R. Xie, X.-J. Ju, L. Liu, Y.-Y. Gu and L.-Y. Chu, Soft Matter, 2013, 9, 4150 RSC.
- S. Boduroglu, J. M. EI Khoury, D. V. Reddy, P. L. Rinaldi and J. Hu, Bioorg. Med. Chem. Lett., 2005, 15, 3974 CrossRef CAS PubMed.
- S. Li, E. N. Davis, J. Anderson, Q. Lin and Q. Wang, Biomacromolecules, 2009, 10, 113 CrossRef CAS PubMed.
- X. Jin, X. Zhang, Z. Wu, D. Teng, X. Zhang, Y. Wang, Z. Wang and C. Li, Biomacromolecules, 2009, 10, 1337 CrossRef CAS PubMed.
- K. Hinds, J. J. Koh, L. Joss, F. Liu, M. Baudyš and S. W. Kim, Bioconjugate Chem., 2000, 11, 195 CrossRef CAS PubMed.
- S. Lee, K. Kim, T. S. Kumar, J. Lee, S. K. Kim, D. Y. Lee, Y.-K. Lee and Y. Byun, Bioconjugate Chem., 2005, 16, 615 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |




