Benzofuran: an emerging scaffold for antimicrobial agents
Asha Hiremathad†
ab,
Mahadeo R. Patil†
a,
Chethana K. R.
a,
Karam Chand
b,
M. Amelia Santosb and
Rangappa S. Keri
*a
aCentre for Nano and Material Sciences, Jain University, Jain Global Campus, Jakkasandra post, Kanakapura Road, Ramanagara District, Bangalore 562112, Karnataka, India. E-mail: keriphd@gmail.com; sk.rangappa@jainuniversity.ac.in; Fax: +91 9620667075; Tel: +91 8027577199
bCentro de Química Estrutural, Instituto Superior Técnico, Universidade de Lisboa, Av. Rovisco Pais 1, 1049-001 Lisboa, Portugal
First published on 27th October 2015
Abstract
Resistance to antibiotics is a major global problem and there is an urgent need to develop new therapeutic agents. Although many classes of active compounds have been established as efficient derivatives in diverse fields of antimicrobial therapy, they have not yet found wide application against a few deadly microbes. In recent years, compounds have been developed that have solved some of the problems posed; for example improved bioavailability is one of the targets achieved with most of the more recent compounds, allowing for once-daily dosing. Benzofuran and its derivatives are found to be suitable structures, existing widely in natural products and unnatural compounds with a wide range of biological and pharmacological applications; thus, considerable attention has been focused on the discovery of new drugs in the fields of drug invention and development. Some benzofuran derivatives, such as psoralen, 8-methoxypsoralen and angelicin have been used in the treatment of skin diseases such as cancer or psoriasis. The unique structural features of benzofuran and its wide array of biological activities make it a privileged structure in the field of drug discovery, especially in the search for efficient antimicrobial candidates. Recently, this scaffold has emerged as a pharmacophore of choice for designing antimicrobial agents that are active toward different clinically approved targets. To pave the way for future research, there is a need to collect the latest information in this promising area. In the present review, we collated the published reports on this versatile core to provide a deeper insight, so that its full therapeutic potential can be utilized for the treatment of microbial diseases. This study systematically provides a comprehensive report on current developments in benzofuran-based compounds as antimicrobial agents and is also helpful for the researchers working on a substitution pattern around the nucleus, with an aim to help medicinal chemists to develop structure activity relationships (SAR) on these derivatives as antimicrobial drugs.
1. Introduction
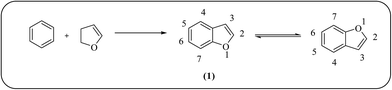
Contagious microbial diseases are increasing with the course of time around the world due to the emergence of new multidrug-resistant bacteria, resulting from the development of mutagenicity.1 They are a major cause of morbidity and mortality, especially in people who are immunosuppressed and patients who acquire them in hospitals.2 The challenge that is more crucial than ever is the conscious usage of the currently marketed antibiotics and also development of novel efficient antibiotic agents.3,4 In view of this, it is imperative to discover new chemotherapeutic agents to prevent the emergence of resistance and ideally shorten the duration of therapy. From this perspective, one of the best ways to design new antimicrobial agents is to synthesize/generate bioactive heterocyclic moieties using a single molecular scaffold.Heterocyclic ring systems are powerful backbones with many biological properties. Among the heterocyclic compounds, benzofuran derivatives are an important class of compounds, occupying a place in numerous bioactive natural products. Benzofuran was synthesized for the first time by Perkin in 1870.5 Subsequently, the research and development of benzofuran-based biologically active compounds has been a rapidly developing and increasingly active field due to their wide potential applications as medicinal drugs (pharmaceuticals), agrochemicals, molecular electronic and functional polymers, man-made materials, artificial acceptors, and supramolecular ligands.6 In particular, the application of benzofuran derivatives in medicinal chemistry has achieved great progress. Many benzofuran-anchored drugs play an important role in the treatment of various types of diseases and new benzofuran derivatives with medicinal value are being actively explored worldwide.7 The accepted name for ring system 1 (shown in Fig. 1) in chemical abstracts is benzo(b)furan. To shorten this, (b) is conveniently dropped and it is commonly known by the generic name benzofuran. The name, coumarone, used in earlier literature for this nucleus is now rejected. The positions of benzofuran ring are numbered starting from heteroatom, as shown in Fig. 1.
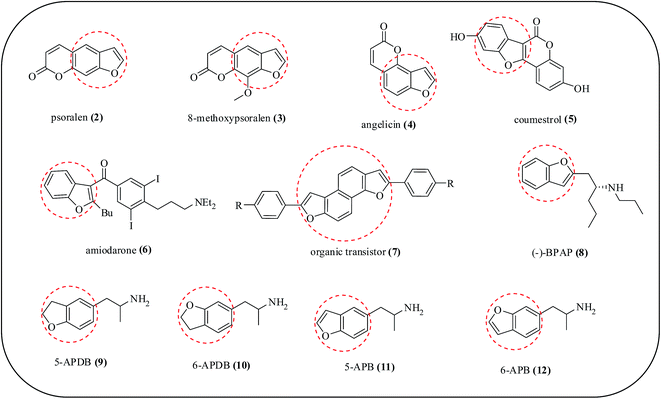
Benzofuran derivatives exhibit significant activity including antifungal,8,9 antiprotozoal,8 antitubercular,10 antiinflammatory,11 anticonvulsant,12 anticancer,13 antiHIV,14 analgesic,15 antiparasitic,16 antihyperlipidemic,17 antioxidant,18 antidiabetic,19 antihypertensive,20 antiplasmodial,21 anti-Alzheimer's,22 vasodilating and hypotensive,23 and antiarrhythmic.24 Moreover, some of the benzofuran derivatives have been demonstrated to be potent topoisomerase I inhibitors,25 sigma receptors,26 Pim-1 inhibitors,27 farnesyl transferase inhibitors,28 histamine H3 receptors29 and carbonic anhydrase inhibitors.30 Moreover, this heterocyclic compound is found in various branches of chemical research, namely, in polymer31,32 and dye industries33,34 and in silver photography.35,36 Benzofuran derivatives are important heterocycles frequently found in both bioactive compounds and organic materials. A few benzofuran derivatives that are actively used in the pharmacological field are illustrated in Fig. 2. The members of the furocoumarin class of natural products including psoralen 2, 8-methoxypsoralen 3 and angelicin 4 can cross-link with DNA upon light irradiation. As a result, they have been used for the treatment of skin diseases such as cancer or psoriasis.37–40 The natural product coumestrol 5 is found especially in soya beans and has estrogenic activity.41 Synthetic bioactive compounds containing benzofurans are also important, as exemplified by amiodarone 6, which is an antiarrhythmic drug.42,43 Recently, benzofurans have also emerged as important structural elements for organic materials such as the organic transistor 7.44 (−)-1-(Benzofuran-2-yl)-2-propylaminopentane {(−)-BPAP} 8 is another benzofuran-based drug used as a catecholaminergic and serotonergic activity enhancer. This stimulates the impulse propagation mediated transmitter release of the neurotransmitters dopamine, norepinephrine and serotonin in the brain.45 5-(2-Aminopropyl)-2,3-dihydrobenzofuran (5-APDB) 9 and 6-(2-aminopropyl)-2,3-dihydrobenzofuran (6-APDB) 10 are putative entactogen drugs of the phenethylamine and amphetamine classes.46 5-(2-Aminopropyl)benzofuran (5-APB) 11 and 6-(2-aminopropyl) benzofuran (6-APB) 12 are triple monoamine reuptake inhibitors47,48 and 5-APB is also an agonist of the 5-HT2B receptor.48 This makes it likely that 5-APB would be cardiotoxic with long term use, as seen in other 5-HT2B agonists such as fenfluramine and MDMA. 6-APB is an entactogenic compound of the phenethylamine and amphetamine classes.49
Therefore, the vast range of biological effects associated with this scaffold has resulted in the benzofuran ring system being considered as a privileged structure. This has resulted in considerable effort being focused on benzofuran-based medicinal agents and the expanding research and developments have become rapidly developing and increasingly active domains of research and are extended to almost the whole range of medicinal field. To develop more effective and less toxic agents to treat infectious diseases is still a challenge for the pharmaceutical chemist. A large amount of effort has been invested in the past decade to develop benzofuran-based compounds as microbial agents that are active on different clinically approved therapeutic targets and show excellent therapeutic potency.
A spectrum of pharmacological activities exhibited by benzofuran and its derivatives has been reviewed by some researchers;50 however, in this review, we focused on the applications of benzofuran scaffolds (as organic molecules, inorganic complexes and naturally occurring compounds) as antimicrobial agents in detail. By looking into the importance of this therapeutic area, we decided to collect literature on the antimicrobial potency of benzofurans, the indispensable anchor in medicinal chemistry. In this review, we have attempted to shed light and compile published reports on benzofuran derivatives along with some opinions on different approaches to help the medicinal chemists in designing future generation potent yet safer antimicrobial agents. The purpose of this review is to update the most recent reports on the development of microbial agents as potential drug candidates for the treatment of infectious diseases.
2. Chemistry of benzofuran
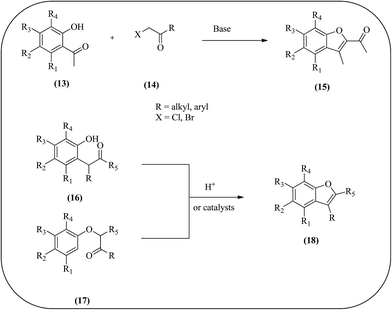
Benzofurans are a class of very important heterocyclic compounds existing widely in natural products and unnatural compounds with biological and pharmacological potentials. It is therefore not surprising that an enormous amount of research has been carried out to develop efficient synthetic methods for their assemblies. The most pertinent and often employed strategies involve the one-pot etherification and dehydrative cyclization of o-hydroxyacetophenones 13 under basic conditions.51 Other strategies include the dehydrative cyclization of o-hydroxybenzyl ketones 16 or R-(phenoxy)alkyl ketones 17 (Fig. 3)52 and the cyclization of aryl acetylenes using transition-metal catalysis.53 Although numerous synthetic approaches have been developed to prepare this family of compounds during the past decades, general protocols for the synthesis of these compounds are still of high interest.503. Structural requirements of benzofuran derivatives for antimicrobial activity
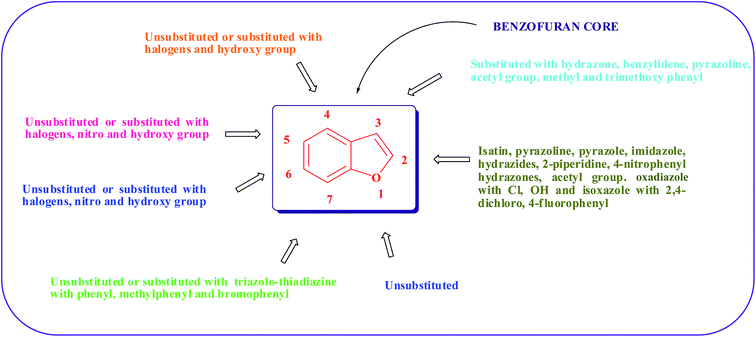
A close proximity exists between the chemistry of furan and benzofuran. The greater stability of benzofuran compared with furan is due to annelation of the benzene ring. Similar to furan, oxygen contributes 2π-electrons to form a 10-π-electron system in the case of benzofuran. This compound belongs to a group that is commonly known as “electron rich” or “π excessive” heteroaromatics. As anticipated of such compounds, the benzofuran ring is highly reactive towards electrophilic substitution; however, the overall reactivity of the furan ring in benzofuran is decreased by the annelated benzene ring. Resonance considerations of such condensed systems indicate that electrophilic substitution should occur at C-3. This is true with the analogous heterocycle, indole and to some extent with thionaphthene. However, benzofuran undergoes electrophilic substitution almost exclusively at the C-2 position, in contrast to the general prediction. This unusual difference in orientation between benzofuran and thionaphthene is associated with the electronegativity of oxygen and sulphur. Because oxygen is more electronegative than sulphur, the unshared electrons around oxygen are held more tightly than those of sulphur. Thus, the strongly electronegative character of oxygen cuts down the extent to which an unshared pair interacts with the two double bonds to form the aromatic system. Therefore, benzofuran behaves to a considerable extent like an olefin. Due to this distortion of electrons, ionic structure 18 is of greater importance in benzofuran (Fig. 3) and consequently electrophilic substitution at the C-2 position is favored. However, in thionaphthene, the electronegativity of sulphur is secondary to the stabilizing influence of benzene resonance, and the ionic structure 20 with a negative charge at the C-2 position is of greater importance (Fig. 4).54From the collected literature, it is found that the benzofuran nucleus substituted at all positions with various substituents produces potent antimicrobial activity; however, the 1-position contains core oxygen and therefore it is unsubstituted. The 2-position of benzofuran may be unsubstituted, or isatin, pyrazoline, pyrazole, imidazole, oxadiazole with –Cl, –OH, hydrazides, 2-piperidine, 4-nitrophenyl hydrazones, acetyl group and 2,4-dichloro-isoxazole, 4-fluorophenyl-isoxazole substituents may enhance the antimicrobial activity. Similarly, good activity was observed when the 3-position was substituted with hydrazone, benzylidene, pyrazoline, acetyl group or methyl group. The 4-position of the benzofuran may be substituted or unsubstituted but good antimicrobial activity was exhibited when the substituent on the 4-position contained halogens or hydroxyl groups. The 5- or 6-position of the nucleus may be unsubstituted, and substituents with halogens, nitro and hydroxyl groups have also displayed potent antibacterial activity. To exhibit antibacterial activity, it is essential that benzofuran contains halogens, nitro and hydroxyl groups at positions 4, 5, and 6. Benzofuran with triazolo-thiadiazine containing phenyl, methylphenyl or bromophenyl at the 7-position displayed good antimicrobial activity (Fig. 5).
4. Benzofuran derivatives as antimicrobial agents
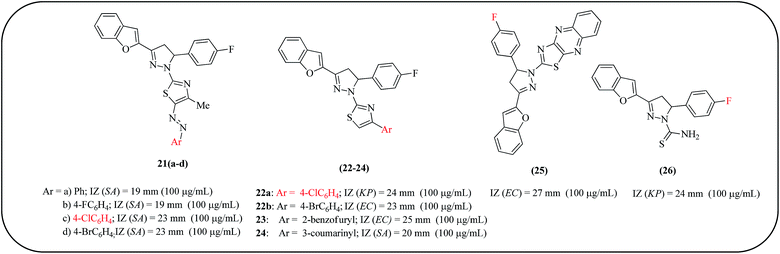
Nowadays, a number of common and uncommon bacteria previously susceptible to common antimicrobials are reported to have developed resistance to diverse antibiotics. Initially, these bacteria caused significant nosocomial infections and were the cause of major morbidity and mortality in patients. More recently, they have spread to the community, causing severe illness in previously healthy and otherwise non-vulnerable patients.55–58 Owing to this increased microbial resistance, new classes of antimicrobial agents with other modes of action are needed to fight against the recent multidrug-resistant infections.Mohamed et al. reported the synthesis of a series of benzofuran-based pyrazoline-thiazoles 21(a–d) (Fig. 6) and fluorinated pyrazole-thiazole (22–26) derivatives and tested their potential antimicrobial activities against four Gram-positive bacteria (Staphylococcus aureus (S. aureus (SA)), Bacillus subtilis (B. subtilis (BS)), Bacillus megaterium (B. megaterium (BM)), Sarcina lutea) and Gram-negative bacteria (Klebsiella pneumoniae (K. pneumoniae (KP)), Pseudomonas aeruginosa (P. aeruginosa (PA)), and Escherichia coli (E. coli (EC))). Among these compounds, 21c displayed excellent antimicrobial activity and highest potency against all the tested organisms with respect to reference drugs (ciprofloxacin and ketoconazole). Compounds 21d and 22b inhibited the growth of S. aureus with inhibition zones (IZ) of 23 and 20 mm, respectively, while compound 22a evidenced promising antifungal activity against K. pneumoniae, P. aeruginosa, and E. coli with an IZ of about 24 mm. Moreover, compound 21c showed the highest activity against S. aureus, S. cerevisiae, and C. albicans with an IZ of about 23 mm. A SAR study revealed that the presence of a -chloro substituent on pyrazoline and pyrazole moieties (21c and 22a) increases the antimicrobial activity (Fig. 6).59
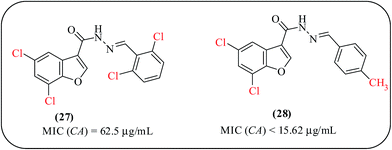
In the literature, we found that the antimicrobial activity of benzofuran derivatives appears to be more dependent on substitution at the heterocyclic furan ring than on substitution at the aromatic moiety.60 Telvekar and co-workers investigated a series of benzofuran-3-carbohydrazide derivatives and screened for their potential antifungal activity against candida albicans (C. albicans). The combination of unsubstituted benzylidene and a chloro di-substituted benzofuran ring exhibited greater activity compared to the activity of other members. In the case of a benzofuran derivative substituted with two electron-withdrawing groups 27, the activity was increased around fourfold. The electron-withdrawing group on the benzofuran ring and the electron-donating group on the para position of the aromatic side chain showed good activity 28 (Fig. 7).61
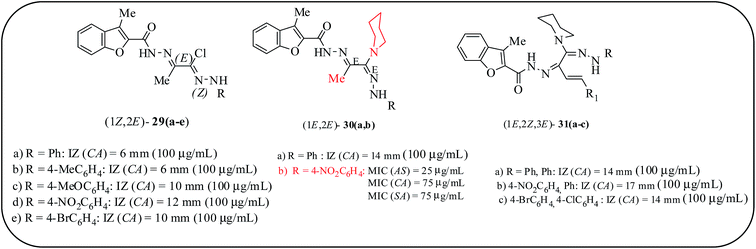
Structural modification of clinically approved benzofuran-based antimicrobial drugs is an effective strategy to increase biological activity and broaden the active spectrum of current drugs. Therefore, there are some reports on amidrazone derivatives that were found to possess remarkable biological activities62,63 and additionally these compounds have been reported as precursors of some effective antifungal azoles.64 Abdel-Aziz and co-workers presented the synthesis of benzofuran-amidrazones 29(a–e), 30a, b and 31(a–c) (Fig. 8) and screened their antifungal/antibacterial activities. These derivatives showed significant antifungal potency, which was more than their antibacterial potency. Compound 30b exhibited the highest potency against all the tested fungal organisms with respect to a reference drug, griseofulvin. C. albicans (MIC = 75 μg mL−1) was the most sensitive fungus to griseofulvin (MIC = 75 μg mL−1), followed by Aspergillus fumigatus (A. fumigatus), Geotrichum candidum (G. candidum) and Syncephalastrum racemosum (S. racemosum). Moreover, the same compound exhibited activity (MIC = 75 μg mL−1) against S. aureus, which is a little less than the standard drug amoxicillin (MIC = 50 μg mL−1). A SAR study revealed that the piperidine moiety and 4-nitrophenylhydazone functions are essential for potential antimicrobial activity. Piperidines, which are frequently found in the side chains of therapeutic agents, are not usually associated with pharmacophores; they simply serve as surrogates for open-chain tertiary amines (Fig. 8).65
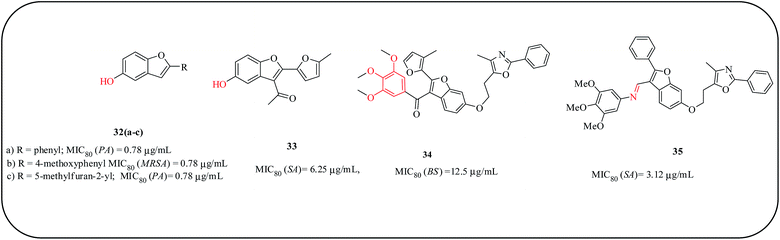
3-Methanone-6-substituted-benzofuran derivatives were synthesized by Liu and co-workers and evaluated for their in vitro antibacterial activities against E. coli, S. aureus, Methicillin-resistant Staphylococcus aureus (MRSA), B. subtilis, and P. aeruginosa. Some of the compounds 32(a–c) and 33 with MIC80 = 0.78–12.5 μg mL−1 displayed excellent antibacterial activities compared to the positive controls (cefotaxime and sodium penicillin). Compounds 34 and 35 displayed strain-specificity to S. aureus with MIC80 = 3.12–12.5 μg mL−1. SAR studies revealed that the hydroxyl group at the C-6 position of benzofuran is essential for antibacterial activity, and the functional groups at the C-3 position play an important role in the antibacterial selectivity of these compounds (Fig. 9).66
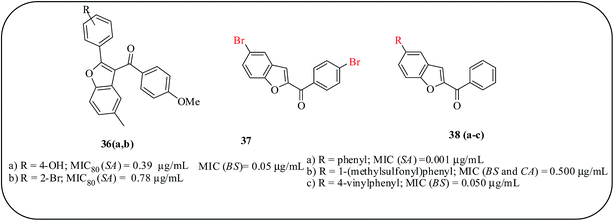
Furthermore, a benzofuran skeleton bearing aryl substituents at the C-3 position through a methanone linker has also been investigated as an antibacterial agent. Compound 36a with a hydroxyl group at the C-4 position showed moderate antibacterial activity against S. aureus (MIC80 = 0.39 mg mL−1) and MRSA (MIC80 = 0.78 mg mL−1), compared to the positive control drugs. On the other hand, compounds with methylation of the hydroxyl group had reduced solubility and decreased antimicrobial abilities, and compounds with halogen substituents showed no antimicrobial activity, whereas compound 36b had favorable activity against B. subtilis (MIC80 = 0.78 mg mL−1).67 Chandrashekar et al. demonstrated the synthesis and antimicrobial applications of 5-phenyl-1-benzofuran-2-yl derivatives and the biphenyl methanones 37 and 38(a–c). These derivatives exhibited antimicrobial activities with MIC values ranging between 0.001 and 0.5 μg mL−1. The carbinols and tertiary alcohols corresponding to methanone exhibited no antimicrobial activity (Fig. 10).68
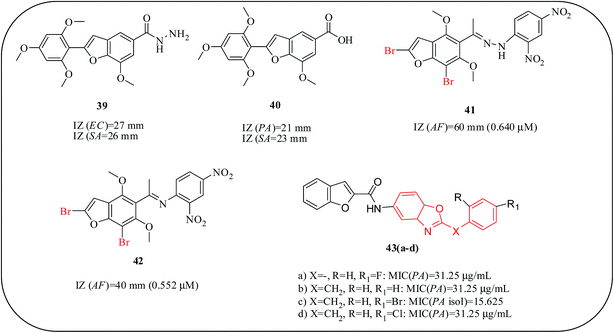
Rao and co-workers reported a series of 2,4,6-trimethoxy benzofuran derivatives as antibacterial agents. The synthesized compound, benzofuran carbohydrazide, 39, displayed excellent activities with IZs of 27 mm and 26 mm against E. coli and S. aureus, respectively, at 25 μg mL−1, when compared with the standard drug norfloxacin (IZ = 25 mm) for the same strains. Moreover, benzofuran carboxylic acid, 40, showed a remarkable activity against P. aeruginosa (IZ = 21 mm) and S. pyogenes (IZ = 23 mm), although a SAR study has not been reported for these compounds.69 Soliman and co-workers described the synthesis of naturally occurring visnagin derivatives and tested for their antimicrobial activity against different bacterial and fungal strains. Surprisingly, these compounds showed better antimycotic activities than the reference drug, fluconazole. In particular, compounds 41 (0.64 μM) and 42 (0.532 μM) demonstrated promising antifungal activity. The tested compounds exhibit higher antifungal activity against A. flavus than against C. albicans.70 A series of benzyl-benzofurylcarboxamido benzoxazole derivatives are reported by Yildiz et al. and their antimicrobial activity was determined against some Gram-positive and Gram-negative bacteria and fungi. Among the tested derivatives, 43(a–d) exhibited higher activity against P. aeruginosa than ampicillin (MIC > 500 μg mL−1), rifampicin (MIC > 500 μg mL−1) and ofloxacin (MIC = 62.5 μg mL−1) and had the same potency as gentamycin (MIC = 31.25 μg mL−1). Compound 43c displayed promising activity with an MIC value of 15.6 μg mL−1 against drug-resistant P. aeruginosa, which is more potent than all standard drugs. Compound 43b, carrying a benzyl group at the second position of the benzoxazole ring, showed a remarkable antifungal activity. In addition, attaching a hydrogen or bromine on position “R” played a role in obtaining good inhibitory potency. However, substituting position R1 with a bromine atom, while the main structure was 2-phenylbenzoxazole, decreased the inhibitory effect (Fig. 11).71
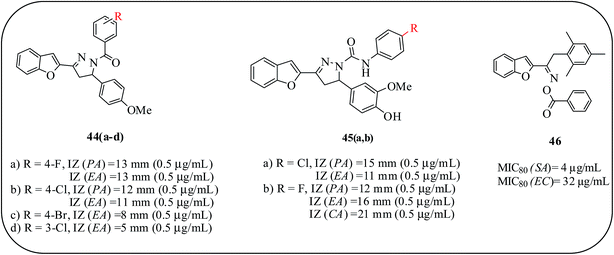
Rangaswamy and co-workers reported the synthesis of a new class of functionalized benzofuranyl methoxy phenyl pyrazole scaffolds. The synthesized compounds were evaluated for antibacterial and antifungal activities by the well plate method in Mueller-Hinton agar. The -chloro, -fluoro, -bromo, and -nitro functionalized derivatives demonstrated better activity, while the others showed less activity against all the tested bacterial strains. Compounds 44a (p-fluoro substituted) and 44b (p-chloro substituted) on the 1-substituted phenyl ring showed significant activity against P. aeruginosa and E. coli at concentrations of 1 and 0.5 μg mL−1, which was almost 15 times better than the standard drug, streptomycin (12 mm and 17 mm at 0.5 μg mL−1). Compounds 44c (p-bromo substituted) and 44d (m-chloro substituted) exhibited similar activity to that of the standard drug against E. coli. As for antibacterial activities, the presence of halogen substituents on the phenyl ring produced better antifungal activity compared to other analogues.72
Furthermore, a series of benzofuran-based pyrazole derivatives as antibacterial/antifungal agents are reported. The introduction of substituted anilines into the pyrazole ring enhanced the antibacterial activity. Compounds 45a and 45b, possessing p-chloro and p-fluoro substituents on the 1-substituted phenyl ring, showed excellent activity against P. aeruginosa and E. coli at concentrations of 1 and 0.5 μg mL−1. The activity is considerably affected by halogen substituents present at the para position of the phenyl ring. In the case of compound 45b, fluoro substituent on the para-position of phenyl ring accounted for the enhanced antifungal activity against C. albicans as compared to the standard drug, fluconazole.73 Kirilmis and co-workers designed mesitylene-substituted benzofuran derivatives for antimicrobial activity against S. aureus, E. coli and C. albicans. Among the synthesized compounds, (E)-1-(1-benzofuran-2-yl)-2-mesitylethanone-O-benzoyloxime (46) was found to be the most effective derivative against S. aureus and E. coli at MIC values of 4 and 32 μg mL−1, respectively (Fig. 12).74
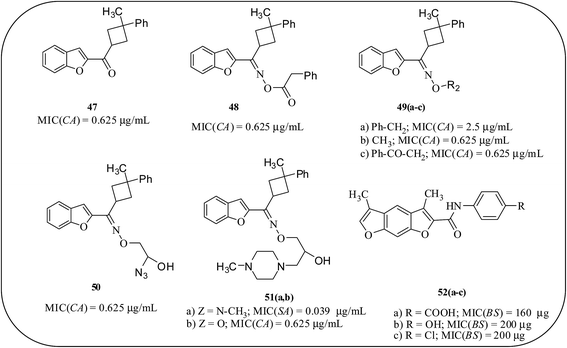
Koca et al., developed a series of benzofuran-ketoxime derivatives as effective antimicrobial agents and among the reported ones, compound 51a exhibited promising activity (MIC = 0.039 μg mL−1) against S. aureus. The compounds 47, 48, 49b, 49c, 50 and 51b revealed almost the same antimicrobial effect against C. albicans (0.625 μg mL−1). Compound 49a displayed a good antimicrobial effect against C. albicans (2.5 μg mL−1).75 The synthesis and antimicrobial efficacies of a series of benzodifuran amide derivatives are reported by Soni et al. These compounds exhibited higher MIC values (600 μg mL−1) against Gram-negative bacteria P. aeruginosa and fungus C. albicans. A SAR study revealed that the presence of an electron-withdrawing group at the para position of amine is responsible for their moderate activity against Gram-positive bacteria B. subtilis (52a–c), while the presence of an electron-releasing group at the para position of the amine resulted in moderate activity against S. aureus (Fig. 13).76
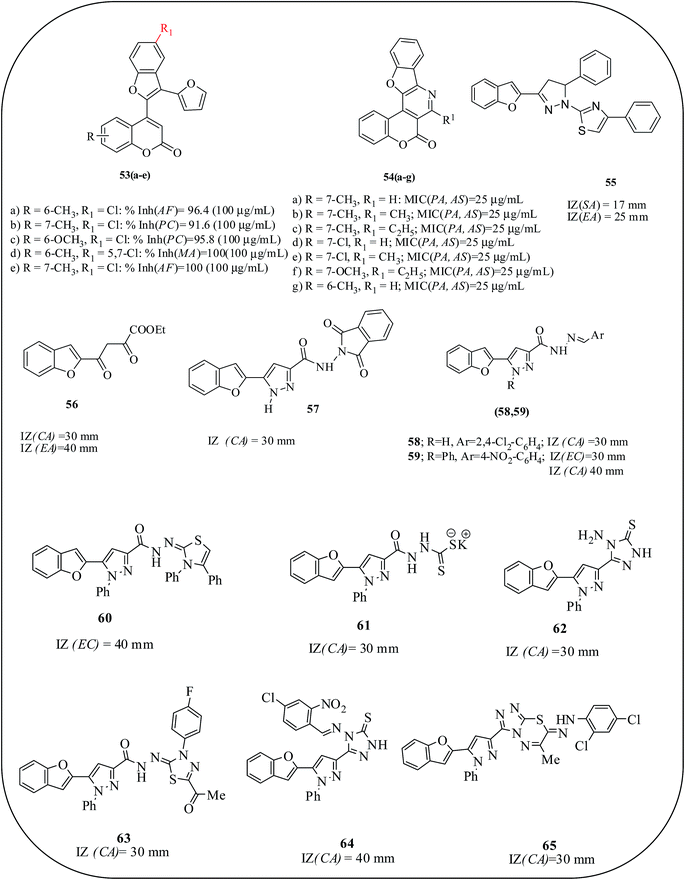
Triheterocycles with coumarin, benzofuran and furan rings are reported by Khan and co-workers. The synthesized compounds were screened against two bacterial and two fungal species by the standard cup plate method. Introduction of monochloro and dichloro substituents on the benzofuran ring enhanced the antimicrobial potency against Pseudomonas chinchori, A. fumigatus, and P. wortmanni. Among the reported compounds, 53a–e exhibited considerable inhibition of the microbial growth of all the species at 50 μg mL−1 concentration. SAR studies showed that the chlorination of the benzofuran ring enhances both the antibacterial and antifungal potencies of the triheterocycles.77 Angularly fused polycyclic heterocycles with coumarin, benzofuran and pyridine rings 54a–f are also reported as antimicrobial agents. All the synthesized compounds exhibited anti-microbial activity against P. chinchori at an MIC of 25 μg mL−1, and for antifungal screening, the growth inhibition was found at 25 μg mL−1 concentration against A. fumigatus, whereas for P. wortmanni, these derivatives were found to be active at a concentration of 100 μg mL−1.78
Abdel-Wahab et al. reported the synthesis of a series of benzofuranyl nitro arylbutanones and benzofuranyl dihydro aryl thiazolyl pyrazole derivatives and their antimicrobial activity at 100 μg concentration. 1-(Thiazol-2-yl) pyrazoline (55) revealed excellent antimicrobial activity against Gram-negative bacteria (with an IZ of 25 mm) and good activity against Gram-positive bacteria (with a IZ of 17 mm). It was concluded that benzofuran, pyrazoline, and thiazole moieties are essential for potential antimicrobial activity.79 Furthermore, they reported a series of benzofuranyl pyrazole derivatives as antimicrobial agents. Compounds 56–59 presented promising antifungal activities against C. albicans, which were more than the reference compound, fluconazole. Moreover, compounds 58 and 59 exhibited noticeable antibacterial activities against B. subtilis, which were better than that of the control, amoxicillin. SAR studies revealed that the benzofuran moieties are essential for antimicrobial activity and an increase in the activity is found with an increase in the number of nitrogen atoms. The phthalimide moieties in compounds are also found to be beneficial for the potential antimicrobial activities.80 On the other hand, benzofuran-thiazole and phenyl pyrazole conjugated compounds as antimicrobial agents are reported. Benzofuran derivatives (60–65), produced the highest IZ of 30–40 mm against C. albicans, therefore it is concluded that benzofuran, pyrazole and thiazole moieties are beneficial for antimicrobial activity (Fig. 14).81
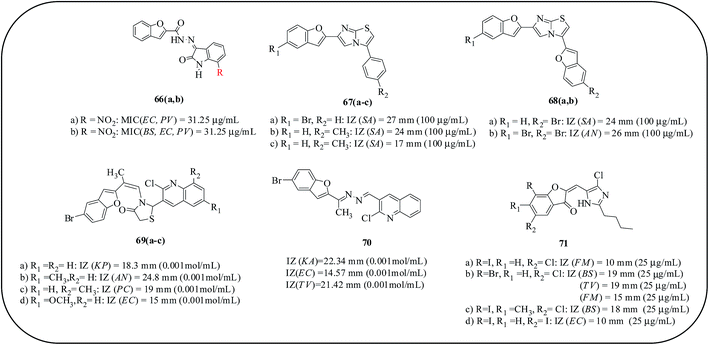
Ugale and co-workers developed benzofurano-isatin derivatives as antimicrobial agents. Compound 66a with a nitro group at C-7 of isatin exhibited significant antibacterial activity against E. coli and P. vulgaris with the same MIC value of 31.25 μg mL−1. Moreover, the presence of a fluoro group at the C-7 position in the case of compound 66b resulted in good activities against B. subtilis, E. coli and P. vulgaris with an MIC value of 31.25 μg mL−1. This derivative was found to be active when compared to the standard drugs, ampicillin and norfloxacin. A SAR study revealed that the introduction of an electron-withdrawing substituent on the isatin nucleus at the C-5 and C-7 positions enhances the antibacterial potential.82 Shanker Rao and co-workers reported the synthesis of imidazole/thiazole-benzofuran-conjugated compounds and their antibacterial and antifungal activities. Compounds 67a and 68a exhibited an excellent effect on S. aureus, P. aeruginosa and Enterococcus feltis (E. feltis) bacterial strains. Compounds 67c and 68a exhibited effective antimicrobial activity against S. aureus and E. coli. Antifungal activity was shown against two human pathogen fungal species, A. niger and T. viride. Compounds 67(a,b) and 68b showed significant activities against both the tested organisms.83 In continuation of this study on benzofuran, they also reported the synthesis of a thiazolidinone nucleus bridged with quinoline and benzofuran. Compounds 69a and 70 showed the highest activities against Klebsiella pneumoniae, whereas compounds 69d and 70 exhibited the highest activities against E. coli. Amongst the antifungal activity results, compound 70 demonstrated the highest activity against A. niger, and compounds 69a, 69d and 70 showed the highest activity against C. albicans. Compound 69c exhibited very good activity against P. chrysozenous, while compounds 69b and 70 displayed the highest activity against T. vridar.84 Imidazole-substituted benzofuran-conjugated compounds are reported along with their antimicrobial potentials. Compounds 71(b,c) displayed an effective IZ against B. subtilis, while compounds 71a, 71c and 71d presented a similar level of activity against F. moniliforme. Compound 71b showed comparatively higher activity against T. viride and F. moniliforme. From the SAR, the activity was increased by the presence of halo (-I, -Br and -Cl) groups as substituents at the R and R2-positions on the benzene ring, and methyl groups at the R1 or R2 positions in combination with –I and –Br at the R position enhanced the activity (Fig. 15).85
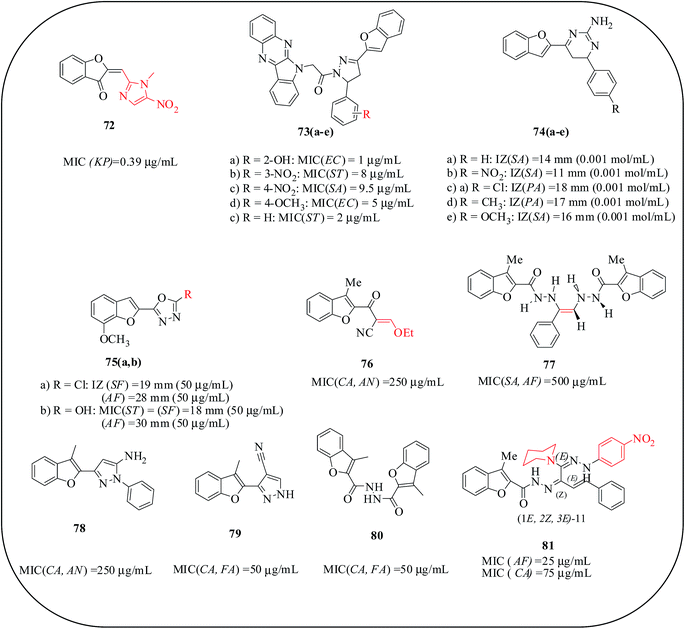
Hadj-Esfandiari and co-workers reported the synthesis of 5-nitroimidazolebenzofuranone and 4-nitroimidazolebenzofuranone derivatives and their antibacterial activity against Gram-positive and Gram-negative bacteria. All 5-nitroimidazole analogues showed remarkable inhibition, evincing their potential antimicrobial activity. Benzofuran with a 4-nitroimidazole core or phenyl group exhibited antibacterial activity with an MIC of 100 μg mL−1. Benzofuranone, 72, was slightly more effective in inhibiting the growth of K. pneumoniae with an MIC of 0.39 μg mL−1, which is 2- to 8-fold higher than the 5-nitrobenzimidazole derivatives against Gram-positive bacteria. The antibacterial activity was enhanced by the negative charge of –NO2 group on the C-5 position of imidazole; a partial charge on the carbonyl oxygen in benzofuran is necessary for the activity.86 Manna and co-workers reported the synthesis of quinoxalino/benzofuran-pyrazole derivatives under microwave conditions; their antibacterial activity was evaluated against seven different multidrug-resistant organisms in various concentrations and results were compared with standard fluoroquinolone drugs. Among the reported compounds, 73(a–e) showed better activities against E. coli, P. aeruginosa, S. typhi and S. aureus. A SAR study revealed that ortho substitution in the phenyl ring with –OH or –NO2 and para substitution on the phenyl ring by –OCH3 at the 5-position of the pyrazole ring evidenced the best antibacterial activity against Gram-negative bacteria.87 Aminopyridine-benzofuran derivatives 74(a–e) were prepared under microwave-assisted synthetic protocols and were tested for their antimicrobial activity against P. aeruginosa and S. aureus; none of these compounds exhibited good antimicrobial activity.88 Basavaraja et al. reported a series of oxadiazole and pyrazole derivatives incorporating a benzofuran core as microbial agents. The compounds bearing chloro-substitution, 75a, and a hydroxyl group, 75b, exhibited potent antimicrobial activity.89 Abdel-Aziz and co-workers synthesized 2-substituted-3-methylbenzofuran derivatives and screened their antimicrobial activity. The highest antifungal and antibacterial activities were demonstrated by an ethoxymethylene derivative, 76, and an ethylene derivative, 77, respectively. The presence of a pyrazole moiety beside the benzofuran ring (in the cases of 78 and 79) imparts high antifungal and antibacterial activities. The significant antimicrobial activity of 77 and 80 may be due to the presence of benzofuran moieties in addition to the hydrazide function in both the derivatives.90 Furthermore, they reported the stereoselective synthesis of benzofuran-based piperidinyl arylamidrazones and tested them for in vitro antimicrobial activity. Compound 81 showed a low MIC (25 μg mL−1) against A. fumigatus and revealed an MIC of 75 μg mL−1 against C. albicans, compared with the standard drug, griseofulvin, which showed MIC of 50 and 75 μg mL−1 against A. fumigatus and C. albicans, respectively. From an SAR study, it is concluded that both the piperidine moiety and the 4-nitrophenylhydazine function were essential for the antimicrobial activity (Fig. 16).91
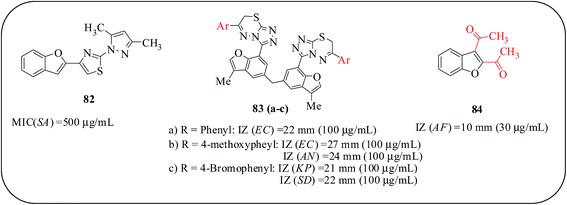
Rida and co-workers studied a series of benzofuran derivatives containing heterocyclic ring substituents linked toa benzofuran nucleus at the C-2 position by a two- to four-atom spacer as potential antimicrobial agents. Compounds were evaluated for their in vitro activity against S. aureus, Gram-positive bacteria, E. coli, Gram-negative bacteria, and C. albicans, a fungus, and ampicillin and clotrimazole were used as reference drugs. Compound 82 exhibited less activity against S. aureus with an MIC of 500 μg mL−1. A SAR study revealed that the presence of a spacer between the heterocyclic substituent and the benzofuran nucleus may be essential for the biological activity.92 Bis-benzofuran-thiadiazine derivatives were reported as antibacterial and antifungal agents. Compounds containing the phenyl (83a), methoxyphenyl (83b) and bromophenyl (83c) substituents at the C-3 position of a triazolo-thiadiazine ring demonstrated promising activity against bacterial and fungal strains. Compound 83b is highly active against all the tested organisms employed and the IZ was found to be greater than the standard drug, neomycin.93 2-Substituted and diacetyl benzofurans are reported by Khan et al., using palladium-catalyzed reactions. The compounds demonstrated mild to significant growth inhibition against Gram-positive and Gram-negative bacteria. An isomeric mixture of diacetylbenzofurans emerged as the most potent microbial agent. In particular, 2,3-diacetylbenzofuran (84) was the most potent compound. From this data, it is concluded that the catalytic sites for benzofurans in the target biomolecule are those with at least one hydrophobic pocket and two H-bond donors—a polar hydroxylated and an imino nitrogen containing amino acid residues. Moreover, substitution at the C4–7 positions of the benzofuran ring and the replacement of a 3-acetyl group by other acyl/lower alkyl functionalities are to be envisaged for functional characterization of the target biomolecule (Fig. 17).94
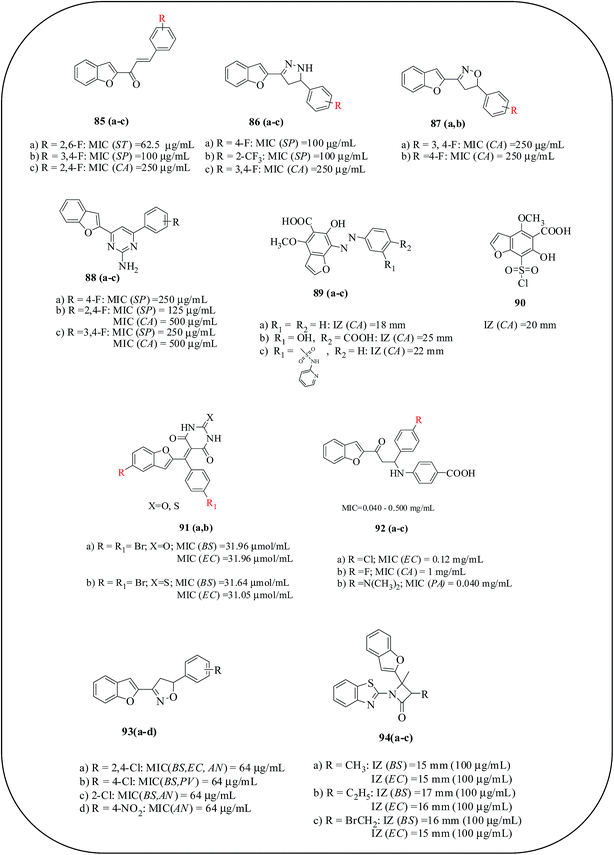
A series of fluorine-substituted benzofuran chalcones, benzofuran-pyrazoles and benzofuran-isoxazoles were synthesized and screened for their antibacterial and antifungal activity, and the results were compared with standards, ampicillin and griseofulvin. Compound 85a displayed good activity with an MIC of 62.5 μg mL−1 against S. typhi and S. pyogenes, whereas compounds 85b and 86(a,b) showed similar antibacterial activity to the reference drug, ampicillin. For antifungal activity, compounds 85c, 86c, 87a and 87b exhibited better activity than the reference drug, griseofulvin, against C. albicans.95 Furthermore, a series of fluorine-containing benzofuran-pyrimidine conjugates as antimicrobial agents are reported. Compounds 88(a–c) showed excellent antibacterial activities against S. pyogenes, which were similar to the activity of the reference drug, ampicillin. With respect to antifungal activity, compounds 88(b,c) showed similar activity against C. albicans.96 Benzofuran-salicylic acid derivatives as antimicrobial agents were described by Hassan and co-workers. Azo derivatives 89(a–c) exhibited good antibacterial activity against both Gram-positive and Gram-negative bacteria and compound 90 demonstrated a promising antifungal activity. A SAR study revealed that the substitution of salicylic acid with an amino group either at position C-5, C-4, or C-3 improved the antimicrobial activity.97 Kenchappa et al. demonstrated the synthesis and antimicrobial activity of benzofuran barbitone/thiobarbitone derivatives. Compounds 91(a,b), having two bromo substituents on C-5 of benzofuran and C-4 of the phenyl ring, respectively, were found to exhibit excellent antibacterial activity against all the tested bacterial strains with MIC values ranging between 29.76 and 31.96 μmol L−1. The same compounds also exhibited equipotent activity against all fungal strains with MIC values in the range of 12.50–66.49 μmol L−1. SAR studies revealed that substitution at the ortho position of benzofuran ring and the para position of aryl ring was responsible for the increased antimicrobial activity. Compounds containing two bromo substituents have increased antimicrobial activity compared to those bearing a single halogen/other substituent group.98 The same group also developed β-amino carbonyl derivatives of benzofuran as antimicrobial agents. From these studies, it is concluded that the electron-withdrawing groups substituted on the aromatic ring, particularly at the para position, 92(a–c), exhibited potential antimicrobial activity.99 Srinivas and co-workers investigated the synthesis of benzofuran-isoxazole and tested its antibacterial and antifungal activity using a serial tube dilution technique; results were compared with ampicillin and fluconazole as a reference standard. Among the tested derivatives, compounds 93a and 93b possessed maximum activity, which may be due to electron-withdrawing substituents such as 2,4-dichlorophenyl and 4-fluorophenyl appended at the C-5 position of isoxazole core. Moreover, the presence of 2-chlorophenyl, 2,4-dichlorophenyl, and 4-nitrophenyl 93a, b and d) at the C-5 position of isoxazole structure resulted in good antifungal activity. This reveals the importance of the electronic effects of the substituents present on the aromatic ring in enhancing the antibacterial/antifungal activity.100 Kumar et al., invented a series of tricyclic compounds containing benzofuran, benzothiazole and lactum derivatives as antimicrobial agents against B. subtilius, E. coli and C. albicans. Compounds 94(a–c) exhibited moderate activities with IZ ranging from 11 to 17 mm (Fig. 18).101
5. Benzofuran-based metal complexes as antimicrobial agents
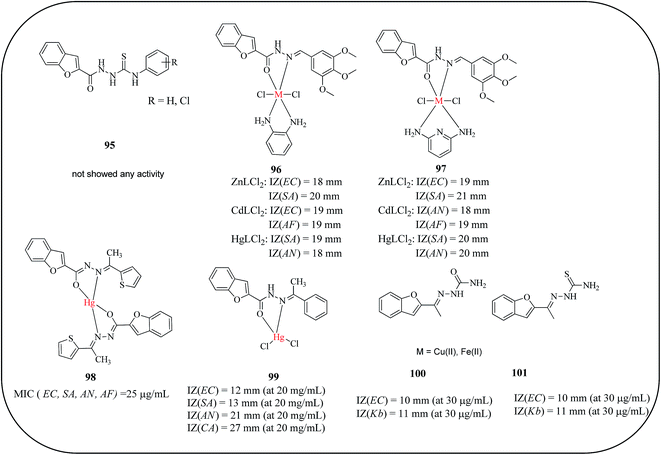
Metal ions play an important role in biochemical processes. Many biochemical reactions depend on the presence of metal ions, which are the part of coordination complexes. Increasing attention has been given in the last decade to the biological applications of benzofuran-based metal complexes including late transition metals such as cobalt(II), nickel(II), copper(II) and zinc(II). Many of these complexes exhibited considerable antibacterial and anticancer properties in in vitro and/or in vivo assays. The choice of a suitable substitution on the benzofuran-based ligand system is found to be crucial in the design of novel target specific bioactive metal complexes. In this section, the important benzofuran-based ligands and their metal complexes reported for potential biological applications are discussed.Halli and co-workers reported the synthesis of benzofuran-2-carbohydrazide, 95, and its Co(II), Cu(II), Ni(II), Cd(II), Hg(II), Zn(II), and UO2(II) complexes and Th(IV) complexes of phenylisocyanate (BCPT)/p-chlorophenylisothiocyanate (BCCIPT). All the complexes and ligands were screened for their potential antimicrobial activity. The Zn(II), Cd(II), Hg(II), Ni(II) and UO2(II) complexes displayed significant growth inhibition activity against Pseudomonas and Wild bacillus bacteria compared with the free ligand BCCIPT and metal ions.102 Furthermore, a series of mixed ligand complexes of the type [MLL′Cl2] of Co(II), Ni(II), Cu(II), Zn(II), Cd(II) and Hg(II) derived from benzofuran-2-carbohydrazide and 3,4,5-trimethoxybenzaldehyde and ortho-phenylenediamine/2,6-diaminopyridine (96, 97) were reported. All the complexes and ligands were screened for potential antimicrobial activity against bacterial strains. The Zn(II), Cd(II) and Hg(II) complexes exhibited potential antimicrobial activity against both bacteria and fungi, having higher activity than ligands against the same microorganisms.103 Furthermore, complexes of Co(II), Ni(II), Zn(II), Cd(II) and Hg(II) from benzofuran-2-carbohydrazide with 2-acetylthiophene/acetophenone were reported as microbial agents. The Schiff bases were inactive against all the bacteria tested, while Hg(II) complexes (98, 99) showed significantly enhanced activity against both the bacterial, E. coli and S. aureus, and fungal, A. niger and A. flavus, strains. All other complexes showed less growth inhibition activity against all bacteria tested.104–106 Goel et al. synthesized Cu(II) and Fe(III) complexes derived from 2-acetylbenzofuran and semicarbazone/thiosemicarbazone ligands and screened their antibacterial activities. The metal complexes (100, 101) displayed effective antibacterial properties against all the pathogenic bacteria; in particular, complexes of thiosemicarbazone demonstrated remarkable antibacterial activity. The metal complexes were found to have higher antibacterial activities compared with their respective Schiff base ligands (Fig. 19).107
6. Benzofuran-based natural products as antimicrobial agents
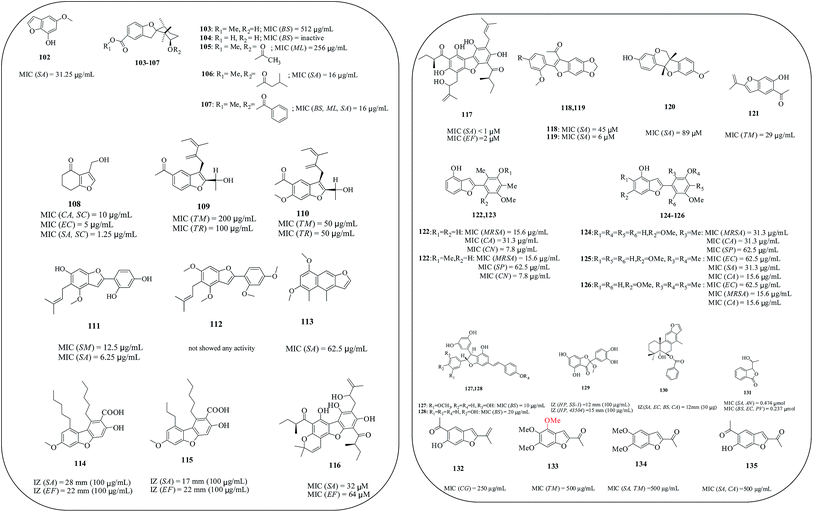
Natural products play an important role in both drug discovery and chemical biology. In fact, many approved therapeutics as well as drug candidates are derived from natural sources. Benzofuran natural products isolated from the Styracaceae family plants, such as Styrax japonica, S. formosanus, S. obassia, S. macranthus and S. officinalis, showed a variety of biological activities including insecticidal, fungicidal, antimicrobial, antiproliferative, cytotoxic and antioxidant properties.108,109 Over the years, several newer/modified approaches were adopted for the isolation of benzofuran and their compounds and were screened for antibacterial/antifungal activities. All the related compounds are listed in Table 1 (Fig. 20).| S. No. | Species | Family | Compound | Reference(s) |
|---|---|---|---|---|
| 1 | Cotula coronopifolia | Asteraceae | 6-Methoxy-1-benzofuran-4-ol (102) | 110 |
| 2 | Heliotropium filifolium | Boraginaceae | Methyl-3′-hydroxy-2′,2′,6′-trimethyl-3H-spiro[1-benzofuran-2,1′-cyclohexane]-5-carboxylate (103), 3′-hydroxy-2′,2′,6′-trimethyl-3H-spiro[1-benzofuran-2,1′-cyclohexane]-5-carboxylic acid (104), methyl-3′-acetyloxy-2′,2′,6′-trimethyl-3H-spiro[1-benzofuran-2,1′-cyclohexane]-5-carboxylate (105), methyl-3′-isopentanoyloxy-2′,2′,6′-trimethyl-3H-spiro[1-benzofuran-2,1′-cyclohexane]-5-carboxylate (106), methyl-3′-benzoyloxy-2′,2′,6′-trimethyl-3H-spiro[1-benzofuran-2,1′-cyclohexane]-5-carboxylate (107) | 111 |
| 3 | Phomopsis sp. | — | 3-(Hydroxymethyl)-6,7-dihydrobenzofuran-4(5H)-one (108) | 112 |
| 4 | Eupatorium aschenbornianum | Asteraceae | 5-Acetyl-3β-angeloyloxy-2β-(1-hydroxyisopropyl)-2,3-dihydrobenzofurane (109), 5-acetyl-3β-angeloyloxy-2β-(1-hydroxyisopropyl)-6-methoxy-2,3-dihydrobenzofurane (110) | 113 |
| 5 | Glycyrrhiza | Xibei licorice | 4-(6-Hydroxy-4-methoxy-5-(3-methylbut-2-enyl)benzofuran-2-yl)benzene-1,3-diol or licocoumarone (111), 2-(2,4-dimethoxyphenyl)-4,6-dimethoxy-5-(3-methylbut-2-enyl)benzofuran (112) | 114 |
| 6 | Ligularia veitchiana | Compositae | 1,3-Dimethoxy-4,6-dimethylnaphthofuran (113) | 115 |
| 7 | Cladonia rangiferina | Cladoniaceae | Didymic acid (114), condidymic acid (115) | 116 |
| 8 | Achyrocline satureioides | Asteraceae | 1′,1′′-[6,7,9-Trihydroxy-8-(2-hydroxy-3-methylbut-3-en-1-yl)-3,3-dimethyl-3H-benzofuro[2,3-f]chromene-5,10-diyl]bis(2-(S)-methylbutan-1-one) (116), 1′,1′′-[6,7,9-trihydroxy-8-(2-hydroxy-3-methylbut-dihydroxy)-3,3-dimethyl-3H-benzofuro[2,3-f]chromene-5,10-diyl]bis(2-(S)-methylbutan-1-one) (117) | 117 |
| 9 | Dalea spinosa (Psorothamnus spinosus) | Fabaceae | Spinosan A (118), spinosan A acetate (119), (+)-medicarpin (120) | 118 |
| 10 | Helianthella quinquenervis | Asteraceae | Euparin (121) | 119 |
| 11 | Stemona aphylla | Stemonaceae | Stemofuran E (122), stemofuran J (123), stemofuran M (124), stemofuran P (125), stemofuran R (126) | 120 |
| 12 | Gnetum gnemon L. | Gnetacea | Stilbenoid (127), gnetin C (128) | 121 |
| 13 | Allium cepa | Alliaceae | 2-(3,4-Dihydroxyphenyl)-4,6-dihydroxy-2-methoxybenzofuran-3-one (129) | 122 |
| 14 | Caesalpinia pulcherrima | Leguminosae | Isovouacapenol D or [(4aα,5β,11bβ)-1,2,3,4,4a,5,6,11b-octahydro-4,4,7,11b-tetramethyl phenanthro[3,2-b]furan-4a,5-diol-5-benzoate] (130) | 123 |
| 15 | Murraya koenigii | Rutaceae | 3ε-(1ε-Hydroxyethyl)-7-hydroxy-1-isobenzofuranone (131) | 124 |
| 16 | Calea platylepis | Asteraceae/Heliantheae | Euparin (132), caleprunin A (133), caleprunin B (134), euparone (135) | 125 |
7. Conclusions and future aspects
Incidences of bacterial and fungal infections have increased significantly in the past 25 years. The evolution of resistance in bacterial strains against currently available antibacterial agents has been an increasing concern in recent years. To overcome the threat of widespread multidrug resistance in Gram positive and Gram negative bacterial strains as well as fungi, there is ongoing demand for new antimicrobial agents. The discovery of novel drugs in many fields, i.e. antibacterial, has been stalled for many years. There is an urgent need for new pharmaceuticals that have a broader spectrum of activity or act through novel mechanisms of action, i.e. to overcome the increasing incidence of microbial resistance observed for currently used drugs. Numerous outstanding achievements revealed that benzofuran-based compounds have extensive potential as antimicrobial agents. To further optimize the complete potential of benzofuran compounds, SAR-based studies are likely to continue to play an important role. It is very likely that optimized benzofuran compounds with excellent potency and few side effects will continue to be created. Some of these benzofuran compounds will undoubtedly be used as antimicrobial therapeutic agents in the near future. The present review, covering literature from the last fifteen years, is expected to provide a bird's eye view of benzofuran-derived compounds for drug designers and medicinal chemists as well as comprehensive and target-oriented information for the development of clinically viable molecules. Furthermore, future research in this field will bring innovative pharmaceutical developments with a considerable spectrum of use.Abbreviations
| A. fumigatus (AF) | Aspergillus fumigatus |
| A. niger (AN) | Aspergillus niger |
| APB | Aminopropyl benzofuran |
| APDB | Aminopropyl-2,3-dihydrobenzofuran |
| B. megaterium (BM) | Bacillus megaterium |
| BPAP | Benzofuran-2-yl-2-propylaminopentane |
| B. subtilis (BS) | Bacillus subtilis |
| C. albicans (CA) | Candida albicans |
| C. glabrata (CG) | Candida glabrata |
| DNA | Deoxyribonucleic acid |
| E. coli (EC) | Escherichia coli |
| E. feltis (EF) | Escherichia feltis |
| F. moniliforme (FM) | Fusarium moniliforme |
| G. candidum (GC) | Geotrichum candidum |
| HIV | Human immunodeficiency virus |
| IZ | Inhibition zone |
| K. pneumoniae (KP) | Klebsiella pneumoniae |
| MIC | Minimum inhibitory concentration |
| μg | Micro gram |
| mL | Milli litre |
| ML | Micrococcus luteus |
| mm | Milli metre |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| P. aeruginosa (PA) | Pseudomonas aeruginosa |
| P. chinchori (PC) | Pseudomonas chinchori |
| P. vulgaris (PV) | Proteus vulgaris |
| P. wortmanni (PW) | Pseudomonas wortmanni |
| S. aureus (SA) | Staphylococcus aureus |
| SAR | Structure activity relationship |
| S. cerevisiae (SC) | Saccharomyces cerevisiae |
| S. pyogenes (SP) | Streptococcus pyogenes |
| S. racemosum (SR) | Syncephalastrum racemosum |
| S. typhi (ST) | Salmonella typhimurium |
| S. mentagrophytes (TM) | Trichophyton mentagrophytes |
| T. viride (TV) | Trichoderma viride |
Acknowledgements
The author RSK thanks Jain University, Bangalore for financial support and author AH is thankful to Erasmus NAMASTE consortium (unique grant number: NAMASTE_20140147) for the award of his doctoral exchange fellowship, while author KC is thankful to Fundação para a Ciência e a Tecnologia (FCT) for funding his postdoctoral research.References
- Y. He, B. Wu, J. Yang, D. Robinson, L. Risen, R. Ranken, L. Blyn, S. Sheng and E. E. Swayze, Bioorg. Med. Chem. Lett., 2003, 13, 3253–3256 CrossRef CAS PubMed.
- B. P. Mathew and M. Nath, ChemMedChem, 2009, 4, 310–323 CrossRef CAS PubMed.
- I. Berber, C. Cokmus and E. Atalan, Microbiol., 2003, 72, 42–47 CrossRef CAS.
- W. S. Sung, H. J. Jung, K. Park, H. S. Kim, L. S. Lee and D. G. Lee, Life Sci., 2007, 80, 586–591 CrossRef CAS PubMed.
- W. H. Perkin, J. Chem. Soc., 1870, 23, 368–371 RSC.
- B. A. Keay, J. M. Hopkins and P. W. Dibble, in Comprehensive heterocyclic chemistry III, ed. A. R. Katritzky, C. A. Ramsden, E. F. V. Scriven and R. J. K. Taylor, Elsevier, Oxford, 2008 Search PubMed.
- (a) S. Shimazu, K. Takahata, H. Katsuki, H. Tsunekawa, A. Tanigawa, F. Yoneda, J. Knoll and A. Akaike, Eur. J. Pharmacol., 2001, 421, 181–189 CrossRef CAS PubMed; (b) S. Shimazu, H. Tsunekawa, F. Yoneda, H. Katsuki, A. Akaike and A. Janowsky, Eur. J. Pharmacol., 2003, 482, 9–16 CrossRef CAS PubMed; (c) J. Knoll, F. Yoneda, B. Knoll, H. Ohde and I. Miklya, Br. J. Pharmacol., 1999, 128, 1723–1732 CrossRef CAS PubMed.
- C. Ryu, A. Song, J. Y. Lee, J. Hong, J. H. Yoon and A. Kim, Bioorg. Med. Chem. Lett., 2010, 20, 6777–6780 CrossRef CAS PubMed.
- (a) B. F. Abdel-Wahab, H. A. Abdel-Aziz and E. M. Ahmed, Eur. J. Med. Chem., 2009, 44, 2632–2635 CrossRef CAS PubMed; (b) F. Pan and T. C. Wang, J. Chin. Chem. Soc., 1961, 8, 220–225 CrossRef CASF. Pan and T. C. Wang, J. Chin. Chem. Soc., 1961, 8, 374–379 CrossRef.
- (a) K. Manna and Y. K. Agrawal, Eur. J. Med. Chem., 2010, 45, 3831–3839 CrossRef CAS PubMed; (b) M. Brandvang, V. Bakken and L. L. Gundersen, Bioorg. Med. Chem. Lett., 2009, 17, 6512–6516 CrossRef PubMedS. M. Bakunova, S. A. Bakunov, T. Wenzler, T. Barszcz, K. A. Werbovetz, J. E. Reto Brun Hall and R. R. Tidwell, J. Med. Chem., 2007, 50, 5807–5823 CrossRef CAS PubMed.
- F. A. Ragab, N. M. Eid, G. S. Hassan and Y. M. Nissan, Chem. Pharm. Bull., 2012, 60, 110–120 CrossRef PubMed.
- K. M. Dawood, H. Abdel-Gawad, E. A. Rageb, M. Ellithey and H. A. Mohamed, Bioorg. Med. Chem., 2006, 14, 3672–3680 CrossRef CAS PubMed.
- M. O. Abdelhafez, K. M. Amin, H. I. Ali, M. M. Abdallad and E. Y. Ahmed, RSC Adv., 2014, 4, 11569–11579 RSC.
- S. M. Rida, S. A. El-Hawash, H. T. Fahmy, A. A. Hazza and M. M. El-Meligy, Arch. Pharmacal Res., 2006, 29, 16–25 CrossRef CAS.
- S. Rádl, P. Hezký, P. Konvička and I. Krejčí, Chem. Commun., 2000, 65, 1093–1108 Search PubMed.
- S. P. Gibson and C. Laurate, Benzofuran antiparasitic agents, WO/2008/102232, 2008.
- K. V. Sashidhara, R. K. Modukuri, R. Sonkar, K. B. Rao and G. Bhatia, Eur. J. Med. Chem., 2013, 68, 38–46 CrossRef CAS PubMed.
- S. S. Rindhe, M. A. Rode and B. K. Karale, Indian J. Pharm. Sci., 2010, 72, 231–235 CrossRef CAS PubMed.
- K. A. Reddy, B. B. Lohray, V. Bhushan, A. C. Bajji, K. V. Reddy, P. R. Reddy, T. H. Krishna, I. N. Rao, H. K. Jajoo, N. V. Rao, R. T. Chakrabarti, D. Kumar and R. Rajagopalan, J. Med. Chem., 1999, 42, 1927–1940 CrossRef CAS PubMed.
- B. C. Ross, D. Middlemiss, D. I. C. Scopes, T. I. M. Jack, K. S. Cardwell, M. D. Dowle, J. G. Montana, M. Pass and D. B. Judd, EP, 0514197 A1, 1992.
- S. Ravikumar, G. Ramanathan and M. Gnanadesigan, Asian Pac. J. Trop. Med., 2012, 5, 358–361 CrossRef CAS.
- M. Ono, M. P. Kung, C. Hou and H. F. Kung, Nucl. Med. Biol., 2002, 29, 633–642 CrossRef CAS PubMed.
- M. B. Isman, P. Proksch and L. Witte, Arch. Insect Biochem. Physiol., 1987, 6, 109–120 CrossRef CAS.
- M. B. Isman, in Insecticides of plant origin, J. T. Arnason, B. J. R. Philogène and P. Morand, ACS Symposium Series 387, Washington, DC, 1989, pp. 44–58 Search PubMed.
- K. Suzuki, T. Okawara, T. Higashijima, K. Yokomizo, T. Mizushima and M. Otsuka, Bioorg. Med. Chem. Lett., 2005, 15, 2065–2068 CrossRef CAS PubMed.
- K. S. C. Marriott, A. Z. Morrison, M. Moore, O. Olubajo and L. E. Stewart, Bioorg. Med. Chem. Lett., 2012, 20, 6856–6861 CrossRef CAS PubMed.
- Y. Xiang, B. Hirth, G. Asmussen, H.-P. Biemann, K. A. Bishop, A. Good, M. Fitzgerald, T. Gladysheva, A. Jain, K. Jancsics, J. Liu, M. Metz, A. Papoulis, R. Skerlj, J. D. Stepp and R. R. Wei, Bioorg. Med. Chem. Lett., 2011, 21, 3050–3056 CrossRef CAS PubMed.
- K. Asoh, M. Kohchi, I. Hyoudoh, T. Ohtsuka, M. Masubuchi, K. Kawasaki, H. Ebiike, Y. Shiratori, T. A. Fukami, O. Kondoh, T. Tsukaguchi, N. Ishii, Y. Aoki, N. Shimma and M. Sakaitani, Bioorg. Med. Chem. Lett., 2009, 19, 1753–1757 CrossRef CAS PubMed.
- M. Sun, C. Zhao, G. A. Gfesser, C. Thiffault, T. R. Miller, K. Marsh, J. Wetter, M. Curtis, R. Faghih, T. A. Esbenshade, A. A. Hancock and M. Cowart, J. Med. Chem., 2005, 48, 6482–6490 CrossRef CAS PubMed.
- S. L. Graham, J. M. Hoffman, P. Gautheron, S. R. Michelson, T. H. Scholz, H. Schwam, K. L. Shepard, A. M. Smith and R. L. Smith, J. Med. Chem., 1990, 33, 749–754 CrossRef CAS PubMed.
- B. Pourabas and A. Banihashemi, Polym. Int., 2002, 51, 1086–1099 CrossRef CAS.
- J. Xu, G. Nie, S. Zhang, X. Han, S. Pu, L. Shen and Q. Xiao, Eur. Polym. J., 2005, 41, 1654–1661 CrossRef CAS.
- C. H. Chen, N. Y. Fairport, L. J. Fox and M. Balmnore, Novel benzofuran dyes, US Pat., 4900831 A, 1990.
- C. H. Chen and J. L. Fox, Novel benzofuran dyes, US Pat., 4948893 A, 1990.
- R. Sato, K. Kato, T. Sasaki and H. Sugit, Silver halide photosensitive materials for color photography, EP, 0148536 A2, 1985.
- H. Odenwalder, H. Langen, J. Hagemann and K. Henseler, Color photographic silver halide, material, US Pat., 5981160 A, 1999.
- P. S. Song and K. J. Tapley Jr, Photochem. Photobiol., 1979, 29, 1177–1197 CrossRef CAS PubMed.
- F. P. Gasparro, R. Dallamico, D. Goldminz, E. Simmons and D. Weingold, Yale J. Biol. Med., 1989, 62, 579–593 CAS.
- S. Caffieri, Photochem. Photobiol. Sci., 2002, 1, 149–157 CAS.
- L. Santana, E. Uriarte, F. Roleira, N. Milhazes and F. Borges, Curr. Med. Chem., 2004, 11, 3239–3261 CrossRef CAS PubMed.
- E. M. Bickoff, A. N. Booth, R. L. Lyman, A. L. Livingston, C. R. Thompson and F. Deeds, Science, 1957, 126, 969–970 CAS.
- J. W. Mason, N. Engl. J. Med., 1987, 316, 455–566 CrossRef CAS PubMed.
- F. Bogazzi, L. Tomisti, L. Bartalena, F. Aghini-Lombardi and E. Martino, J. Endocrinol. Invest., 2012, 35, 340–348 CAS.
- C. Mitsui, J. Soeda, K. Miwa, H. Tsuji, J. Takeya and E. Nakamura, J. Am. Chem. Soc., 2012, 134, 5448–5451 CrossRef CAS PubMed.
- F. Yoneda, J. Knoll, H. Ode, M. Sakae, M. Katurada, T. Moto, T. Ando, S. Shimazu, K. Takahata and M. Fujimoto, Ethylamine derivatives, US Pat., 6214859, 2001.
- A. P. Monte, D. Marona-Lewicka, N. V. Cozzi and D. E. Nichols, J. Med. Chem., 1993, 36, 3700–3706 CrossRef CAS PubMed.
- P. Dawson, J. Opacka-Juffry, J. D. Moffatt, Y. Daniju, N. Dutta, J. Ramsey and C. Davidson, Prog. Neuro-Psychopharmacol. Biol. Psychiatry, 2014, 48, 57–63 CrossRef CAS PubMed.
- L. Iversen, S. Gibbons, R. Treble, V. Setola, X. P. Huang and B. L. Roth, Eur. J. Pharmacol., 2012, 700, 147–151 CrossRef PubMed.
- K. Briner, J. P. Burkhart, T. P. Burkholder, M. J. Fisher, W. H. Gritton, D. T. Kohlman, S. X. Liang, S. C. Miller, J. T. Mullaney, Y. C. Xu and Y. Xu, US Pat., 7045545, 2006.
- (a) R. Naik, D. S. Harmalkar, X. Xu, K. Jang and K. Lee, Eur. J. Med. Chem., 2015, 90, 379–393 CrossRef CAS PubMed; (b) A. Radadiya and A. Shah, Eur. J. Med. Chem., 2015, 97, 356–376 CrossRef CAS PubMed; (c) R. J. Nevagi, S. N. Dighe and S. N. Dighe, Eur. J. Med. Chem., 2015, 5, 561–581 CrossRef PubMed; (d) K. M. Dawood, Expert Opin. Ther. Pat., 2013, 23, 1133–1156 CrossRef CAS PubMed; (e) M. G. Kadieva and É. T. Oganesyan, Chem. Heterocycl. Compd., 1997, 33, 1245–1258 CrossRef CAS; (f) H. Khanam and Shamsuzzaman, Eur. J. Med. Chem., 2015, 5, 483–504 CrossRef PubMed.
- (a) N. G. Karaburun, K. Benkli, Y. Tunali, Ü. Uçucu and Ş. Demirayak, Eur. J. Med. Chem., 2006, 41, 651–656 CrossRef PubMed; (b) D. Bogdal and M. Warzala, Tetrahedron, 2000, 56, 8769–8773 CrossRef CAS; (c) A. R. Katrizky, Y. Ji, Y. Fang and I. Prakash, J. Org. Chem., 2001, 66, 5613–5615 CrossRef; (d) K. Ando, Y. Kawamura, Y. Akai, J. Kunitomo, T. Yokomizo, M. Yamashita, S. Ohta, T. Ohishi and Y. Ohishi, Org. Biomol. Chem., 2008, 6, 296–307 RSC.
- (a) W. Boehme, Org. Synth., 1953, 33, 43–46 CrossRef CAS; (b) R. Adams and L. Whitaker, Quinone Imides. XXXIX. Adducts of quinone monoimides and conversion of active methylene adducts to benzofurans, J. Am. Chem. Soc., 1956, 78, 658–663 CrossRef CAS.
- (a) A. Arcadi, S. Cacchi, M. D. Rosario, G. Fabrizi and F. Marinelli, J. Org. Chem., 1996, 61, 9280–9288 CrossRef CAS; (b) C. Amatore, E. Blart, J. P. Genet, A. Jutand, S. Lemaire-Audoire and M. Savignac, J. Org. Chem., 1995, 60, 6829–6839 CrossRef CAS; (c) A. Sogawa, M. Tsukayama, H. Nozaki and M. Nakayama, Heterocycles, 1996, 43, 101–111 CrossRef CAS; (d) S. Cacchi, G. Fabrizi and L. Moro, Tetrahedron Lett., 1998, 39, 5101–5104 CrossRef CAS; (e) A. Furstner and P. W. Davies, J. Am. Chem. Soc., 2005, 127, 15024–15025 CrossRef PubMed; (f) J. Oppenheimer, W. Johnson, M. Tracey, R. Hsung, P. Y. Yao, R. Liu and K. Zhao, Org. Lett., 2007, 9, 2361–2364 CrossRef CAS PubMed; (g) V. Patel, G. Pattenden and J. Russell, Tetrahedron Lett., 1986, 27, 2303–2306 CrossRef CAS.
- A. Weissberger and E. C. Taylor, Chemistry of heterocyclic compounds, John Wiley & Sons, New York, vol. 29, 1974 Search PubMed.
- P. Frohberg, C. Kupfer, P. Stenger, U. Baumeister and P. Nuhn, Arch. Pharm., 1995, 328, 505–516 CrossRef CAS PubMed.
- J. Debord, P. N. Diaye, J. C. Bollinger, K. Fikri, B. Penicaut, J. M. Robert, P. S. Robert and G. le-Baut, J. Enzyme Inhib. Med. Chem., 1997, 12, 13–26 CrossRef CAS.
- E. S. H. El Ashry, N. A. Rashed and H. S. Shobier, Pharmazie, 2000, 55, 403–415 CAS.
- J. M. Robert, O. Rideau, S. R. Piessard, M. Duflos, G. LeBaut, N. Grimaud, M. Juge and J. Y. Petit, Arzneim.-Forsch./Drug Res., 1997, 47, 635–642 CAS.
- H. A. Mohamed, E. Abdel-Latif, B. F. Abdel-Wahab and G. E. A. Awad, Int. J. Med. Chem., 2013 DOI:10.1155/2013/986536.
- E. P. Davies, J. D. Pittam, K. B. Mallion and N. P. Taylor, Process for preparing an antifungal azole with hydrazino and amidrazone intermediates, US Pat., 5861516, 1999, p. 19.
- V. N. Telvekar, A. Belubbi, V. K. Bairwa and K. Satardekar, Bioorg. Med. Chem. Lett., 2012, 22, 2343–2346 CrossRef CAS PubMed.
- M. G. Mamolo, V. Falagiani, L. Vio and E. Banfi, Farmaco, 1999, 54, 761–767 CrossRef CAS PubMed.
- D. Ranft, G. Lehwark-Yvetot, K. J. Schaper and A. Buge, Arch. Pharm., 1997, 330, 169–172 CrossRef CAS PubMed.
- D. Ranft, T. Seyfarth, K. Schaper, G. Lehwark-Yvetot, C. Bruhn and A. Buge, Arch. Pharm., 1999, 332, 427–430 CrossRef CAS.
- H. A. Abdel-Aziz and A. A. I. Mekawey, Eur. J. Med. Chem., 2009, 44, 4985–4997 CrossRef CAS PubMed.
- J. Liu, F. Jiang, X. Jiang, W. Zhang, J. Liu, W. Liu and L. Fu, Eur. J. Med. Chem., 2012, 54, 879–886 CrossRef CAS PubMed.
- X. Jiang, W. Liu, W. Zhang, F. Jiang, Z. Gao, H. Zhuang and L. Fu, Eur. J. Med. Chem., 2011, 46, 3526–3530 CrossRef CAS PubMed.
- A. Chandrashekar, E. Bheemappa, Y. D. Bodke, V. K. Bhovi, R. Ningegowda, M. C. Shivakumar, S. K. Peethambar and S. Telkar, Med. Chem. Res., 2013, 22, 78–87 CrossRef.
- V. K. Reddy, J. V. Rao, L. B. Reddy, B. Ram and B. Balram, Pharma Chem., 2013, 5, 237–242 CAS.
- S. S. EI-Nakkady, H. F. Roaiah, W. S. El-Serwy, A. M. Soliman, S. I. Abd-EI-Moez and A. A. Abdel-Rahman, Acta Pol. Pharm., 2012, 69, 645–655 Search PubMed.
- S. Alper-Hayta, M. Arisoy, Ö. Temiz-Arpaci, I. Yildiz, E. Aki, S. Özkan and F. Kaynak, Eur. J. Med. Chem., 2008, 43, 2568–2578 CrossRef CAS PubMed.
- J. Rangaswamy, H. Vijay Kumar, S. T. Harini and N. Naik, Arabian J. Chem., 2013 DOI:10.1016/j.arabjc.2013.10.012.
- J. Rangaswamy, H. Vijay Kumar, S. T. Harini and N. Naik, Bioorg. Med. Chem. Lett., 2012, 22, 4773–4777 CrossRef CAS PubMed.
- C. Kirilmis, M. Ahmedzade, S. Servi, M. Koca, A. Kizirgil and C. Kazaz, Eur. J. Med. Chem., 2008, 43, 300–308 CrossRef CAS PubMed.
- M. Koca, S. Servi, C. Kirilmis, M. Ahmedzade, C. Kazaz, B. Özbek and G. Ötük, Eur. J. Med. Chem., 2005, 40, 1351–1358 CrossRef CAS PubMed.
- J. N. Soni and S. S. Soman, Eur. J. Med. Chem., 2014, 75, 77–81 CrossRef CAS PubMed.
- I. A. Khan, M. V. Kulkarni and C. M. Sun, Eur. J. Med. Chem., 2005, 40, 1168–1172 CrossRef CAS PubMed.
- I. A. Khan, M. V. Kulkarni, M. Gopal, M. S. Shahabuddin and C. M. Sun, Bioorg. Med. Chem. Lett., 2005, 15, 3584–3587 CrossRef CAS PubMed.
- B. F. Abdel-Wahab, H. A. Abdel-Aziz and E. M. Ahmed, Eur. J. Med. Chem., 2009, 44, 2632–2635 CrossRef CAS PubMed.
- B. F. Abdel-Waha, H. A. Abdel-Aziz and E. M. Ahmed, Arch. Pharm. Chem. Life Sci., 2008, 341, 734–739 CrossRef PubMed.
- B. F. Abdel-Wahab, H. A. Abdel-Aziz and E. M. Ahmed, Monatsh. Chem., 2009, 140, 601–605 CrossRef CAS.
- V. Ugale, H. Patel, B. Patel and S. Bari, Arabian J. Chem., 2012 DOI:10.1016/j.arabjc.2012.09.011.
- S. Shankerrao, Y. D. Bodke and S. Santoshkumar, Arabian J. Chem., 2012 DOI:10.1016/j.arabjc.2012.10.018.
- V. K. Bhovia, Y. D. Bodke, S. Biradar, B. E. Kumaraswamy and S. Umesh, Phosphorus, Sulfur Silicon Relat. Elem., 2010, 185, 110–116 CrossRef.
- B. S. Dawane, S. G. Konda, N. T. Khandare, S. S. Chobe, B. M. Shaikh, R. G. Bodade and V. D. Joshi, Org. Commun., 2010, 3, 22–29 CAS.
- N. Hadj-Esfandiari, L. Navidpour, H. Shadnia, M. Amini, N. Samadi, M. A. Faramarzi and A. Shafiee, Bioorg. Med. Chem. Lett., 2007, 17, 6354–6363 CrossRef CAS PubMed.
- K. Manna and Y. K. Agrawal, Bioorg. Med. Chem. Lett., 2009, 19, 2688–2692 CrossRef CAS PubMed.
- D. B. Arun Kumar, G. K. Prakash, M. N. Kumaraswamy, B. P. Nandeshwarappa, B. S. Shrigara and K. M. Mahadevan, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2006, 45, 1699–1703 Search PubMed.
- H. Hanumanagoud and K. M. Basavaraj, Pharma Chem., 2013, 5, 87–98 CAS.
- H. A. Abdel-Aziz, A. A. I. Mekawey and K. M. Dawood, Eur. J. Med. Chem., 2009, 44, 3637–3644 CrossRef CAS PubMed.
- H. A. Abdel-Aziz and A. A. I. Mekawey, Eur. J. Med. Chem., 2009, 44, 4985–4997 CrossRef CAS PubMed.
- S. M. Rida, S. A. M. EI-Hawash, H. T. Y. Fahmy, A. A. Hazza and M. M. M. EI-Meligy, Arch. Pharmacal Res., 2006, 29, 826–833 CrossRef CAS.
- C. S. Reddy, D. Chandrasekhar Rao, V. Yakub and A. Nagaraj, Acta Chim. Slov., 2011, 58, 582–589 CAS.
- M. W. Khan, M. J. Alam, M. A. Rashid and R. Chowdhury, Bioorg. Med. Chem., 2005, 13, 4796–4805 CrossRef CAS PubMed.
- T. S. Chundawat, N. Sharma and S. Bhagat, Med. Chem. Res., 2014, 23, 1350–1359 CrossRef CAS.
- T. S. Chundawat, N. Sharma and S. Bhagat, Med. Chem., 2014, 10, 409–417 CrossRef CAS.
- G. S. Hassan and G. A. Soliman, Eur. J. Med. Chem., 2010, 45, 4104–4112 CrossRef CAS PubMed.
- R. Kenchappa, Y. D. Bodke, B. Asha, S. Telkar and M. A. Sindhe, Med. Chem. Res., 2014, 23, 3065–3081 CrossRef CAS.
- R. Kenchappa, Y. D. Bodke, S. K. Peethambar, S. Telkar and V. K. Bhovi, Med. Chem. Res., 2013, 22, 4787–4797 CrossRef CAS.
- M. Srinivas, D. Swapna, K. Haritha and K. Rao, PHARMANEST, 2013, 4, 1219–1228 Search PubMed.
- D. R. Harish Kumar and M. D. Karvelar, Eur. J. Chem., 2010, 7, 636–640 CAS.
- M. B. Halli and Z. S. Qureshi, Indian J. Chem., Sect. A: Inorg., Bio-inorg., Phys., Theor. Anal. Chem., 2004, 43, 2347–2351 Search PubMed.
- M. B. Halli, V. B. Patil, M. Kinni and R. B. Sumathi, J. Coord. Chem., 2011, 64, 651–662 CrossRef CAS.
- M. B. Halli and R. S. Malipatil, Pharma Chem., 2011, 3, 146–157 CAS.
- M. B. Halli, V. B. Patil and S. R. Bevinamaradha, Turk. J. Chem., 2011, 35, 393–404 CAS.
- M. B. Halli, R. S. Malipatil and R. B. Sumathi, Int. J. Pharma Bio Sci., 2012, 3, 547–557 CAS.
- S. Goel, S. Chandra and S. D. Dwivedi, J. Saudi Chem. Soc., 2013 DOI:10.1016/j.jscs.2013.07.005.
- (a) M. Takanashi, Y. Takizawa and T. Mitsuhashi, Chem. Lett., 1974, 8, 869–871 CrossRef; (b) Y. Luo, Z. He and H. Li, Fitoterapia, 2007, 78, 211–214 CrossRef CAS PubMed.
- D. H. Choi, J. W. Hwang, H. S. Lee, D. M. Yang and J. G. Jun, Bull. Korean Chem. Soc., 2008, 29, 1594–1596 CrossRef CAS.
- K. Liouane, K. B. H. Salah, H. B. Abdel-kader, M. A. Mahjoub, M. Aouni, K. Said and Z. Mighri, Afr. J. Microbiol. Res., 2012, 6, 4662–4666 CAS.
- A. Urzúa, J. Echeverría, M. C. Rezende and M. Wilkens, Molecules, 2008, 13, 2385–2393 CrossRef.
- X. Du, C. Lu, Y. Li, Z. Zheng, W. Su and Y. Shen, J. Antibiot., 2008, 61, 250–253 CrossRef CAS PubMed.
- M. Y. Rios, A. B. Aguilar-guadarrama and V. Navarro, Planta Med., 2003, 69, 967–970 CrossRef CAS PubMed.
- S. Demizu, K. Kajiyama, K. Takahashi, Y. Hiraga, S. Yamamoto, Y. Tamura, K. Okada and T. Kinoshita, Chem. Pharm. Bull., 1988, 36, 3474–3479 CrossRef CAS PubMed.
- Q. Liu, L. Shen, T.-T. Wang, J. Chen, W. Y. Qi and K. Gao, Food Chem., 2010, 122, 55–59 CrossRef CAS.
- K. Yoshikawa, N. Kokudo, M. Tanaka, T. Nakano, H. Shibata, N. Aragaki, T. Higuchi and T. Hashimoto, Chem. Pharm. Bull., 2008, 56, 89–92 CrossRef CAS PubMed.
- C. Casero, F. Machín, S. Méndez-Álvarez, M. Demo, A. G. Ravelo, N. Pérez-Hernández, P. Joseph-Nathan and A. Estévez-Braun, J. Nat. Prod., 2015, 78, 93–102 CrossRef CAS PubMed.
- G. Belofsky, R. Carreno, K. Lewis, A. Ball, G. Casadei and G. P. Tegos, J. Nat. Prod., 2006, 69, 261–264 CrossRef CAS PubMed.
- P. Castaneda, L. Gomez and R. Mata, J. Nat. Prod., 1996, 59, 323–326 CrossRef CAS PubMed.
- T. Sastraruji, S. Chaiyong, A. Jatisatienr, S. G. Pyne, A. T. Ung and W. Lie, J. Nat. Prod., 2011, 74, 60–64 CrossRef CAS PubMed.
- E. Kato, Y. Tokunaga and F. Sakan, J. Agric. Food Chem., 2009, 57, 2544–2549 CrossRef CAS PubMed.
- F. A. Ramos, Y. Takaishi, M. Shirotori, Y. Kawaguchi, K. Tsuchiya, H. Shibata, T. Higuti, T. Tadokoro and M. Takeuchi, J. Agric. Food Chem., 2006, 54, 3551–3557 CrossRef CAS PubMed.
- C. Y. Ragasa, J. G. Hofilena and J. A. Rideout, J. Nat. Prod., 2002, 65, 1107–1110 CrossRef CAS.
- M. M. Rahman and A. I. Gray, Phytochemistry, 2005, 66, 1601–1606 CrossRef CAS PubMed.
- A. M. Do Nascimento, M. J. Salvador, R. C. Candidoc, I. Y. Ito and D. C. R. de Oliveira, Fitoterapia, 2004, 75, 514–519 CrossRef CAS PubMed.
Footnote |
| † Equal contribution from both the authors. |
| This journal is © The Royal Society of Chemistry 2015 |