Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photoinduced single-crystal-to-single-crystal phase transition and photosalient effect of a gold(I) isocyanide complex with shortening of intermolecular aurophilic bonds†
Tomohiro
Seki
,
Kenta
Sakurada
,
Mai
Muromoto
and
Hajime
Ito
*
Division of Chemical Process Engineering and Frontier Chemistry Center (FCC), Graduate School of Engineering, Hokkaido University, Sapporo, Hokkaido 060-8628, Japan. E-mail: hajito@eng.hokudai.ac.jp
First published on 27th November 2014
Abstract
We report the first photoinduced single-crystal-to-single-crystal (SCSC) phase transition of a gold complex that involves the shortening of intermolecular aurophilic bonds. This is also the first solid state photochromism of a gold complex. Upon UV irradiation, the blue-emitting crystals of the gold(I) isocyanide complex 1 (1B) transform into the weakly yellow-emitting polymorph 1Y. X-ray diffraction analyses reveal that this phase transition proceeds in an SCSC manner. After phase transition from 1B to 1Y, the intermolecular Au⋯Au separation decreases from 3.5041(14) to 3.2955(6) Å, resulting in a red-shifted emission. The photoinduced shortening of the aurophilic bond in the excited state initiates the change in the crystal structure from 1B to 1Y. Moreover, the crystal 1B showed a photosalient effect: the 1B crystals jump upon irradiation with strong UV light owing to the phase transition into 1Y. The aurophilic bond formation in the crystal generates the mechanical movement of the crystal.
Introduction
Photochromic organic solid materials attract considerable attention because of their wide applicability in sensing and recording devices.1 Changes in the solid structures of these materials are generally based on photochromic components exhibiting intramolecular photoinduced configurational alterations (e.g., stilbene2 and azobenzene3) and intramolecular covalent bond formation/cleavage (e.g., diarylethene4 and spiropyran5). Moreover, some organometallic complexes show solid-state photoinduced molecular structural alteration through the isomerization of their ligands.6 However, photochromic materials whose properties are based on changes in intermolecular noncovalent bonding interactions without chemical transformation or configurational alternation upon photoirradiation remain rare.The luminescence properties of many gold complexes are sensitive to their molecular arrangements and intermolecular interaction patterns.7 In particular, aurophilic interactions, which are noncovalent interactions between Au atoms, strongly affect the emission properties of gold complexes in both the solid state and solution.8 Gold complexes are currently considered to be an important research field because of their unique structural and luminescence properties and responses to stimuli.9 Single-crystal-to-single-crystal (SCSC) phase transitions of gold complexes can be induced by heating,10 pressure,11 and contact with a solvent.12 We recently reported that mechanical stimulation or solid seeding can also induce SCSC phase transitions of gold(I) isocyanide complexes.13 Theoretically, photoirradiation can change the aurophilic bonding structure.14 Indeed, transient shortening and rapid relaxation of aurophilic bonding in solution has been reported.15 However, solid-state phase changes of gold complexes where the photoinduced change of aurophilic interactions is a dominant factor have not been reported.16
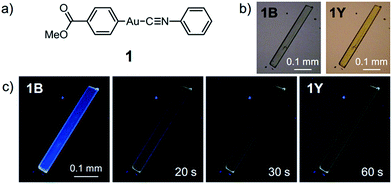
Here, we report the first photoinduced SCSC phase transition of a gold(I) complex that is accompanied by a decrease of the Au⋯Au distance. To the best of our knowledge, this report is also the first observation of solid-state photochromism in a gold complex.16 The colorless 1B phase, a crystallized form of the gold(I) isocyanide complex 1, exhibits blue photoluminescence (Fig. 1). Strong UV irradiation transforms 1B to the yellow 1Y phase, which exhibits weak yellow emission upon excitation (Fig. 1b and c). X-ray diffraction (XRD) analyses confirmed the SCSC phase transition from 1B to 1Y. A comparison of these crystal structures revealed the shortening of the distance between the Au atoms after the photoinduced SCSC phase transition. It is proposed that the SCSC phase transition of 1 proceeds through tightening of the aurophilic bonds in the excited state, which has not been observed previously. Indeed, the DFT calculations based on the crystal structures indicate that the shortening of the Au⋯Au distance caused the red-shifted emission in the 1Y phase generated after the photoirradiation of 1B. In addition, 1B exhibits a surprising “photosalient effect” upon irradiation with stronger UV light. To our knowledge, this report is the first observation of the photosalient effect which occurs through phase transition between polymorphs without chemical transformation or configurational alternations.17
Results and discussion
Photoinduced single-crystal-to-single-crystal phase transition
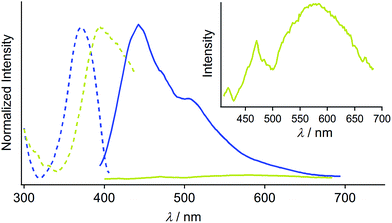
Complex 1 was prepared by modification of our reported procedures (Scheme 1).13,18 Briefly, the (4-methoxycarbonylphenyl)zinc(II) iodide reagent19 was mixed with chloro(phenyl isocyanide)gold(I) at 0 °C under a nitrogen atmosphere for 1 h to afford analytically pure 1 in 98% yield after subsequent purification by column chromatography and recrystallization from CH2Cl2/hexane. 1 was fully characterized using 1H and 13C NMR spectroscopy, high-resolution mass spectrometry and elemental analysis [see the ESI†]. The monomer of 1 in CH2Cl2 shows photoluminescence in the UV region and does not exhibit photochromism (Fig. S1 and S2†).Crystallization of complex 1 from CH2Cl2/hexane gave the blue-emitting polymorph 1B (Fig. 1c). The photoluminescence spectrum of 1B obtained upon excitation at 370 nm shows an emission band ranging from 400 to 700 nm with a maximum at 448 nm (blue solid line in Fig. 2).20 The absolute luminescence quantum yield Φem and average luminescence lifetime τav of 1B were determined to be 2.2% and 34.2 μs, respectively (Fig. S3 and Table S2†). The excitation spectrum of 1B detected at 450 nm confirmed the contribution from the absorption band at 371 nm (blue dashed line in Fig. 2). Both excitation and emission bands of 1B are red shifted from those of monomeric 1 in solution because of the crystal packing in the solid phase.
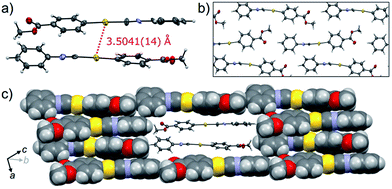
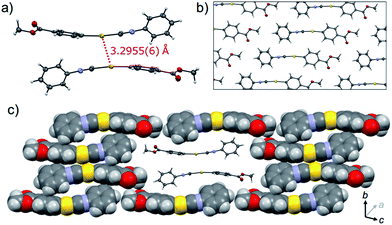
The crystalline structure of the polymorph 1B was characterized using single-crystal X-ray analysis. A single crystal of 1B suitable for the XRD required crystallization in the dark.21,221B crystallized in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) [R1 = 8.67%, wR2 = 24.96%, GOF = 1.106, a = 7.381(2) Å, b = 11.755(2) Å, c = 15.940(3) Å, α = 102.912(5)°, β = 92.025(5)°, γ = 100.595(5)°, Z = 4, V = 1320.8(4) Å3, Dcalc = 2.189 g cm−3] (Table 1 and S3†). In many cases, the R1 and wR2 values observed in 1B were slightly worse than those observed in 1Y, even when the same crystal was used for 1B and 1Y. This result could be attributed to the small contamination of the photoinduced phase 1Y in the original 1B. X-ray, elemental and thermal gravimetric analyses and 1H NMR measurements revealed that no solvent was included in the crystalline lattice of 1B (Fig. S5 and S6 and Table S3 and S4†). There are two crystallographically independent molecules in the lattice that exhibit dihedral angles of 26.76° and 56.04° between two intramolecular benzene rings (Fig. 3a). These molecules form dimers with a head-to-tail orientation and an intermolecular Au⋯Au distance of 3.5041(14) Å, indicating the formation of a very weak aurophilic bond.7,8 These dimers further stack with a longer Au⋯Au distance of 4.451(2) Å and contact with adjacent columns to form flat sheet-like layer structures (Fig. 3b and c).
[R1 = 8.67%, wR2 = 24.96%, GOF = 1.106, a = 7.381(2) Å, b = 11.755(2) Å, c = 15.940(3) Å, α = 102.912(5)°, β = 92.025(5)°, γ = 100.595(5)°, Z = 4, V = 1320.8(4) Å3, Dcalc = 2.189 g cm−3] (Table 1 and S3†). In many cases, the R1 and wR2 values observed in 1B were slightly worse than those observed in 1Y, even when the same crystal was used for 1B and 1Y. This result could be attributed to the small contamination of the photoinduced phase 1Y in the original 1B. X-ray, elemental and thermal gravimetric analyses and 1H NMR measurements revealed that no solvent was included in the crystalline lattice of 1B (Fig. S5 and S6 and Table S3 and S4†). There are two crystallographically independent molecules in the lattice that exhibit dihedral angles of 26.76° and 56.04° between two intramolecular benzene rings (Fig. 3a). These molecules form dimers with a head-to-tail orientation and an intermolecular Au⋯Au distance of 3.5041(14) Å, indicating the formation of a very weak aurophilic bond.7,8 These dimers further stack with a longer Au⋯Au distance of 4.451(2) Å and contact with adjacent columns to form flat sheet-like layer structures (Fig. 3b and c).
| 1B | 1Y | |
|---|---|---|
| a For data with I > 2.00σ(I). b For all reflection data. c Goodness of Fit. Residual electron density is mostly located near Au atoms because Au atoms have many electrons. See ESI Table S3 for more details. | ||
| CCDC name | CCDC 987280 | CCDC 987281 |
| Crystal system | Triclinic | Triclinic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (#2) (#2) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (#2) (#2) |
| a/Å | 7.381(2) | 6.0552(5) |
| b/Å | 11.755(2) | 7.0297(6) |
| c/Å | 15.940(3) | 15.969(2) |
| α/° | 102.912(5) | 96.315(3) |
| β/° | 92.025(5) | 93.979(3) |
| γ/° | 100.595(5) | 90.279(3) |
| V/Å3 | 1320.8(4) | 673.9(1) |
| Z Value | 4 | 2 |
| V/Z/Å3 | 330.2 | 337.0 |
| D calc/g cm−3 | 2.189 | 2.145 |
| Residual R1a/% | 8.67 | 5.42 |
| Residual wR2b/% | 24.96 | 13.30 |
| GOFc | 1.106 | 1.045 |
The photophysical properties of the polymorph 1B were altered upon photoirradiation. Upon photoirradiation of 1B using a fluorescence microscope equipped with an ultrahigh-pressure mercury lamp (367 nm, approx. 100 mW cm−2) for 60 s at room temperature, the emission color of the crystals gradually changed from blue to yellow because of the formation of the polymorph 1Y (Fig. 1c). During this process, the diffraction pattern also gradually changed (Fig. S7†), and the transparency of the crystal was retained (Fig. 1b and S8†). UV light with a lower power density necessitates a longer irradiation time to induce a transformation from 1B to 1Y (Table S1†). As shown in Fig. 2, the intensity of the emission from 1B decreases under strong UV irradiation and becomes almost zero after 60 s under strong UV irradiation. The resulting 1Y phase exhibits weak emission with a structureless maximum at 580 nm (inset in Fig. 2). This band is red shifted from that of 1B by 132 nm. The polymorph 1Y exhibits a very low Φem of ∼0.5% (Table S2†). The emission lifetime τav of 1Y is 0.685 μs, which is a decrease of ∼2% upon phase transition (Fig. S3 and Table S2†). The excitation spectrum of 1Y detected at 590 nm revealed the contribution from the absorption band at 394 nm (greenish yellow dashed line in Fig. 2). Because the excitation maximum is red shifted by 23 nm upon phase transition, the crystal becomes yellow (Fig. 1b).
XRD analyses indicate that 1Y is formed through the SCSC phase transition of 1B. The photoinduced SCSC phase transition reproducibly occurred from single crystals of 1B to give 1Y crystals of suitable quality for single-crystal X-ray analysis. After the single crystal X-ray analysis of 1B, it was photoirradiated (367 nm, approx. 100 mW cm−2, 60 s) to yield a crystal of 1Y, and this sample was of sufficient quality for single crystal X-ray analysis [P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , R1 = 5.42%, wR2 = 13.30%, GOF = 1.045, a = 6.0552(5) Å, b = 7.0297(6) Å, c = 15.969(2) Å, α = 96.315(3)°, β = 93.979(3)°, γ = 90.279(3)°, Z = 2, V = 673.9(1) Å3, Dcalc = 2.145 g cm−3] (Table 1 and S3†). X-ray, elemental, and thermal gravimetric analyses and 1H NMR measurements revealed that decomposition of these compounds does not take place and that solvent is not included (Fig. S5 and S6 and Table S3 and S4†). 1Y has a triclinic space group P
, R1 = 5.42%, wR2 = 13.30%, GOF = 1.045, a = 6.0552(5) Å, b = 7.0297(6) Å, c = 15.969(2) Å, α = 96.315(3)°, β = 93.979(3)°, γ = 90.279(3)°, Z = 2, V = 673.9(1) Å3, Dcalc = 2.145 g cm−3] (Table 1 and S3†). X-ray, elemental, and thermal gravimetric analyses and 1H NMR measurements revealed that decomposition of these compounds does not take place and that solvent is not included (Fig. S5 and S6 and Table S3 and S4†). 1Y has a triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . Like 1B, the molecules form dimers with a head-to-tail orientation. The intermolecular Au⋯Au distance in the dimer of 1Y is 3.2955(6) Å, which is a marked decrease of ca. 0.2 Å upon crystalline structural transformation. This shorter distance indicates a stronger aurophilic bond in 1Y compared with that in 1B. The dihedral angle between the two benzene rings in a molecule is 47.01° (Fig. 4a). The isocyanide benzene ring participates in weak CH/π interactions with the adjacent benzene ring on the Au atom. The molecules adopt a severely distorted conformation: the structures of the –Au–C
. Like 1B, the molecules form dimers with a head-to-tail orientation. The intermolecular Au⋯Au distance in the dimer of 1Y is 3.2955(6) Å, which is a marked decrease of ca. 0.2 Å upon crystalline structural transformation. This shorter distance indicates a stronger aurophilic bond in 1Y compared with that in 1B. The dihedral angle between the two benzene rings in a molecule is 47.01° (Fig. 4a). The isocyanide benzene ring participates in weak CH/π interactions with the adjacent benzene ring on the Au atom. The molecules adopt a severely distorted conformation: the structures of the –Au–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N– moieties deviate markedly from linearity, unlike in 1B. The thermodynamic destabilization arising from the distortion of the molecule is probably compensated for by the formation of aurophilic bonds and weak CH/π interactions in the solid lattice (Fig. 4b and c).
N– moieties deviate markedly from linearity, unlike in 1B. The thermodynamic destabilization arising from the distortion of the molecule is probably compensated for by the formation of aurophilic bonds and weak CH/π interactions in the solid lattice (Fig. 4b and c).
Mechanistic studies of the photoinduced single-crystal-to-single-crystal phase transition
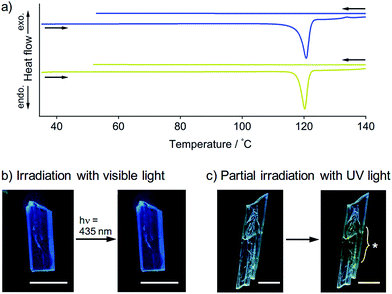
To obtain further insights into the SCSC phase transition of 1, a series of control experiments was performed. Upon heating, neither 1B nor 1Y showed thermal polymorph transformation, and both chemically decomposed at ca. 120 °C (Fig. S9†). The corresponding endothermic peaks were found in the differential scanning calorimetry profiles at 121 °C for 1B and 120 °C for 1Y, and no other peaks were observed even in the cooling profiles (Fig. 5a). This result confirms the absence of a thermal phase transition between 1B and 1Y. Thermal analyses using a thermography camera also confirmed that the photogenerated heat was negligible (less than 2 °C, Fig. S10 and S11†). We also confirmed that the photoinduced SCSC phase transition of 1B was not initiated by visible light (435 nm, approx. 200 mW cm−2, Fig. 5b and S12†). Moreover, upon UV irradiation (367 nm, approx. 100 mW cm−2) of a local area of a 1B crystal, the phase transition occurred only in the photoirradiated area and did not expand into the unirradiated region (Fig. 5c), indicating the absence of a “domino-like” mechanism.13 These control experiments confirmed that the phase transition of 1B was induced by the UV photoexcitation of the crystals.A schematic representation of aurophilic bonds and their simplified orbital levels is shown in Fig. 6. Aurophilic interactions arising from the correlation and relativistic effects of Au atoms produce filled dz2σ and dz2σ* molecular orbitals and result in decreased excitation energy [(i) and (ii) in Fig. 6a].7 Aurophilic bonds shorten in the excited state because of the electronic transition from the filled antibonding dz2σ* orbital located on the aurophilic bond to an empty orbital of higher energy [(iii) in Fig. 6a]. Although theoretical studies on this topic have been reported,14 it has seldom been observed experimentally. Tahara's group observed aurophilic bond shortening using time-resolved spectroscopy of aqueous solutions of [Au(CN)2−]n complexes.15 In this study, the trimeric species [Au(CN)2−]3 predominantly present in the ground state associated into larger oligomers upon photoexcitation through the excited [Au(CN)2−]3* state with shortening of the aurophilic bonds (Fig. 6b). However, in this case, the original ground state recovered when photoirradiation ceased (Fig. 6b) because the molecules have enough mobility in aqueous solution to reorient to their original structure.
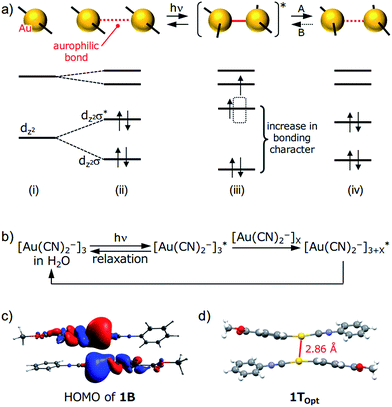 | ||
| Fig. 6 (a) A schematic representation of aurophilic bonds and their simplified orbital levels. An aurophilic bond produces dz2σ and dz2σ* orbitals and shortens upon photoexcitation. (b) A schematic representation of the relaxation pathway upon photoirradiation of [Au(CN)2−]n complexes. See ref. 15 for details. (c) HOMO of the dimer derived from the single-crystal structure of 1B (PBEPBE/SDD). (d) The structure of 1TOpt obtained using triplet excited-state optimization of a 1B dimer in a vacuum (PBEPBE/SDD). | ||
Although the detailed mechanism for the photoinduced SCSC phase transition of 1 is still unknown, we can provide some mechanistic insights by computational investigation (Fig. 6). A DFT calculation (PBEPBE/SDD) of the dimer of 1 derived from the single-crystal structure of 1B possesses a HOMO with antibonding character located between the two Au atoms even though the separation between the Au atoms is on the limit of an aurophilic bond (Fig. 6c). TDDFT calculations based on the crystal structures of 1B and 1Y qualitatively matched the experimental excitation and emission spectra (see the ESI and Fig. S14†). Because the structural optimization of the crystal structures of 1B and 1Y is very difficult in terms of the computational cost, quantitative estimations of the thermodynamic stability and luminescence properties could not be achieved. Geometry optimization of the dimer derived from 1B in the triplet state (1TOpt) in a vacuum gave a marked decrease in the Au⋯Au separation (2.86 Å, Fig. 6d). The structures and molecular orbitals of 1TOpt are more similar to those of the dimer of 1Y compared with those in 1B (Fig. S13 and S15 and Table S7†). These results indicate that the structure transformation from 1B to 1Y can be facilitated through the photoexcited state of 1B [(ii)–(iv) in Fig. 6a]. Although the structural conversion from the photoexcited state to 1Y (“A” in Fig. 6a) would compete with the relaxation to the original ground state 1B, once the phase transformation to 1Y occurred in a local area, the reverse phase transition from 1Y to 1B (“B” in Fig. 6a) does not proceed thermally, probably because the movement of the molecules in the 1Y polymorph is restricted by the relatively strong intermolecular interactions, such as CH/π interactions and aurophilic interactions (Fig. 4). Irradiation to 1Y could not cause it to revert to 1B because the structure of the excited state would be more similar to 1Y than the structure of 1B. Thus, the entire 1B crystal eventually transformed into the new phase 1Y after a certain period of photoirradiation. Although many studies on the transient shortening of metallophilic bonds (Pt, Rh, and Ir) in the photoexcited state have been reported, these structural changes reverted back to the original structures after photoirradiation ceased.23 The present study is the first example of a photoinduced SCSC transformation with a shortening of metallophilic bonds.
The first photosalient effect induced by a phase transition between polymorphs
Recently, a rare dynamic phenomenon of organic crystals, the “salient effect”, has been attracting much attention.17 The thermosalient effect, in which organic crystals jump after the crystal is heated, was first reported in 1983.24 A related phenomenon in which a crystal jumps due to irradiation with light is referred to as the “photosalient effect”.17 The “salient effect” is produced by the internal crystal constraints imposed by changes in the crystalline shape and/or volume at the interface between the two phases upon the thermo- or photoinduced crystalline structural change. Recently, intense explorations of thermo- and photosalient effects have been made by Naumov and colleagues;17,25,26 however, compounds that show the photosalient effect are still very rare.17,26We found that the 1B crystals show the “salient effect” upon strong UV light irradiation (367 nm, approx. 400 mW cm−2). As shown in Fig. 7a–f and the ESI Movie S1,† the blue emission of the surface of the 1B crystal becomes yellow after 5 s of photoirradiation through the phase transition from 1B to 1Y (see Fig. 7a and b), and after 21 s the crystal jumps (see Fig. 7c–e). Not all crystals of 1B showed this jumping phenomenon; approximately 1% of the irradiated crystals of 1B jumped (see also the ESI Movie S2 and Fig. 7g–l and S16†) and approximately 10% of the irradiated crystals split (ESI Movie S3 and Fig. S17†). Furthermore, more than 80% of the irradiated crystals showed cracking (Fig. S18†). These mechanical responses upon photoirradiation suggested that the transformation from 1B to 1Y is a martensitic-type transition, similar to most salient-active materials, rather than a phase transition through a nucleation–elongation mechanism. In Naumov's articles,17,25 the authors discussed the idea that the thermosalient phase transition generally occurs between two phases with an identical symmetry and space group. Thus, the overall packing and unit cell parameters of the two phases are only slightly, yet distinctly changed upon applying the external stimuli. This notion can also be applied to the photosalient phase transition of 1 because the crystalline structures of 1B and 1Y fulfil these requirements: the space groups of 1B and 1Y are both P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , and their V/Z values are almost the same (1B: 330.2 Å3; 1Y: 337.0 Å3, Table 1). The photosalient effect is generally induced by a photochemical reaction (chemical structural changes, e.g., a [2 + 2] cycloaddition, a ring closing reaction, etc.).26 Contrary, the present study is the first example of the photosalient effect induced by a phototriggered phase transition between polymorphs (a packing structural change or molecular arrangement change) without chemical structural changes. As shown in the aforementioned control experiments, the heating effect that would accompany photoexcitation17 has negligible influence on the phase transition of 1 (Fig. S10 and S11†), and thus is not related to its photosalient effect. This conclusion is also supported by the fact that 1B does not show any thermal phase change below 120 °C (Fig. 5a). The aurophilic bonding formation in the crystal was induced by light irradiation and generated the macro-scale mechanical power that can sputter the crystals.
, and their V/Z values are almost the same (1B: 330.2 Å3; 1Y: 337.0 Å3, Table 1). The photosalient effect is generally induced by a photochemical reaction (chemical structural changes, e.g., a [2 + 2] cycloaddition, a ring closing reaction, etc.).26 Contrary, the present study is the first example of the photosalient effect induced by a phototriggered phase transition between polymorphs (a packing structural change or molecular arrangement change) without chemical structural changes. As shown in the aforementioned control experiments, the heating effect that would accompany photoexcitation17 has negligible influence on the phase transition of 1 (Fig. S10 and S11†), and thus is not related to its photosalient effect. This conclusion is also supported by the fact that 1B does not show any thermal phase change below 120 °C (Fig. 5a). The aurophilic bonding formation in the crystal was induced by light irradiation and generated the macro-scale mechanical power that can sputter the crystals.
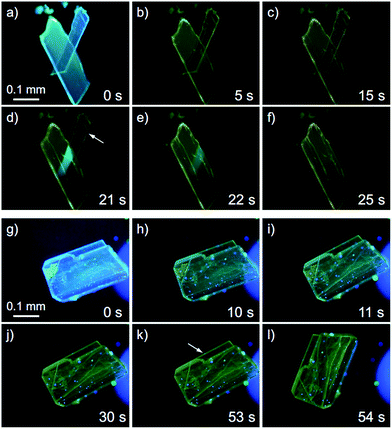 | ||
| Fig. 7 A series of photographs of the photosalient effect of 1B through the transformation into 1Y induced by strong photoirradiation (367 nm, approx. 400 mW cm−2). Photographs from (a) to (f) and (g) to (l) were cropped from the ESI Movies S1 and S2,† respectively. The arrows in (d) and (k) indicate the crystals just before the jump. The irradiation times are shown. | ||
Conclusion
In summary, we have described the first photoinduced SCSC phase transition of the gold(I) isocyanide complex 1 with a shortening of the aurophilic bond. This phase transformation was accompanied by a defined color change under UV light. We propose that the shortening of the aurophilic bond in the excited state is the driving force behind this transition; structural relaxation from the excited state to 1Y is followed by immobilization of the structural change, including the short aurophilic bond, which inhibits reversal to the original 1B. The first photosalient effect induced by a polymorph transformation was also discovered. These findings could be an important lead in the design of novel photosensitive materials that exhibit reorganization of intermolecular interactions induced by photoirradiation.Acknowledgements
This work was financially supported by the Funding Program for Next Generation World-Leading Researchers (NEXT Program, no. G002) from the Japan Society for the Promotion of Science (JSPS) and the MEXT (Japan) program “Strategic Molecular and Materials Chemistry through Innovative Coupling Reactions” of Hokkaido University. JSPS KAKENHI Grant-in-Aid for Young Scientists (B), 26810042.Notes and references
- J. Zhang, Q. Zou and H. Tian, Adv. Mater., 2013, 25, 378–399 CrossRef CAS PubMed
.
- J. W. Chung, S.-J. Yoon, B.-K. An and S. Y. Park, J. Phys. Chem. C, 2013, 117, 11285–11291 CAS
.
- O. S. Bushuyev, A. Tomberg, T. Friščić and C. J. Barrett, J. Am. Chem. Soc., 2013, 135, 12556–12559 CrossRef CAS PubMed
.
-
(a) S. Kobatake, S. Takami, H. Muto, T. Ishikawa and M. Irie, Nature, 2007, 446, 778–781 CrossRef CAS PubMed
; (b) M. Morimoto and M. Irie, J. Am. Chem. Soc., 2010, 132, 14172–14178 CrossRef CAS PubMed
; (c) D. Kitagawa, H. Nishi and S. Kobatake, Angew. Chem., Int. Ed., 2013, 52, 9320–9322 CrossRef CAS PubMed
.
- S. Iyengar and M. C. Biewer, Cryst. Growth Des., 2005, 5, 2043–2045 CAS
.
-
(a) A. Y. Kovalevsky, K. A. Bagley and P. Coppens, J. Am. Chem. Soc., 2002, 124, 9241–9248 CrossRef CAS PubMed
; (b) H. Nakai, M. Mizuno, T. Nishioka, N. Koga, K. Shiomi, Y. Miyano, M. Irie, B. K. Breedlove, I. Kinoshita, Y. Hayashi, Y. Ozawa, T. Yonezawa, K. Toriumi and K. Isobe, Angew. Chem., Int. Ed., 2006, 45, 6473–6476 CrossRef CAS PubMed
; (c) A. Kobayashi, K. Komatsu, H. Ohara, W. Kamada, Y. Chishina, K. Tsuge, H. C. Chang and M. Kato, Inorg. Chem., 2013, 52, 13188–13198 CrossRef CAS PubMed
; (d) L. E. Hatcher and P. R. Raithby, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2013, 69, 1448–1456 CrossRef CAS PubMed
.
- For selected reviews and books on gold complexes, see:
(a) A. L. Balch, Gold Bull., 2004, 37, 45–50 CrossRef CAS
; (b) P. Pyykkö, Angew. Chem., Int. Ed., 2004, 43, 4412–4456 CrossRef PubMed
; (c) V. W.-W. Yam, in Photofunctional Transition Metal Complexes, Springer-Verlag, Berlin/Heidelberg, 2007 Search PubMed
; (d) J. M. López-de-Luzuriaga, in Modern Supramolecular Gold Chemistry, ed. A. Laguna, Wiley-VCH, Weinheim, 2008 Search PubMed
; (e) V. W.-W. Yam and E. C. Cheng, Chem. Soc. Rev., 2008, 37, 1806–1813 RSC
; (f) M. J. Katz, K. Sakai and D. B. Leznoff, Chem. Soc. Rev., 2008, 37, 1884–1895 RSC
; (g) H. Schmidbaur and A. Schier, Chem. Soc. Rev., 2008, 37, 1931–1951 RSC
; (h) A. L. Balch, Angew. Chem., Int. Ed., 2009, 48, 2641–2644 CrossRef CAS PubMed
; (i) X. He and V. W.-W. Yam, Coord. Chem. Rev., 2011, 255, 2111–2123 CrossRef CAS PubMed
; (j) H. Schmidbaur and A. Schier, Chem. Soc. Rev., 2012, 41, 370–412 RSC
.
- For selected reports on the correlation between the aurophilic interactions and emission properties of gold complexes, see:
(a) M. A. Rawashdeh-Omary, M. A. Omary, H. H. Patterson and J. P. Fackler, J. Am. Chem. Soc., 2001, 123, 11237–11247 CrossRef CAS PubMed
; (b) W. Lu, N. Zhu and C. M. Che, J. Am. Chem. Soc., 2003, 125, 16081–16088 CrossRef CAS PubMed
; (c) L. Rodríguez, M. Ferrer, R. Crehuet, J. Anglada and J. C. Lima, Inorg. Chem., 2012, 51, 7636–7641 CrossRef PubMed
; (d) S. H. Lim, J. C. Schmitt, J. Shearer, J. Jia, M. M. Olmstead, J. C. Fettinger and A. L. Balch, Inorg. Chem., 2013, 52, 823–831 CrossRef CAS PubMed
. For related studies, see: (e) E. C.-C. Cheng, K.-H. Leung, V. M. Miskowski, V. W.-W. Yam and D. L. Phillips, Inorg. Chem., 2000, 39, 3690–3695 CrossRef CAS
; (f) R. L. White-Morris, M. M. Olmstead, A. L. Balch, O. Elbjeirami and M. A. Omary, Inorg. Chem., 2003, 42, 6741–6748 CrossRef CAS PubMed
.
-
(a) J. C. Vickery, M. M. Olmstead, E. Y. Fung and A. L. Balch, Angew. Chem., Int. Ed., 1997, 36, 1179–1181 CrossRef CAS
; (b) S. H. Lim, M. M. Olmstead and A. L. Balch, J. Am. Chem. Soc., 2011, 133, 10229–10238 CrossRef CAS PubMed
; (c) M. A. Malwitz, S. H. Lim, R. L. White-Morris, D. M. Pham, M. M. Olmstead and A. L. Balch, J. Am. Chem. Soc., 2012, 134, 10885–10893 CrossRef CAS PubMed
.
-
(a) E. M. Gussenhoven, J. C. Fettinger, D. M. Pham, M. A. Malwitz and A. L. Balch, J. Am. Chem. Soc., 2005, 127, 10838–10839 CrossRef CAS PubMed
. For the thermo-induced SCSC phase transition of a gold complex without an emission color change, see: (b) A. Deák, T. Tunyogi, Z. Károly, S. Klébert and G. Pálinkás, J. Am. Chem. Soc., 2010, 132, 13627–13629 CrossRef PubMed
.
- C. H. Woodall, C. M. Beavers, J. Christensen, L. E. Hatcher, M. Intissar, A. Parlett, S. J. Teat, C. Reber and P. R. Raithby, Angew. Chem., Int. Ed., 2013, 52, 9691–9694 CrossRef CAS PubMed
.
- S. H. Lim, M. M. Olmstead and A. L. Balch, Chem. Sci., 2013, 4, 311–318 RSC
.
-
(a) T. Seki, K. Sakurada and H. Ito, Angew. Chem., Int. Ed., 2013, 52, 12828–12832 CrossRef CAS PubMed
; (b) H. Ito, M. Muromoto, S. Kurenuma, S. Ishizaka, N. Kitamura, H. Sato and T. Seki, Nat. Commun., 2013, 4, 2009 Search PubMed
.
- For selected reports on theoretical studies of tightening aurophilic bonds under photoexcitation, see:
(a) Q.-J. Pan and H.-X. Zhang, Inorg. Chem., 2004, 43, 593–601 CrossRef CAS PubMed
; (b) R. K. Arvapally, P. Sinha, S. R. Hettiarachchi, N. L. Coker, C. E. Bedel, H. H. Patterson, R. Elder, A. K. Wilson and M. A. Omary, J. Phys. Chem. C, 2007, 111, 10689–10691 CrossRef CAS
; (c) G. Cui, X. Y. Cao, W. H. Fang, M. Dolg and W. Thiel, Angew. Chem., Int. Ed., 2013, 52, 10281–10285 CrossRef CAS PubMed
.
- M. Iwamura, K. Nozaki, S. Takeuchi and T. Tahara, J. Am. Chem. Soc., 2013, 135, 538–541 CrossRef CAS PubMed
.
- For a gold complex exhibiting photochromism in solution, see: J. B. Foley, A. Herring, B. Li and E. V. Dikarev, Inorg. Chim. Acta, 2012, 392, 300–310 CrossRef CAS PubMed
.
- For a highlight article on the thermo- and photosalient effects of organic crystals as well as related mechanical responses to external stimuli, see: N. K. Nath, M. K. Panda, S. C. Sahoo and P. Naumov, CrystEngComm, 2014, 16, 1850–1858 RSC
.
- T. Seki, S. Kurenuma and H. Ito, Chem.–Eur. J., 2013, 19, 16214–16220 CrossRef CAS PubMed
.
- A. Krasovskiy, V. Malakhov, A. Gavryushin and P. Knochel, Angew. Chem., Int. Ed., 2006, 45, 6040–6044 CrossRef CAS PubMed
.
- The power density of the UV light for measuring the photoluminescence spectra was less than 0.1 mW cm−2, under which 1B is inert for hours (Table S1†).
- Crystallization of 1 under ambient light gave blue-emitting crystals that were not suitable for single-crystal X-ray diffraction analysis, probably because ambient light, containing both UV and visible light, may create a small amount of contamination of 1Y during the course of the crystallization. To prepare 1B crystals of sufficient quality for X-ray analysis, crystallization should be conducted in the dark.
- Single crystal X-ray diffraction analyses of 1B and 1Y were performed at 123 K. Lowering the temperature of 1B and 1Y does not induce their polymorph transformation. This is confirmed by the luminescence spectroscopy of 1B and 1Y at low temperature, exhibiting spectra similar to those obtained at ambient temperature (Fig. S4†).
- For selected examples of the transient shortening of metallophilic bonds, see:
(a) N. Yasuda, H. Uekusa and Y. Ohashi, Bull. Chem. Soc. Jpn., 2004, 77, 933–944 CrossRef CAS
; (b) M. Christensen, K. Haldrup, K. Bechgaard, R. Feidenhans'l, Q. Kong, M. Cammarata, M. L. Russo, M. Wulff, N. Harrit and M. M. Nielsen, J. Am. Chem. Soc., 2009, 131, 502–508 CrossRef CAS PubMed
; (c) R. M. van der Veen, C. J. Milne, A. E. Nahhas, F. A. Lima, V. T. Pham, J. Best, J. A. Weinstein, C. N. Borca, R. Abela, C. Bressler and M. Chergui, Angew. Chem., Int. Ed., 2009, 48, 2711–2714 CrossRef CAS PubMed
; (d) K. Haldrup, T. Harlang, M. Christensen, A. Dohn, T. B. van Driel, K. S. Kjaer, N. Harrit, J. Vibenholt, L. Guerin, M. Wulff and M. M. Nielsen, Inorg. Chem., 2011, 50, 9329–9336 CrossRef CAS PubMed
. For the transient shortening of the bonds between Au and ligands in gold complexes, see: (e) M. Hoshino, H. Uekusa, S. Ishii, T. Otsuka, Y. Kaizu, Y. Ozawa and K. Toriumi, Inorg. Chem., 2010, 49, 7257–7265 CrossRef CAS PubMed
; (f) As a related study, for reports on the photocleavage of argentophilic (Ag⋯Ag) bonds in the solid state which was mediated by the 2 + 2 photocyclization of olefins, see: Q. Chu, D. C. Swenson and L. R. MacGillivray, Angew. Chem., Int. Ed., 2005, 44, 3569–3572 CrossRef CAS PubMed
.
- M. C. Etter and A. R. Siedle, J. Am. Chem. Soc., 1983, 105, 641–643 CrossRef CAS
.
- For a comprehensive article on the thermosalient effect, see: S. C. Sahoo, M. K. Panda, N. K. Nath and P. Naumov, J. Am. Chem. Soc., 2013, 135, 12241–12251 CrossRef CAS PubMed
.
- For photosalient effect, see:
(a) P. Naumov, J. Kowalik, K. M. Solntsev, A. Baldridge, J.-S. Moon, C. Kranz and L. M. Tolbert, J. Am. Chem. Soc., 2010, 132, 5845–5857 CrossRef CAS PubMed
; (b) P. Naumov, S. C. Sahoo, B. A. Zakharov and E. V. Boldyreva, Angew. Chem., Int. Ed., 2013, 52, 9990–9995 CrossRef CAS PubMed
; (c) R. Medishetty, A. Husain, Z. Bai, T. Runčevski, R. E. Dinnebier, P. Naumov and J. J. Vittal, Angew. Chem., Int. Ed., 2014, 53, 5907–5911 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray crystallographic data, optical properties, DFT calculations, and other additional information. CCDC [987280 and 987281]. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4sc02676d |
| This journal is © The Royal Society of Chemistry 2015 |