Open Access Article
Open Access ArticleAnnihilation electrogenerated chemiluminescence of mixed metal chelates in solution: modulating emission colour by manipulating the energetics
Emily
Kerr
a,
Egan H.
Doeven
a,
Gregory J.
Barbante
*a,
Conor F.
Hogan
*b,
David J.
Bower
b,
Paul S.
Donnelly
c,
Timothy U.
Connell
c and
Paul S.
Francis
*a
aCentre for Chemistry and Biotechnology, School of Life and Environmental Sciences, Faculty of Science, Engineering and Built Environment, Deakin University, Geelong, Victoria 3220, Australia. E-mail: paul.francis@deakin.edu.au; g.barbante@deakin.edu.au
bDepartment of Chemistry, La Trobe Institute for Molecular Science, La Trobe University, Melbourne, Victoria 3086, Australia. E-mail: C.Hogan@latrobe.edu.au
cSchool of Chemistry and Bio21 Molecular Science and Biotechnology Institute, University of Melbourne, Melbourne 3010, Australia
First published on 15th October 2014
Abstract
We demonstrate the mixed annihilation electrogenerated chemiluminescence of tris(2,2′-bipyridine)ruthenium(II) with various cyclometalated iridium(III) chelates. Compared to mixed ECL systems comprising organic luminophores, the absence of T-route pathways enables effective predictions of the observed ECL based on simple estimations of the exergonicity of the reactions leading to excited state production. Moreover, the multiple, closely spaced reductions and oxidations of the metal chelates provide the ability to finely tune the energetics and therefore the observed emission colour. Distinct emissions from multiple luminophores in the same solution are observed in numerous systems. The relative intensity of these emissions and the overall emission colour are dependent on the particular oxidized and reduced species selected by the applied electrochemical potentials. Finally, these studies offer insights into the importance of electronic factors in the question of whether the reduced or oxidized partner becomes excited in annihilation ECL.
Introduction
Electrogenerated chemiluminescence (ECL) is the luminescence arising from electron transfer reactions in which at least one reactant has been electrochemically generated. This phenomenon has not only provided an excellent means for highly sensitive chemical detection,1–3 but also enabled extensive exploration of exergonic electron-transfer reactions in solution.4,5 In fact, ECL investigations provided the first experimental verifications of the ‘inverted region’ of Marcus electron transfer theory,6,7 where the electron transfer rates of highly exergonic reactions decrease with increasing free energy. Thus, under certain circumstances, the formation of (luminescent) excited states occur at much faster rates than the thermodynamically favoured ground state products.7Central to ECL is the ‘annihilation’ pathway, in which oxidized and reduced species are formed, usually sequentially, at two different electrode potentials,1,3 with the subsequent comproportionation of these species generating an emissive excited state. For example, the singlet excited state of an organic luminophore may be generated via recombination of its electrogenerated radical anion and cation as in reaction 1–4 (known as the S-route). The oxidized and reduced species can also be generated from different parent compounds, which is referred to as a ‘mixed’ ECL system.
| R + e− → R˙− | (1) |
| R − e− → R˙+ | (2) |
| R˙− + R˙+ → R + 1R* | (3) |
| 1R* → 1R + hν | (4) |
For organic ECL systems, the above excitation process is frequently in competition with generation of the lower-lying triplet excited-state (5). In solution at room temperature, organic triplets are generally non-emissive, but triplet–triplet annihilation can generate the emissive excited state (known as the T-route) (6), even in reactions that lack sufficient energy for direct singlet population (3).5,8
| R˙− + R˙+ → R + 3R* | (5) |
| 3R* + 3R* → R + 1R* | (6) |
Accordingly, annihilation ECL is somewhat simplified for luminophores exhibiting efficient phosphorescence at room temperature. This includes various transition metal complexes such as tris(2,2′-bipyridine)ruthenium(II) (Ru(bpy)32+),9,10 in which the emission of light from the lowest lying triplet state is facilitated by spin–orbit coupling induced by the heavy-metal ion.
Mixed annihilation systems combining more than one organic compound,5 or a transition metal chelate with a non-emissive organic compounds,11–13 have been widely used to study bimolecular electron-transfer reactions and the competitive generation of excited states. Demonstrations of ECL from cyclometalated iridium(III) chelates in recent years,12–15 with emission maxima spanning the entire visible region, has created new opportunities for multi-colour ECL. Several reports of mixed metal chelate co-reactant ECL systems incorporating ruthenium(II) and iridium(III) complexes have emerged, where excitation is achieved solely by applied oxidative potentials.16–19 This includes cases in which the emissions were resolved by selective excitation at different potentials.17–19 Similarly, the co-reactant ECL of Ru(bpy)32+ and peroxydisulfate has recently been used in conjunction with luminol ECL detection in a potential-resolved immunoassay of two different antigens at a cell surface.20
Ruthenium(II) and iridium(III) chelates have also been extensively utilized in light-emitting devices,3,21 and several research groups have combined an electrochemiluminescent Ru(bpy)32+ derivative with an electroluminescent material for bias- or potential-controlled switching between emission colours.22 The use of multiple transition metal chelates in this context offers several major advantages, as demonstrated by Su et al.,23 who combined a blue-green and red emitting iridium(III) complex in a solid-state electrochemical cell to generate white electroluminescence, and Moon et al.,24 who recently created emissive plastic displays based on the mixed annihilation ECL of Ru(bpy)32+ and Ir(df-ppy)2(bpy)+ in block-copolymer-based ion-gels. This approach enabled Moon et al. to set the emission colour from orange-red to green, based on the mole ratio of the incorporated complexes.24
Despite these impressive advances towards multiplexed ECL detection systems and colour-tuneable light-emitting technologies, annihilation ECL from mixed transition metal-chelate systems in simple solution is yet to be explored. This is surprising, as the fundamental understanding gained from such studies may underpin new developments in these areas. One basic mechanistic question that has remained unanswered in relation to annihilation ECL is whether the reduced or oxidized partner becomes excited following the comproportionation reaction. In the case of ruthenium complexes, formation of an excited state from the reduced parent requires a metal-to-metal electron transfer, whereas formation of an excited state from the oxidized species involves a ligand-to-ligand electron transfer. This suggests that the latter route ought to predominate due to more a favourable electronic factor. By exploring mixed ECL systems where the components have differing localizations of electron density associated with their frontier orbitals, we hope to gain insight into the importance of electronic factors in annihilation ECL.
Utilizing an electrochemical cell coupled with a CCD spectrometer for instantaneous collection of emission spectra, we have examined the multi-colour emissions from a series of mixed annihilation ECL systems containing Ru(bpy)32+ and various cyclometalated iridium(III) chelates exhibiting green or blue luminescence, to understand and control the relative emission intensities of these novel ECL systems.
Experimental section
Chemicals
Acetonitrile (Ajax Finechem, Australia) was distilled over calcium hydride under grade 5 argon. Solutions were degassed with argon prior to analysis. The chemical structure and luminescence chromaticity of each ruthenium and iridium complex used in this study is shown in Fig. 1.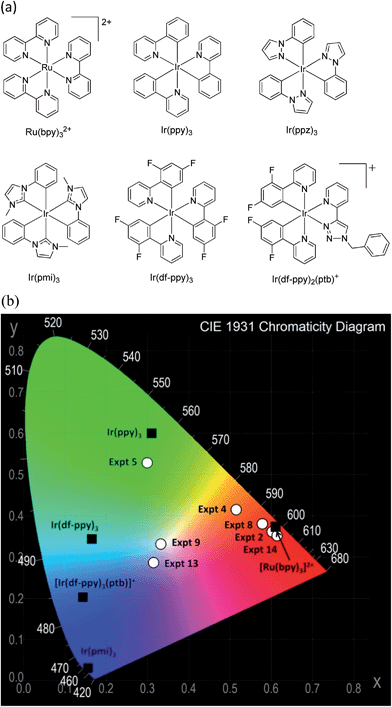 | ||
| Fig. 1 (a) Ruthenium and iridium complexes used in this study. (b) CIE chromaticity characterization of the photoluminescence of individual complexes (black squares) and the ECL from mixtures of complexes (white circles). The photoluminescence CIE coordinates were obtained using a fluorescence spectrophotometer with integrating sphere and corrected CCD detector.15 The ECL CIE coordinates were calculated using the mean RGB values19 for the circular area of the electrode in the photographs shown in subsequent figures. The colour space representation was generated with efg's Computer Lab software. | ||
Tetrabutylammonium hexafluorophosphate (TBAPF6, 99.5%, electrochemical grade) was purchased from Sigma-Aldrich (Australia). The hexafluorophosphate salt of tris(2,2′-bipyridine-κN1,κN1′)ruthenium(2+) ([Ru(bpy)3](PF6)2) was prepared from Ru(bpy)3Cl2·6H2O (Strem Chemicals, USA). fac-Tris[2-(2-pyridinyl-κN)phenyl-κC]iridium (tris(2-phenylpyridinato-C2,N)iridium(III); Ir(ppy)3, 99%) and tris[4,6-difluoro-2-(2-pyridyl)phenyl-C2,N]iridium(III) (Ir(df-ppy)3) were purchased from Sigma-Aldrich (Australia). Tris[2-(1H-pyrazol-1-yl-κN2)phenyl-κC]iridium (tris(phenylpyrazole)iridium(III); Ir(ppz)3, >99%) and fac-tris(1-phenyl-3-methylimidazolin-2-ylidene-C,C(2)′)iridium(III) (Ir(pmi)3, >99%) were purchased from LumTech (Taiwan). Bis[3,5-difluoro-2-(2-pyridinyl-κN)phenyl-κC][2-[1-(phenylmethyl)-1H-1,2,3-triazol-4-yl-κN3]pyridine-κN]iridium(1+) hexafluorophosphate(1−) ([Ir(df-ppy)2(ptb)](PF6)), was synthesized and characterized as previously described.15
Experimental procedure
An Autolab PGSTA12 potentiostat was used to perform chronoamperometry and cyclic voltammetry experiments (Metrohm Autolab B.V., Netherlands). A custom-built, light-tight faraday cage encased the electrochemical cell, which consisted of a cylindrical glass cell with a quartz window base and Teflon cover with spill tray. A conventional three-electrode assembly was used throughout, consisting of a glassy carbon (3 mm diameter) working electrode shrouded in Teflon (CH Instruments, Austin, USA), Ag/AgNO3 (0.02 M) reference electrode and platinum wire counter electrode. The glassy carbon working electrode was polished using 0.30 and 0.05 μm alumina on a felt pad with water, rinsed in freshly distilled acetonitrile and dried with nitrogen. The electrode was positioned ∼2 mm from the bottom of the cell.For cyclic voltammetry measurements, the complexes were prepared at equal concentrations in acetonitrile (0.1 M TBAPF6 supporting electrolyte) and the potentials obtained were referenced to the formal potential of the ferrocene/ferrocenium couple (1 mM); measured in situ in each case. Prior to analysis, solutions were degassed with grade 5 argon. ECL spectra were obtained using a model QE65pro CCD spectrometer (Ocean Optics). The spectrometer was interfaced with the electrochemical cell through an optic fibre (1 m, 1 mm core diameter) and collimating lens using a custom-built electrochemical cell holder. A HR 4000 Break-Out box was programmed to initiate acquisition at the initiation of the experiment using NOVA software. For annihilation ECL experiments, appropriate concentrations of the complexes (in freshly distilled acetonitrile with 0.1 M TBAPF6 supporting electrolyte) were selected to generate similar emission intensities. Solutions were degassed for 15 min prior to analysis using grade 5 argon. ECL spectra were recorded using a 14 s integration time with Spectra Suite software. NOVA software was employed to configure the potentiostat to apply a 12 s 2-step chronoamperometry pulse to the appropriate applied potentials. Oxidative and reductive potentials for chronoamperometry were determined by cyclic voltammograms prior to each set of ECL experiments.
Results and discussion
Preliminary experiments. Ru(bpy)32+ with Ir(ppy)3
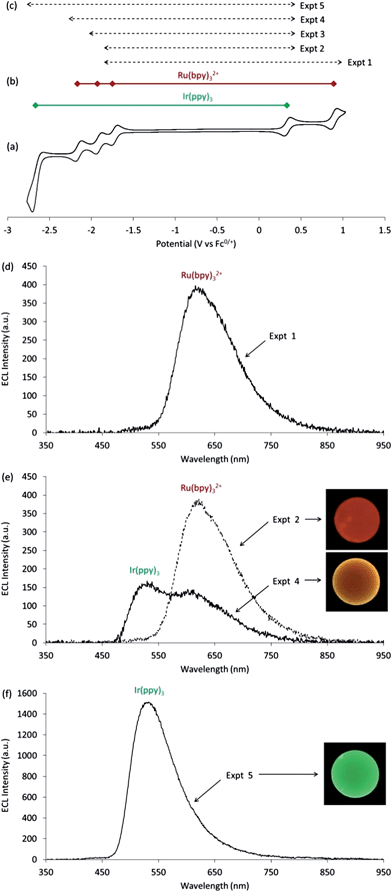
Cyclic voltammetric scans of a mixture of Ru(bpy)32+ and Ir(ppy)3 in acetonitrile (containing 0.1 M TBAPF6) show a combination of the characteristic electron-transfer processes of the two metal chelates (Fig. 2b).12,15,25 This system offers numerous possible reactants for annihilation ECL, which will depend on the applied oxidation and reduction potentials of the alternating electrochemical process. For example, when pulsing 0.1 V beyond the first reduction and oxidation potentials of Ru(bpy)32+ (as in Expt 1; Fig. 2c), we form not only Ru(bpy)3+ and Ru(bpy)33+, but also Ir(ppy)3+, for which a series of subsequent reactions to form ground and excited state products can be considered. The free energy (ΔG) of each reaction can be estimated from the separation of the formal potentials of the reactants, and for excited states, from the emission energy (eqn (I) and (II)).5,26,27| ΔGgs ≈ EoA/A− − EoD+/D | (I) |
| ΔGes ≈ (EoA/A− − EoD+/D) + Ees | (II) |
Ground-state products:
| Ru(bpy)3+ + Ru(bpy)33+ → 2 Ru(bpy)32+ | (1) |
| Ru(bpy)3+ + Ir(ppy)3+ → Ru(bpy)32+ + Ir(ppy)3 | (2) |
One excited-state product:
| Ru(bpy)3+ + Ru(bpy)33+ → [Ru(bpy)32+]* + Ru(bpy)32+ | (3) |
| Ru(bpy)3+ + Ru(bpy)33+ → Ru(bpy)32+ + [Ru(bpy)32+]* | (4) |
| Ru(bpy)3+ + Ir(ppy)3+ → [Ru(bpy)32+]* + Ir(ppy)3 | (5) |
| Ru(bpy)3+ + Ir(ppy)3+ → Ru(bpy)32+ + [Ir(ppy)3]* | (6) |
Two excited-state products:
| Ru(bpy)3+ + Ru(bpy)33+ → 2 [Ru(bpy)32+]* | (7) |
| Ru(bpy)3+ + Ir(ppy)3+ → [Ru(bpy)32+]* + [Ir(ppy)3]* | (8) |
Reactions 1 and 2, which form only ground state products, are so exergonic (ΔG ≪ 0) that they fall into the Marcus inverted region, and thus are kinetically unfavourable compared to the generation of excited states.9 On the other hand, reactions 7 and 8, which would form two excited state products, are not thermodynamically feasible (ΔG ≫ 0).
The formation of [Ru(bpy)32+]* from the annihilation of Ru(bpy)3+ and Ru(bpy)33+ is well-known.1,25 There has been ongoing interest in the subtle question of which of the two parent species forms the excited state,9 as reactions 3 and 4 are thermodynamically equivalent, and co-reactant ‘oxidative-reduction’29 and ‘reductive-oxidation’30 ECL show that either reactant is capable of forming the excited state.1 The question of HOMO → HOMO versus LUMO → LUMO electron transfer is not easily resolved by experiment, but in the case of the annihilation mechanism, simple orbital overlap arguments suggest that the formation of [Ru(bpy)32+]* from the oxidized parent (involving ligand-to-ligand electron transfer) will be kinetically favoured over formation from the reduced parent (which requires metal-to-metal electron transfer).9 The investigation of mixed inorganic ECL systems involving iridium complexes offer an interesting means to gain insight into this question because iridium complexes of the type investigated here often have the electron density of their HOMO delocalized over their ligands as well as on the metal. For example, the HOMO of Ir(ppy)3 has been estimated to be less than 50% metal based, on the basis of DFT calculations.31 Therefore, the HOMO → HOMO electron transfer route ought to be relatively less disfavoured when an iridium complex is the oxidant compared to the case where a ruthenium complex with a purely metal-based HOMO is used.
On the basis of eqn (II), the reaction of Ru(bpy)3+ with Ir(ppy)3+ is sufficiently exergonic to attain [Ru(bpy)32+]* (ΔGes < 0), but not [Ir(ppy)3]* (ΔGes > 0). Indeed, only the characteristic orange-red emission of [Ru(bpy)32+]* was observed in Expt 1 (Fig. 2d). We can isolate reaction 5 by applying suitable voltages to generate only Ru(bpy)3+ and Ir(ppy)3+ (Expt 2). Although this isolated mixed system is less exergonic than conventional Ru(bpy)32+ annihilation ECL, the orange-red emission of [Ru(bpy)32+]* is still observed as predicted (Fig. 2e). The observation of intense orange-red ECL from Expt 2–4 of comparable intensity to Expt 1, shows that the HOMO → HOMO electron transfer route to the excited state is not significantly inhibited in this system.
The reaction of Ru(bpy)3+ with Ir(ppy)3+ does not generate [Ir(ppy)3]*, but the ruthenium(II) chelate exhibits two additional closely spaced ligand reductions that could be exploited to increase the exergonicity of the Ir(ppy)3+ reduction. Pulsing between the potentials required for the oxidation of Ir(ppy)3 and the second reduction of Ru(bpy)32+ (Expt 3) did not change the spectral distribution, but pulsing between potentials suitable for the oxidation of Ir(ppy)3 and the third reduction of Ru(bpy)32+ (Expt 4) provided sufficient energy to populate the [Ir(ppy)3]* state (ΔGes < 0; reaction 10). Experimentally, we observed the combined orange-red ECL of [Ru(bpy)32+]* and green ECL of [Ir(ppy)3]* as a yellow emission (Fig. 2e, Expt 4), which can be confidently ascribed to reactions 5 and 10.
| Ru(bpy)3 + Ir(ppy)3+ → Ru(bpy)3+ + [Ir(ppy)3]* | (9) |
| Ru(bpy)3− + Ir(ppy)3+ → Ru(bpy)3 + [Ir(ppy)3]* | (10) |
In the previously explored annihilation-ECL systems containing mixtures of organic molecules,5 the luminescence was often found to emanate from the lowest lying singlet excited state of one of the emitters, after either direct population or efficient energy transfer. One explanation for the simultaneous ECL from two distinct emitters in Expt 4 could be the large difference in the concentration of the two metal chelates, and thus an insufficient concentration of Ru(bpy)32+ for significant energy transfer from the higher energy [Ir(ppy)3]* emitter. However, there is very little overlap in their respective absorption and emission bands (due in part to the large Stokes shift of their phosphorescent emissions). Furthermore, mixed electrochemiluminophore co-reactant ECL experiments have shown that the emission from [Ir(ppy)3]* can occur in the presence of Ru(bpy)32+ without significant energy transfer.18,19
In Expt 1–4, the limiting reactants for annihilation ECL in terms of concentration are the reduced ruthenium complexes. However, when Ir(ppy)3 was also reduced (e.g. Expt 5), relatively high concentrations of both Ir(ppy)3+ and Ir(ppy)3− were formed, and the characteristic green emission of [Ir(ppy)3]* was dominant (reaction 11 and Fig. 2f).
| Ir(ppy)3− + Ir(ppy)3+ → [Ir(ppy)3]* + Ir(ppy)3 | (11) |
The mean RGB data from the photographs of the mixed annihilation ECL near the electrode surface were used to calculate the CIE chromaticity. For experiments in which the emission was found to occur from only one metal complex (e.g., [Ru(bpy)32+]* in Expt 2, or [Ir(ppy)3]* in Expt 5), the CIE coordinates were in reasonable agreement with those obtained from photoluminescence experiments using a spectrometer with integrating sphere and corrected CCD detector (Fig. 1b), despite difference in the responses of the digital camera and CCD detector over the visible region. For experiments that led to more than one emitting species (e.g., [Ru(bpy)32+]* and [Ir(ppy)3]* in Expt 4), intermediate CIE coordinates were obtained (Fig. 1b).
Mixing emissive and non-emissive metal chelate species. Ru(bpy)32+ with Ir(ppz)3
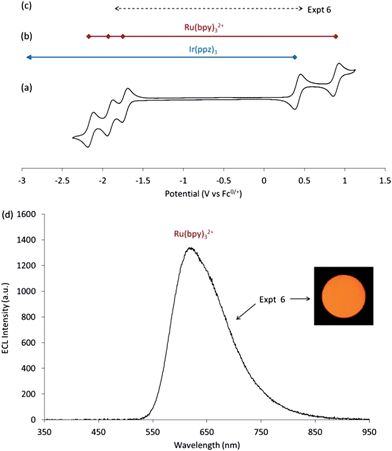
The above findings suggest that annihilation ECL should also be possible using a mixture of Ru(bpy)32+ and a non-emissive iridium complex with similar oxidation potential to Ir(ppy)3, without needing to generate the oxidized Ru(bpy)33+ species. For this experiment, we selected Ir(ppz)3, which has a photoluminescence quantum yield below 0.01 at room temperature, due to efficient population of a non-emissive metal centred (3MC) excited state.32 The reduction potential of Ir(ppz)3 is outside the potential window of the acetonitrile solvent, but its oxidation potential (0.38 V vs. Fc0/+) is marginally higher than that of Ir(ppy)3 (0.33 V vs. Fc0/+), ensuring ΔG < 0 for the generation of [Ru(bpy)3]2+* (reaction 12). The application of alternating potentials sufficient to create these precursors (Expt 6, Fig. 3) resulted in the characteristic emission from [Ru(bpy)3]2+* (Fig. 3d).| Ru(bpy)3+ + Ir(ppz)3+ → [Ru(bpy)32+]* + Ir(ppz)3 | (12) |
Population of the [Ru(bpy)32+]* excited state via an energy transfer pathway can effectively be ruled out due to the low luminescent quantum yield of Ir(ppz)3. Therefore the intense orange-red emission observed in this system is due to efficient HOMO → HOMO electron transfer in reaction 12.
An energy insufficient metal chelate system. Ru(bpy)32+ with Ir(pmi)3
Although the reactions of Ru(bpy)3+ with Ir(ppy)3+ (Expt 2) or Ir(ppz)3+ (Expt 6) are less exergonic than that of Ru(bpy)3+ with Ru(bpy)33+, they can each directly populate the [Ru(bpy)3]2+* excited state. The oxidation potential of Ir(pmi)3 (0.22 V vs. Fc0/+), however, is lower than that of Ir(ppy)3, and although both species are photoluminescent, the generation of [Ru(bpy)3]2+* or [Ir(pmi)3]* from the reaction of Ru(bpy)3+ with Ir(pmi)3+ (Expt 7; data not shown) is not feasible (reactions 13 and 14).| Ru(bpy)3+ + Ir(pmi)3+ → [Ru(bpy)32+]* + Ir(pmi)3 | (13) |
| Ru(bpy)3+ + Ir(pmi)3+ → Ru(bpy)32+ + [Ir(pmi)3]* | (14) |
As predicted, no significant ECL was observed from this experiment. Unlike many ‘energy-insufficient’ systems comprising organic molecules,5 the initial population of lower energy excited states, followed by up-conversion to generate the emissive excited state, is not possible in these mixed metal-chelate systems where the emission occurs from short-lived triplets.9,10,33
Manipulating emission in mixed metal chelate ECL systems
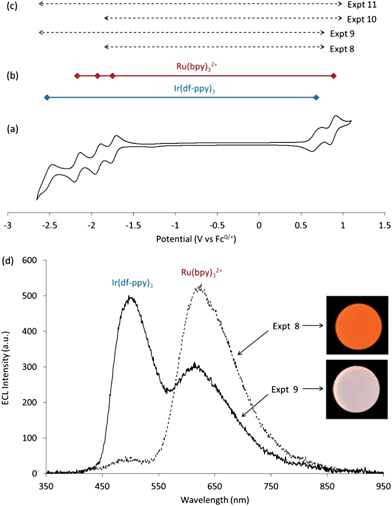
In both Expt 8 and 9, [Ru(bpy)32+]* is formed by the oxidation of Ru(bpy)3+ (reaction 15), but in Expt 9 this immediate precursor is not initially the dominant form of the ruthenium chelate at the electrode. In Expt 8, small amounts of [Ir(df-ppy)3]* are formed from the reduction of Ir(df-ppy)3+ (reaction 16, ΔGes ≈ 0), but in Expt 9 this emitter may be formed from reactions 17 and 18. At these metal chelate concentrations, [Ru(bpy)32+]* dominates in Expt 8, and the emission from [Ir(df-ppy)3]* is greater in Expt 9 (Fig. 4d). Similar reasoning can be presented for Expt 10 and 11. The dominant emission colour in this system is therefore largely determined by the applied reduction potential of the electrochemical process, if the applied oxidation potential is at least sufficient to achieve the first metal-chelate oxidation.
| Ru(bpy)3+ + Ir(df-ppy)3+ → [Ru(bpy)32+]* + Ir(df-ppy)3 | (15) |
| Ru(bpy)3+ + Ir(df-ppy)3+ → Ru(bpy)32+ + [Ir(df-ppy)3]* | (16) |
| Ru(bpy)3− + Ir(df-ppy)3+ → Ru(bpy)32+ + [Ir(df-ppy)3]* | (17) |
| Ir(df-ppy)3− + Ir(df-ppy)3+ → Ir(df-ppy)3 + [Ir(df-ppy)3]* | (18) |
ΔGes = −0.63, +0.07, −0.35, and −0.71 eV, respectively.
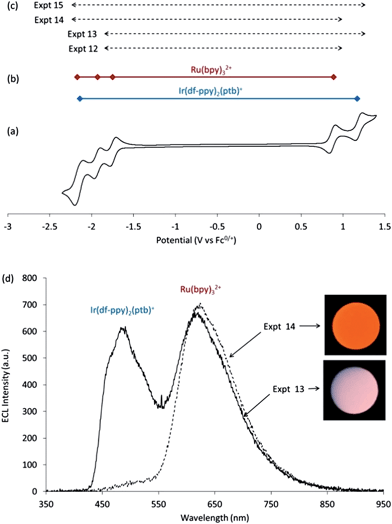
| Ru(bpy)3+ + Ir(df-ppy)2(ptb)2+ → Ru(bpy)32+ + [Ir(df-ppy)2(ptb)+]* | (19) |
| Ru(bpy)3+ + Ir(df-ppy)2(ptb)2+ → [Ru(bpy)32+]* + Ir(df-ppy)2(ptb)+ | (20) |
| Ru(bpy)33+ + Ir(df-ppy)2(ptb) → Ru(bpy)32+ + [Ir(df-ppy)2(ptb)+]* | (21) |
| Ru(bpy)33+ + Ir(df-ppy)2(ptb) → [Ru(bpy)32+]* + Ir(df-ppy)2(ptb)+ | (22) |
Notably in Expt 14, it is the iridium excited state that is populated by electron transfer from the HOMO of the ruthenium complex to the HOMO of the iridium complex, though with seemingly lower efficiency than the previously cases where the iridium HOMO is the donor.
Further extending the applied potential range to include both the reduction and oxidation of Ir(df-ppy)2(ptb)+ (Expt 15) resulted in a similar ECL spectral distribution to that of Expt 13. Therefore, in contrast to the above system, the dominant emission in this case is largely determined by the applied oxidation potential, so long as the other applied potential was at least beyond the first reduction. In Expt 13 and Expt 9, the combined emissions spanned the entire visible region, resulting in near-white luminescence (Fig. 1b, 4d, and 5d).
Conclusions
The mixed annihilation ECL of metal chelates provides an alternative approach to multi-colour ECL, in which the relative intensity of the emissions from multiple luminophores (and hence overall emission colour) can be controlled initially by selection of the electrochemical and spectroscopic properties of the complexes, and then the applied electrochemical potentials. The absence of the up-conversion processes often encountered in ‘energy insufficient’ organic mixed systems simplifies predictions of excited state generation. Furthermore, the numerous closely spaced reductions and oxidations of the mixed systems enable fine tuning of the reaction energy and hence control of the resulting ECL emission colour. Apart from the relevance of these studies to research into voltage controllable light emitting devices, the observation of efficient HOMO → HOMO electron transfer pathways in these mixed systems offers interesting insights into the somewhat intractable question of whether the reduced or oxidized partner becomes the excited state in classic annihilation ECL experiments. In the case of ruthenium complexes where the HOMO is almost exclusively metal-based, it is generally believed that electron transfer between LUMOs is strongly preferred due to more favourable orbital overlap compared with the alternative HOMO → HOMO transfer where the reduced partner becomes excited. Our results tend to support this analysis, because the delocalized nature of the HOMO in the case of the iridium complexes studied here renders this electronic factor less unfavourable, resulting in higher than expected ECL intensities in cases where Ru(bpy)3+ forms an excited state by loss of an electron from its HOMO.Acknowledgements
This research was funded by the Australian Research Council (FT100100646).Notes and references
- M. M. Richter, Chem. Rev., 2004, 104, 3003–3036 CrossRef CAS PubMed; W. Miao, Chem. Rev., 2008, 108, 2506–2553 CrossRef PubMed.
- N. D. Danielson, in Electrogenerated Chemiluminescence, ed. A. J. Bard, Marcel Dekker, New York, 2004, pp. 397–444 RSC; B. A. Gorman, P. S. Francis and N. W. Barnett, Analyst, 2006, 131, 616–639 RSC; R. J. Forster, P. Bertoncello and T. E. Keyes, Annu. Rev. Anal. Chem., 2009, 2, 359–385 CrossRef CAS PubMed; T. Joshi, G. J. Barbante, P. S. Francis, C. F. Hogan, A. M. Bond, G. Gasser and L. Spiccia, Inorg. Chem., 2012, 51, 3302–3315 CrossRef PubMed; X. Zhou, D. Zhu, Y. Liao, W. Liu, H. Liu, Z. Ma and D. Xing, Nat. Protoc., 2014, 9, 1146–1159 CrossRef PubMed.
- L. Hu and G. Xu, Chem. Soc. Rev., 2010, 39, 3275–3304 RSC.
- A. Kapturkiewicz, in Electrogenerated Chemiluminescence, ed. A. J. Bard, Marcel Dekker, New York, 2004, pp. 163–211 Search PubMed.
- S. P. Forry and R. M. Wightman, in Electrogenerated Chemiluminescence, ed. A. J. Bard, Marcel Dekker, New York, 2004, pp. 273–299 Search PubMed.
- R. A. Marcus, J. Chem. Phys., 1956, 24, 966–978 CrossRef CAS PubMed; R. A. Marcus, J. Chem. Phys., 1965, 43, 2654–2657 CrossRef PubMed; Y. Zu, F.-R. F. Fan and A. J. Bard, J. Phys. Chem. B, 1999, 103, 6272–6276 CrossRef.
- A. Kapturkiewicz, Adv. Electrochem. Sci. Eng., 1997, 5, 1–60 CAS.
- D. J. Freed and L. R. Faulkner, J. Am. Chem. Soc., 1971, 93, 2097–2102 CrossRef CAS; E. M. Gross, J. D. Anderson, A. F. Slaterbeck, S. Thayumanavan, S. Barlow, Y. Zhang, S. R. Marder, H. K. Hall, M. F. Nabor, J.-F. Wang, E. A. Mash, N. R. Armstrong and R. M. Wightman, J. Am. Chem. Soc., 2000, 122, 4972–4979 CrossRef.
- A. Juris, V. Balzani, F. Barigelletti, S. Campagna, P. Belser and A. Von Zelewsky, Coord. Chem. Rev., 1988, 84, 85–277 CrossRef CAS.
- S. Campagna, F. Puntoriero, F. Nastasi, G. Bergamini and V. Balzani, Top. Curr. Chem., 2007, 280, 117–214 CrossRef CAS.
- A. Kapturkiewicz, P. Szrebowaty, G. Angulo and G. Grampp, J. Phys. Chem. A, 2002, 106, 1678–1685 CrossRef CAS; A. Kapturkiewicz and P. Szrebowaty, J. Chem. Soc., Dalton Trans., 2002, 3219–3225 RSC; A. Kapturkiewicz, T.-M. Chen, I. R. Laskar and J. Nowacki, Electrochem. Commun., 2004, 6, 827–831 CrossRef PubMed.
- A. Kapturkiewicz and G. Angulo, Dalton Trans., 2003, 3907–3913 RSC.
- A. Kapturkiewicz, J. Nowacki and P. Borowicz, Electrochim. Acta, 2005, 50, 3395–3400 CrossRef CAS PubMed.
- A. Vogler and H. Kunkely, ACS Symp. Ser., 1987, 333, 155–168 CrossRef CAS PubMed; S. Zanarini, M. Felici, G. Valenti, M. Marcaccio, L. Prodi, S. Bonacchi, P. Contreras-Carballada, R. M. Williams, M. C. Feiters, R. J. M. Nolte, L. De Cola and F. Paolucci, Chem.–Eur. J., 2011, 17, 4640–4647 CrossRef PubMed.
- G. J. Barbante, E. H. Doeven, E. Kerr, T. U. Connell, P. S. Donnelly, J. M. White, T. Lópes, S. Laird, C. F. Hogan, D. J. D. Wilson, P. J. Barnard and P. S. Francis, Chem.–Eur. J., 2014, 20, 3322–3332 CrossRef CAS PubMed.
- D. Bruce and M. M. Richter, Anal. Chem., 2002, 74, 1340–1342 CrossRef CAS; B. D. Muegge and M. M. Richter, Anal. Chem., 2004, 76, 73–77 CrossRef.
- E. H. Doeven, E. M. Zammit, G. J. Barbante, C. F. Hogan, N. W. Barnett and P. S. Francis, Angew. Chem., Int. Ed., 2012, 51, 4354–4357 CrossRef CAS PubMed.
- E. H. Doeven, E. M. Zammit, G. J. Barbante, P. S. Francis, N. W. Barnett and C. F. Hogan, Chem. Sci., 2013, 4, 977–982 RSC.
- E. H. Doeven, G. J. Barbante, E. Kerr, C. F. Hogan, J. A. Endler and P. S. Francis, Anal. Chem., 2014, 86, 2727–2732 CrossRef CAS PubMed.
- F. Han, H. Jiang, D. Fang and D. Jiang, Anal. Chem., 2014, 86, 6896–6902 CrossRef CAS PubMed.
- C. H. Lyons, E. D. Abbas, J. K. Lee and M. F. Rubner, J. Am. Chem. Soc., 1998, 120, 12100–12107 CrossRef CAS; E. S. Handy, A. J. Pal and M. F. Rubner, J. Am. Chem. Soc., 1999, 121, 3525–3528 CrossRef; F. G. Gao and A. J. Bard, J. Am. Chem. Soc., 2000, 122, 7426–7427 CrossRef; M. K. Nazeeruddin, R. Humphry-Baker, D. Berner, S. Rivier, L. Zuppiroli and M. Graetzel, J. Am. Chem. Soc., 2003, 125, 8790–8797 CrossRef PubMed; H. J. Bolink, L. Cappelli, E. Coronado, M. Grätzel, E. Orti, R. D. Costa, P. M. Viruela and M. K. Nazeeruddin, J. Am. Chem. Soc., 2006, 128, 14786–14787 CrossRef PubMed; L. He, L. Duan, J. Qiao, R. Wang, P. Wei, L. Wang and Y. Qiu, Adv. Funct. Mater., 2008, 18, 2123–2131 CrossRef; M. Mydlak, C. Bizzarri, D. Hartmann, W. Sarfert, G. Schmid and L. De Cola, Adv. Funct. Mater., 2010, 20, 1812–1820 CrossRef.
- S. Welter, K. Brunner, J. W. Hofstraat and L. De Cola, Nature, 2003, 421, 54–57 CrossRef CAS PubMed; C. Zhen, Y. Chuai, C. Lao, L. Huang, D. Zou, D. N. Lee and B. H. Kim, Appl. Phys. Lett., 2005, 87, 093508 CrossRef PubMed; F. Wang, P. Wang, X. Fan, X. Dang, C. Zhen, D. Zou, E. H. Kim, D. N. Lee and B. H. Kim, Appl. Phys. Lett., 2006, 89, 183519 CrossRef PubMed.
- H.-C. Su, H.-F. Chen, F.-C. Fang, C.-C. Liu, C.-C. Wu, K.-T. Wong, Y.-H. Liu and S.-M. Peng, J. Am. Chem. Soc., 2008, 130, 3413–3419 CrossRef CAS PubMed.
- H. C. Moon, T. P. Lodge and C. D. Frisbie, J. Am. Chem. Soc., 2014, 136, 3705–3712 CrossRef CAS PubMed.
- N. E. Tokel and A. J. Bard, J. Am. Chem. Soc., 1972, 94, 2862–2863 CrossRef CAS.
- A. Vogler, H. Kunkely and S. Schäffl, ACS Symp. Ser., 1986, 307, 120–134 CrossRef CAS PubMed.
- A. Kapturkiewicz, Chem. Phys. Lett., 1995, 236, 389–394 CrossRef CAS.
- G. J. Barbante, C. F. Hogan, D. J. D. Wilson, N. A. Lewcenko, F. M. Pfeffer, N. W. Barnett and P. S. Francis, Analyst, 2011, 136, 1329–1338 RSC.
- M.-M. Chang, T. Saji and A. J. Bard, J. Am. Chem. Soc., 1977, 99, 5399–5403 CrossRef CAS.
- H. S. White and A. J. Bard, J. Am. Chem. Soc., 1982, 104, 6891–6895 CrossRef CAS.
- R. V. Kiran, C. F. Hogan, B. D. James and D. J. D. Wilson, Eur. J. Inorg. Chem., 2011, 4816–4825 CrossRef CAS.
- T. Sajoto, P. I. Djurovich, A. B. Tamayo, J. Oxgaard, W. A. Goddard and M. E. Thompson, J. Am. Chem. Soc., 2009, 131, 9813–9822 CrossRef CAS PubMed.
- L. Flamigni, A. Barbieri, C. Sabatini, B. Ventura and F. Barigelletti, Top. Curr. Chem., 2007, 281, 143–203 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |