Open Access Article
Open Access ArticleHighly efficient one-pot/one-step synthesis of multiblock copolymers from three-component polymerization of carbon dioxide, epoxide and lactone†
Yang
Li
,
Jiali
Hong
,
Renjian
Wei
,
Yingying
Zhang
,
Zaizai
Tong
,
Xinghong
Zhang
*,
Binyang
Du
,
Junting
Xu
and
Zhiqiang
Fan
MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, 38 Zheda Road, Hangzhou, 310027, China. E-mail: xhzhang@zju.edu.cn
First published on 8th December 2014
Abstract
It is a long-standing challenge to combine mixed monomers into multiblock copolymer (MBC) in a one-pot/one-step polymerization manner. We report the first example of MBC with biodegradable polycarbonate and polyester blocks that were synthesized from highly efficient one-pot/one-step polymerization of cyclohexene oxide (CHO), CO2 and ε-caprolactone (ε-CL) in the presence of zinc–cobalt double metal cyanide complex and stannous octoate. In this protocol, two cross-chain exchange reactions (CCER) occurred at dual catalysts respectively and connected two independent chain propagation procedures (i.e., polycarbonate formation and polyester formation) simultaneously in a block-by-block manner, affording MBC without tapering structure. The multiblock structure of MBC was determined by the rate ratio of CCER to the two chain propagations and could be simply tuned by various kinetic factors. This protocol is also of significance due to partial utilization of renewable CO2 and improved mechanical properties of the resultant MBC.
Introduction
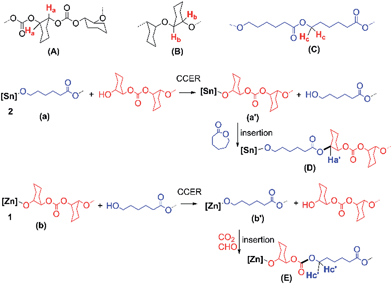
The big concerns on the global energy and environmental issues prompt us to develop efficient methods to prepare new polymeric materials that can be derived from renewable resources. Aliphatic polycarbonates (APCs) and polyesters are two classes of biodegradable polymers with a bright future because of their practical advantages. APCs can be synthesized by copolymerization of epoxide with carbon dioxide (CO2),1 which is one of the most attractive renewable C1 resources and abundant, non-toxic and low-cost, while polyesters can be prepared by ring-opening polymerization of lactones2 that can also be obtained from the renewable resources.3 In contrast to the most of commercialized polycarbonates and polyesters via condensation polymerization, which requires long reaction time as well as high energy supply and releases small molecules as by-products, both CO2/epoxide copolymerization and ring-opening polymerization of lactone are addition polymerizations and undergo a sustainable and environmentally benign process, which meets the principle of atom economy.Another virtue of both ring-opening polymerization of lactone and CO2/epoxide copolymerization is that they could be used to make CO2-based di- and tri-block copolymers, which were rarely reported.4–6 Generally, polycarbonate with one or two hydroxyl (–OH) end groups was at first synthesized by copolymerization of epoxide with CO2 and then used as the macroinitiator for ring-opening polymerization of lactone. For example, Darensbourg and co-workers reported the syntheses of di- and tri-block copolymers from CO2, epoxide and lactone via macroinitiator intermediate by tandem using a (Salen)Co(III) complex plus an organic base 1,8-diazabicyclo[5.4.0]undec-7-ene.4 Williams et al. synthesized a di-block copolymer from epoxide, CO2 and lactone by using a dizinc catalyst, which could be reversibly switched from a polycarbonate catalyst to a polyester catalyst by adding switching reagents.5 These works elegantly provided di- and tri-block copolymers by multi-step or sequential operations. In this context, we present a one-pot/one-step synthesis of a new CO2-based multiblock copolymer (MBC) without tapering from cyclohexene oxide (CHO), CO2 and ε-caprolactone (ε-CL) via cross-chain exchange reaction (CCER) that bridged two independent chain propagations generated by two appropriately selected catalysts (Fig. 1) simultaneously.
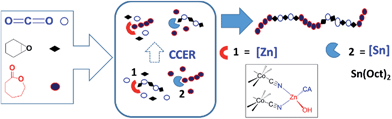 | ||
| Fig. 1 Proposed cross-chain exchange polymerization of CO2, CHO and ε-CL by using Zn–Co(III) DMCC (1, Scheme S1†)7 and stannous octoate [2, Sn(Oct)2] together. | ||
CCER is a kind of chain-transfer reaction in which the propagating chain exchanged with a dormant chain with different structures. Indeed, many metal-catalyzed ring-opening polymerizations of lactone and CO2/epoxide copolymerizations are chain-transfer polymerizations, namely, immortal polymerization.7,8 In such mode, a propagating chain could be converted to a dormant chain with at least one hydroxyl end group (macromolecular chain-transfer agent) via chain-transfer reaction to the proton compounds (e.g., trace water) with much higher rate than those of the chain propagations.7 The generated dormant chains could participate in the chain propagation again as macromolecular chain-transfer agents.2,7,8 Therefore, it was possible to produce MBC by bridging CO2/epoxide copolymerization and ring-opening polymerization of lactone via in situ generation of macromolecular chain-transfer agents during terpolymerization of CHO, CO2 and ε-CL in one reactor.
Results and discussion
To this end, two catalysts, zinc–cobalt double metal cyanide complex (Zn–Co(III) DMCC, 1) and stannous octoate [Sn(Oct)2, 2], were screened out (Fig. 1).9 A nanolamellar Zn–Co(III) DMCC [Fig. S1, its synthesis and characterization are given in the ESI†] is a highly active catalyst for CO2/epoxide copolymerization without producing the byproduct of cyclic carbonate at 50–110 °C.7,10 The initiating site of 1 is zinc–hydroxyl group (Zn–OH, Scheme S1†), which could afford poly(cyclohexene carbonate) with two hydroxyl end groups (HO–PCHC–OH) resulted from Zn–OH initiation and chain-transfer reaction, respectively.72-Catalyzed ring-opening polymerization of ε-CL is a typical chain-transfer polymerization at ca. 80–130 °C.11 When CO2/CHO copolymerization and ring-opening polymerization of ε-CL were combined into one reactor in the presence of 1 and 2 simultaneously, CCER would occur, as shown in Scheme 1, when the dormant polycarbonate exchanged with (a), a new propagating species (a′) was generated so that polycaprolactone (PCL) block was produced via consecutive ε-CL insertion and a junction unit D was formed. Thermodynamically, the insertion of ε-CL into (a′) made the equilibrium reaction to the right hand. Similarly, a propagating species (b′) would be generated via CCER along with the formation of PCHC block and the junction unit E. Hence, the resulted MBC would have the main units (A and C) and new junction units D and E. The production of the ether unit (B) was minor due to the catalytic behavior of Zn–Co(III) DMCC for CO2/CHO copolymerization according to our previous report.7The prerequisite for the formation of MBC is that 1-catalyzed CO2/CHO copolymerization and 2-catalyzed ring-opening polymerization of ε-CL could occur independently with matched polymerization rates. The control experiments indicated that 1 was completely inactive to ring-opening polymerization of ε-CL in the presence or absence of CO2 (Table S3, runs S1 and S2†). When 1 was used for CHO, CO2 and ε-CL (Table S3, run-S3†), only PCHC with fully alternating structure was obtained (Fig. S2†), indicating the complete inhibition of CHO/ε-CL copolymerization in this case. 2 Failed to catalyze either CHO/CO2 copolymerization or ring-opening polymerization of CHO (Table S3, runs S4 and S5†). When 2 was used for CHO and ε-CL in the presence or absence of CO2 (Table S3, runs S6 and S7†), only PCL was obtained. Therefore, the crossed polymerization of three monomers with either catalysts 1 or 2 was kinetically precluded. CO2 self-polymerization and CO2/ε-CL copolymerization were also thermodynamically inhibited.1 Furthermore, 1 could catalyze CHO/CO2 copolymerization independently in the presence of 2, vice versa (Table S3, runs S8 and S9†) with close-up monomer conversions (CHO: 95%; ε-CL: 86%), indicating that both CHO/CO2 copolymerization and ring-opening polymerization of ε-CL could proceed independently with matched polymerization rates.
A series of one-pot polymerizations with mixed monomers of CHO, CO2 and ε-CL in the presence of 1 and 2 were carried out (runs 3–6, Table 1) under mechanical stirring with 500 rpm. GPC results showed that the resultant MBCs had single elution curves (Fig. 2) with PDIs of 1.8–2.0. The number-average molecular weights (Mn) increased from 9.7 to 35.2 kg mol−1 with decreasing the [BnOH]/[ε-CL] molar ratios from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 to 0. Note that BnOH could initiate ring-opening polymerization of ε-CL8 and be used to tune the molecular weights of the resultant MBCs. 97–99% CHO and 94–96% ε-CL were converted within 4.0 h according to the 1H NMR spectra of the crude products, indicating that two catalysts presented high efficiency towards this terpolymerization. Moreover, the ether units of MBCs obtained at 100 °C were dramatically inhibited (3.4–9.9% for runs 3–5 in Table 1) in contrast to the pure PCHC with the ether unit of 19.0% (run-1, Table 1, bulk polymerization). This could be attributed to the solvent-assisted depression effect (herein, ε-CL).12 With such small amounts of the ether units in PCHC block, the possible junction units linked with consecutive ether units could be literally minimized (Schemes S2 and S3†).
40 to 0. Note that BnOH could initiate ring-opening polymerization of ε-CL8 and be used to tune the molecular weights of the resultant MBCs. 97–99% CHO and 94–96% ε-CL were converted within 4.0 h according to the 1H NMR spectra of the crude products, indicating that two catalysts presented high efficiency towards this terpolymerization. Moreover, the ether units of MBCs obtained at 100 °C were dramatically inhibited (3.4–9.9% for runs 3–5 in Table 1) in contrast to the pure PCHC with the ether unit of 19.0% (run-1, Table 1, bulk polymerization). This could be attributed to the solvent-assisted depression effect (herein, ε-CL).12 With such small amounts of the ether units in PCHC block, the possible junction units linked with consecutive ether units could be literally minimized (Schemes S2 and S3†).
| Run | [OH]/[ε-CL] | M n/PDIb kg mol−1 | Compositionc (%) | N | Conv.e (%) CHO/ε-CL | ||
|---|---|---|---|---|---|---|---|
| C | A | B | |||||
| a Reaction conditions of runs 3–5: 100 °C, 4.0 MPa, 35.0 mg of 1, [OH]/[2] = 2/1, 4.0 h, 30.0 mL CHO, 30.0 mL ε-CL, 20.0 mL THF, [OH] was benzyl alcohol (BnOH) for ring-opening polymerization of ε-CL. b Determined by gel permeation chromatography (GPC) of the purified product calibrated with polystyrene standards in THF. c Determined by 1H NMR spectroscopy, C (polyester) = A2.31/(A4.67 + A3.2–3.5 + A2.31), A (polycarbonate) = A4.67/(A4.67 + A3.2–3.5 + A2.31), B (polyether) = A3.2–3.5/(A4.67 + A3.2–3.5 + A2.31). d Determined by 1H NMR spectroscopy, N = (2A4.79 + A4.13)/(A4.79 + A4.67 + A4.50 + A3.2–3.5 + A2.31) (see Fig. S3). e Based on 1H NMR spectroscopy of the crude products. f Bulk. g In a flask under magnetic stirring. h Pentaerythritol, [pentaerythritol]/[ε-CL] = 1/500, 2.0 MPa CO2 pressure. | |||||||
| 1f | — | 29.9/1.8 | — | 81.0 | 19.0 | — | 99/— |
| 2g | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 150 |
22.7/1.7 | 100 | — | — | — | —/84 |
| 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
9.7/2.0 | 52.1 | 38.1 | 9.9 | 10 | 97/94 |
| 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 150 |
18.7/1.8 | 49.5 | 46.6 | 3.9 | 9 | 99/95 |
| 5 | 0 | 35.2/1.9 | 49.2 | 47.5 | 3.4 | 3 | 98/96 |
| 6h | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 125 125 |
14.9/3.7 | 50.2 | 40.4 | 9.4 | 10 | 99/92 |
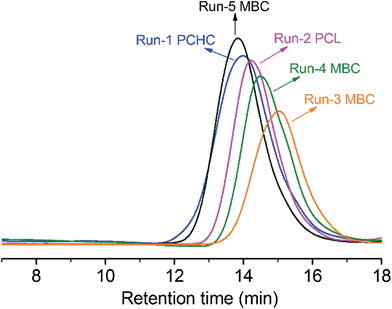 | ||
| Fig. 2 GPC traces of the purified PCHC, PCL and the resultant terpolymers from runs 1–5 in Table 1. | ||
The junction units D and E between PCHC and PCL blocks were confirmed by 1H (13C) NMR spectra of the resultant MBCs (Fig. 3-A and S4†). In contrast to the 1H NMR spectra of PCL and PCHC, two small shoulder peaks at 4.12 and 4.79 ppm were clearly observed in Fig. 3-A. Both peaks could be ascribed to the proton signals of E (H′c) and D (H′a) respectively. Fig. 3-B shows the high-resolution 1H–13C heteronuclear single-quantum coherence (HSQC) spectrum of the run-3 MBC in Table 1. Both H′c of E (4.11 ppm) and H′a of D (4.78 ppm) in the 1H NMR spectrum were directly correlated to the carbon of carbonate unit (67.70 ppm) and the ester unit (73.25 ppm) in 13C NMR spectrum, respectively. In comparison, 1H–13C HSQC spectrum (Fig. S5-C†) of the PCHC/PCL blend (weight ratio of 1/1), which was prepared by using two catalysts under 100 °C and 4.0 MPa CO2 pressure for 4 h in the autoclave, showed no junction units. As a result, no transesterification between PCHC and PCL occurred during terpolymerization.
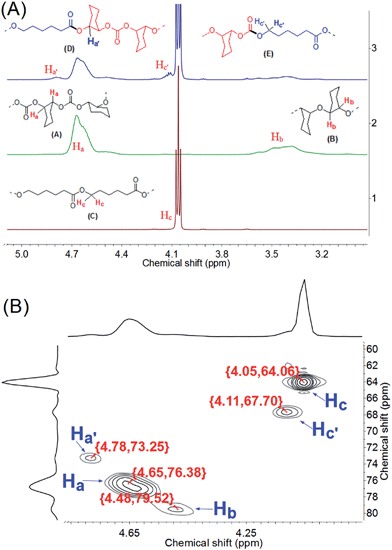 | ||
| Fig. 3 (A) 1H NMR results (500 MHz, CDCl3) of PCL, PCHC and the run-3 terpolymer in Table 1 (spectra 1, 2 and 3 respectively), 1H NMR test with rotating the tube was performed for the run-3 terpolymer; (B) 1H–13C HSQC spectrum (500 MHz NMR instrument) of the run-3 terpolymer in Table 1. | ||
Furthermore, CCERs with two catalysts were clearly disclosed by the observation that two junction units E and D were produced at Zn and Sn sites, respectively. Firstly, HO–PCL–OH with a Mn of 1700 (run-S10 in Table S3 and Fig. S6†) was introduced into the 1-catalyzed CO2/CHO copolymerization system; the 1H–13C HSQC NMR spectrum of the resultant polymers (Fig. S5-D†) showed only one junction unit E {4.12 ppm, 67.83 ppm}, which was solely caused by the chain exchange reaction of HO–PCL–OH on Zn site. Moreover, when HO–PCHC–OH with a Mn of 700 was introduced into the 2-catalyzed ring-opening polymerization of ε-CL system under 4.0 MPa CO2 pressure (run-S11 in Table S3 and Fig. S6†), 1H–13C HSQC NMR spectrum of the product showed only one junction unit D {4.80, 73.52 ppm} (Fig. S5-E†). This result confirmed that the CCER only occurred on the Sn site.
In order to form multiblocks, the total rates of two CCERs should be smaller than those of corresponding propagation processes. The rate percentage of two CCERs to two propagations (N) could be estimated by the ratio of the integral area of D and E to the total carbonate (including small amounts of ether unit) and ester units based on 1H NMR spectra. N of runs 3–5 MBCs in Table 1 was calculated to be ca. 3–10%, indicating that the total formation rate of the junction units D and E were ca. 3–10% of the MBC formation. Such rate difference between CCER and chain propagations led to the formation of polycarbonate and polyester multiblocks. Moreover, the rate ratio of D formation to the polycarbonate formation was approximately equal to that of E formation to the polyester formation based on the 1H NMR spectra (Fig. S3†), suggesting that CCERs at the Zn and Sn site had nearly the same reactivity.
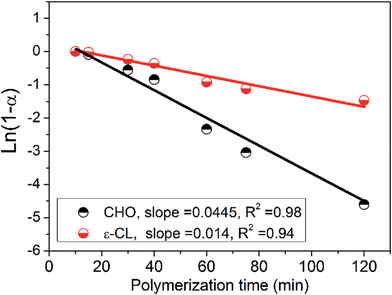
The evolution of the block structure of MBC was further monitored by the apparent kinetic study of the terpolymerization (Fig. 4 and S7–S10 and Table S4†). Fig. 4 shows the semi-logarithmic plots of the conversions of CHO and ε-CL (Table S4†) vs. the reaction time with the assumption of the first-order dependence on monomer concentration for two polymerizations. The rate ratio of CHO/CO2 copolymerization to ring-opening polymerization of ε-CL was estimated to be ca. 3, suggesting that the rate constant of CHO/CO2 copolymerization was three times than that of ring-opening polymerization of ε-CL. As a result, the average block length of PCHC block was longer than that of PCL block in the resultant MBC. The ratio of the integral area of the junction units (D + E) to the total units (A + C + B) of MBC in 1H NMR spectrum kept in the range of 11–14% (Table S4†) in the whole polymerization time, which ensured the continuous production of multiblocks at nearly stable rate. Moreover, Mn increased with the conversion of CHO and ε-CL in a nearly linear manner (Fig. S9†). In this sense, the obtained MBCs are statistical multiblock copolymers.
There are only a few examples of MBC synthesized by using two catalysts.13,14 In the previous reports, two catalysts of the same type were used to catalyze two monomers with same functionality (e.g., double bond)13 or one monomer with R and S enantiomers.14 In these cases, the transition of one block to another via chain shuttling obeyed the same propagation manner, which might cause tapering structure in the resultant multiblock copolymers. Our example reported in the present work provides a novel CCER route between two independent chain propagation processes catalyzed by two different types of catalysts for three monomers with different functionalities in a one-pot/one-step way, affording MBCs without tapering.
The multiblock structure of MBCs was also evidenced by the differential scanning calorimetry (DSC) results of the runs 3–5 MBCs from Table 1 with heating and cooling rates of 20 °C min−1 (Fig. 5) and 10 °C min−1 (Fig. S11†). As shown in Fig. 5-A, the melting temperatures (Tm) of the PCL block of runs 3–5 MBCs were observed (all samples were kept at 0 °C for at least 24 h before testing and complete crystallization). Since MBC with smaller N had a longer average block length, Tm values of runs 3–5 MBCs increased from 45.7 to 54.2 °C with decreasing N value and were lower than that of the PCHC/PCL blend (58.8 °C, Fig. 5-A). Tg values of run-5 and run-4 MBCs were found to be 79.3 and 71.8 °C (see inserted chart in Fig. 5-A), respectively. Both were lower than that of the PCL/PCHC blend (115.0 °C). However, no Tg was observed for the run-3 MBC (Fig. 5-A), in which the glass transition of the run-3 MBC might be neutralized by the melting process with strong enthalpy of the PCL block.
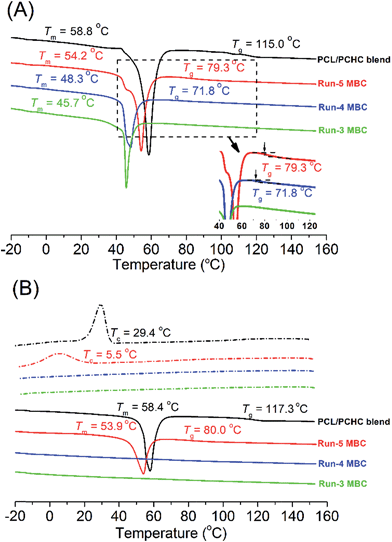 | ||
| Fig. 5 DSC traces of MBCs from runs 3–5 in Table 1 and PCL/PCHC blend (Mn: 26.4 kg mol−1) obtained with a heating rate of 20 °C min−1 in N2 atmosphere, ca. 10 mg sample was used. The curves were shifted vertically for clarity. (A) Samples were kept at 0 °C for at least 24 h before testing for complete crystallization, samples were then heated from −20 to 160 °C; (B) the same samples were kept at 160 °C for 10 min, then were cooled to −20 °C and heated to 160 °C again. | ||
Subsequent DSC measurements were further carried out for the samples with heat treatment at 160 °C for 10 min. As shown in Fig. 5-B, the cooling curves of the PCL/PCHC blend and run-5 MBC presented crystallization temperatures (Tc) at 29.4 and 5.5 °C, and Tg at 117.3 and 80.0 °C, respectively. The low Tc of run-5 MBC was caused by the restricted crystallization of PCL blocks, which are covalently linked with PCHC blocks, with relatively high Tg.15 However, no Tc and Tm were observed for runs 3–4 MBCs. The disappearance of crystallization and melting peaks in the rapidly cooled runs 3–4 MBCs suggested that the crystallization rate of these two samples was very slow. This is also one of the characteristics of restricted crystallization, which is frequently observed in semicrystalline block copolymers.15Tg values of runs 3–4 MBCs could be observed at ca. 69.0 °C when their DSC curves were magnified and the base line subtracted (Fig. S12†). It is reasonable that both MBCs had nearly the same chain compositions (Table 1). Moreover, when DSC measurement was carried out for runs 3–5 and the PCL/PCHC blend with a heating and cooling rate of 10 °C min−1, Tc and Tm values of the sample were similar with those in Fig. 5 (Fig. S11†). The above DSC results confirmed the production of multiblock structure of MBCs via one-pot/one-step reaction of three monomers catalyzed by two different catalysts.
The restricted crystallization behavior of PCL blocks in MBCs was also confirmed by the comparative study of small-angle X-ray scattering (SAXS) profiles of run-5 MBC in Table 1 and PCL/PCHC blend. As seen in Fig. 6-A, run-5 MBC presented a lamellar crystal thickness (lc) of 3.4 nm, which was smaller than that of PCL/PCHC blend (4.6 nm). Due to the multiblock structure, the run-5 MBC in Table 1 showed improved elongation at break of 22.8% relative to those of PCHC (3.3%)16 and PCHC/PCL blend (1.8%) (Fig. 6-B), which meant that the run-5 MBC was tougher than the pure PCHC and PCHC/PCL blend.
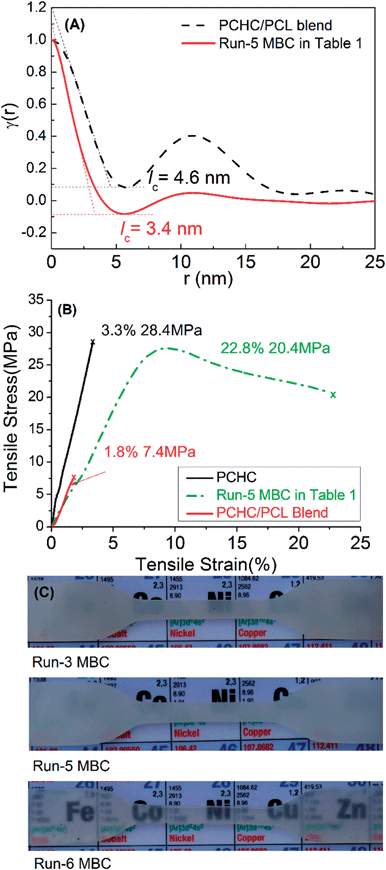 | ||
| Fig. 6 (A) SAXS results: one-dimensional correlation functions for run-5 MBC in Table 1 (solid line) and the PCL/PCHC blend (dashed line); (B) stress–strain curves of run-5 MBC in Table 1, PCL/PCHC blend and PCHC (Mn: 37.4 kg mol−1) at room temperature and 10 mm min−1, * denotes failure point. (C) Images of run-3, run-5 and run-6 MBCs (dog bone sample, thickness of 2.0 mm) in Table 1 synthesized under different conditions. | ||
We also examined the effect of CO2 pressure, CHO/ε-CL feeding ratio, reaction temperature, the type as well as the amount of the initiator on the structure of the resultant MBCs (Tables 1, S6 and S7†). With the fixed molar ratios of 1 to CHO and 2 to ε-CL, the variation of CHO/ε-CL ratio had a strong impact on the chain composition of the resultant polymers. When CHO/ε-CL volume ratio was 1/4 (run-S26 in Table S7†), an MBC with shorter PCHC block was obtained with a Tm of 56.9 °C that was less than that of the PCL/PCHC blend (58.4 °C, Fig. S13†), indicating that even very short PCHC blocks in MBC could result in restricted crystallization of the PCL block. The suitable temperatures and CO2 pressure for synthesizing MBCs were 90–110 °C and ≥2.0 MPa, respectively. PCL block in MBCs in Table S6† kept nearly in the range of 44.7–51.9%. Remarkably, the average block lengths of MBCs could be tuned by changing the type and amounts of the initiator. MBCs from run-3 and run-5 (thickness of 2.0 mm, Table 1) were semi-transparent and non-transparent, respectively (Fig. 6-C). Larger amount of initiator caused shorter average block lengths, which formed relatively thinner PCL lamellar crystals in the sample. When pentaerythritol was used as the initiator (run-6, Table 1), soft and transparent sample was obtained, suggesting that the crystallization of PCL blocks in the armed MBCs was more severely restricted.
Conclusions
In summary, we described a convenient method to synthesize MBCs with high efficiency from a one-pot/one-step polymerization of CO2, CHO and ε-CL by bridging two independent chain propagations via CCER in one system. This reaction is also of significance because it produced multiblock copolymers without tapering by partially using renewable CO2. Such MBCs with improved mechanical properties have a CO2 uptake up to 15 mol% when [CHO]/[ε-CL] feeding ratio was 1.0. The ongoing work will be directed towards MBCs with tunable properties by precise kinetic control.Experimental
Typical terpolymerization of CHO, CO2 and ε-CL in a one-pot/one-step procedure: the terpolymerization was conducted in a Büchi autoclave, which had been pre-dried at 80 °C under vacuum for 2 h. Desired amounts of Zn–Co(III) DMCC, Sn(Oct)2 (in dried THF), BnOH, CHO and ε-CL were transferred into the autoclave equipped with a mechanical stirrer (500 rpm) and a pressure gauge, CO2 was then pressurized to the target pressure. The autoclave was heated by a cyclic oil heating bath with designed temperature (e.g., 100 °C) and kept stirring for a certain time (e.g., 4 h). After reaction, the autoclave was cooled down to room temperature and CO2 was slowly vented. A small amount of the crude product was taken out for 1H NMR measurement. The remaining sample was dissolved in CH2Cl2 and precipitated from methanol. This process was repeated three times to give the purified polymers.Acknowledgements
The authors are grateful for the financial support by the National Science Foundation of China (no. 21474083 and 21274123); the authors also thank the beamline BL16B1 (Shanghai Synchrotron Radiation Facility) for providing the beam time.Notes and references
- (a) S. Inoue, H. Koinuma and T. Tsuruta, J. Polym. Sci., Part B: Polym. Lett., 1969, 7, 287 CrossRef CAS; (b) D. J. Darensbourg and M. W. Holtcamp, Coord. Chem. Rev., 1996, 153, 155 CrossRef CAS; (c) G. W. Coates and D. R. Moore, Angew. Chem., Int. Ed., 2004, 43, 6618 CrossRef CAS PubMed; (d) D. J. Darensbourg, R. M. Mackiewicz, A. L. Phelps and D. R. Billodeaux, Acc. Chem. Res., 2004, 37, 836 CrossRef CAS PubMed; (e) H. Sugimoto and S. Inoue, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 5561 CrossRef CAS; (f) D. J. Darensbourg, Chem. Rev., 2007, 107, 2388 CrossRef CAS PubMed; (g) S. Klaus, M. W. Lehenmeier, C. E. Anderson and B. Rieger, Coord. Chem. Rev., 2011, 255, 1460 CrossRef CAS PubMed; (h) M. R. Kember, A. Buchard and C. K. Williams, Chem. Commun., 2011, 47, 141 RSC; (i) X.-B. Lu, W.-M. Ren and G.-P. Wu, Acc. Chem. Res., 2012, 45, 1721 CrossRef CAS PubMed; (j) X.-B. Lu and D. J. Darensbourg, Chem. Soc. Rev., 2012, 41, 1462 RSC.
- (a) M. Labet and W. Thielemans, Chem. Soc. Rev., 2009, 38, 3484 RSC; (b) R. H. Platel, L. M. Hodgson and C. K. Williams, Polym. Rev., 2008, 48, 11 CrossRef CAS; (c) C. K. Williams and M. A. Hillmyer, Polym. Rev., 2008, 48, 1 CrossRef CAS; (d) M. H. Chisholm and Z. P. Zhou, J. Mater. Chem., 2004, 14, 3081 RSC; (e) O. Dechy-Cabaret, B. Martin-Vaca and D. Bourissou, Chem. Rev., 2004, 104, 6147 CrossRef CAS PubMed.
- (a) R. R. A. Kitson, A. Millemaggi and R. J. K. Taylor, Angew. Chem., Int. Ed., 2009, 48, 9426 CrossRef CAS PubMed; (b) H. Miao and E. Y.-X. Chen, Macromolecules, 2014, 47, 3614 CrossRef.
- (a) G.-P. Wu, D. J. Darensbourg and X.-B. Lu, J. Am. Chem. Soc., 2012, 134, 17739 CrossRef CAS PubMed; (b) D. J. Darensbourg and G.-P. Wu, Angew. Chem., Int. Ed., 2013, 52, 10602 CrossRef CAS PubMed.
- (a) M. R. Kember, J. Copley, A. Buchard and C. K. Williams, Polym. Chem., 2012, 3, 1196 RSC; (b) C. Romain and C. K. Williams, Angew. Chem., Int. Ed., 2014, 53, 1607 CrossRef CAS PubMed; (c) M. R. Kember and C. K. Williams, J. Am. Chem. Soc., 2012, 134, 15676 CrossRef CAS PubMed.
- J.-F. Zhang, W.-M. Ren, X.-K. Sun, Y. Meng, B.-Y. Du and X.-H. Zhang, Macromolecules, 2011, 44, 9882 CrossRef CAS.
- X.-K. Sun, X.-H. Zhang, R.-J. Wei, B.-Y. Du, Q. Wang, Z.-Q. Fan and G.-R. Qi, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 2924 CrossRef CAS.
- (a) S. Inoue, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 2861 CrossRef CAS; (b) A. Cyriac, S. H. Lee, J. K. Varghese, E. S. Park, J. H. Park and B. Y. Lee, Macromolecules, 2010, 43, 7398 CrossRef CAS.
- Catalyst pairs of (Salen)Co(or Cr) complexes/Sn(Oct)2(PPNCl, TBD and DBU) and zinc glutarate/Sn(Oct)2, were applied to the terpolymerization of CO2, CHO and ε-CL, but failed to produce MBCs because of the unmatched polymerization rates, and poisoning of catalysts.
- X.-K. Sun, X.-H. Zhang, F. Liu, S. Chen, B.-Y. Du, Q. Wang, Z.-Q. Fan and G.-R. Qi, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3128 CrossRef CAS.
- (a) A. Duda, Macromolecules, 1994, 27, 576 CrossRef CAS; (b) M. R. Dorn, J. D. Lennon, G. L. Glish and M. R. Gagné, Macromolecules, 1999, 32, 5149 CrossRef; (c) A. Kowalski, J. Libiszowski, T. Biela, M. Cypryk, A. Duda and S. Penczek, Macromolecules, 2005, 38, 8170 CrossRef CAS; (d) R. F. Storey and J. W. Sherman, Macromolecules, 2002, 35, 1504 CrossRef CAS.
- X.-K. Sun, X.-H. Zhang, S. Chen, B.-Y. Du, Q. Wang, Z.-Q. Fan and G.-R. Qi, Polymer, 2010, 51, 5719 CrossRef CAS PubMed.
- D. J. Arriola, E. M. Carnahan, P. D. Hustad, R. L. Kuhlman and T. T. Wenzel, Science, 2006, 312, 714 CrossRef CAS PubMed.
- W. Zhao, Y. Wang, X.-L. Liu, X.-S. Chen, D.-M. Cui and E. Y.-X. Chen, Chem. Commun., 2012, 48, 6375 RSC.
- Z.-Z. Tong, B. Zhou, J. Huang, J.-T. Xu and Z.-Q. Fan, Macromolecules, 2014, 47, 333 CrossRef CAS.
- C. Koning, J. Wildeson, R. Parton, B. Plum, P. Steeman and D. J. Darensbourg, Polymer, 2001, 42, 3995 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Text, figures and tables giving general experimental procedures and characterization data for multiblock copolymers. See DOI: 10.1039/c4sc03593c |
| This journal is © The Royal Society of Chemistry 2015 |