Open Access Article
Open Access ArticleChemical assay-guided natural product isolation via solid-supported chemodosimetric fluorescent probe†
Hongjun
Jeon
,
Chaemin
Lim
,
Ji Min
Lee
and
Sanghee
Kim
*
College of Pharmacy, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul 151-742, Korea. E-mail: pennkim@snu.ac.kr; Fax: +82-2-888-0649; Tel: +82-2-880-2487
First published on 25th March 2015
Abstract
As a new model of chemical assay-guided natural product isolation, an effective chemodosimetric assay system was devised. Our chemical assay system features a fluorogenic chemodosimeter immobilized on a solid support, which offers advantages in identifying the desired compounds in complex natural product mixtures. To isolate only compounds with the target functional groups, the click chemistry concept was adopted. The model system presented here was developed for natural products with a terminal alkyne. Using our newly designed alkyne sensing beads with the aforementioned features, we have readily identified, quantified, and isolated compounds with a terminal alkyne group from plant extracts.
Introduction
Despite the advent of modern drug discovery technologies, natural products still remain a significant source of new drugs.1 In addition, they are valuable tools with which to study biology.2 At present, bioassay-guided isolation is the favored procedure for identifying new natural substances.3 Although modern analytical and bioassay techniques have made it considerably competitive,4 bioassay-guided isolation is a time-consuming process and it still has some limitations caused by false assay signals arising from compounds with unspecific activities.5 Thus, alternative and complementary approaches are needed to further advance natural product research.The isolation of natural products can also be guided by chemical cues associated with functional groups.6 The chemical reactions of certain functional groups can produce a detectable signal and this has long been used in the natural product isolation process.7 For example, the phenolic group of tannins was identified by FeCl3, and the tertiary amino group of alkaloids was detected by Dragendorff's reagent. However, this approach has become obsolete and is not considered useful, primarily because of the low sensitivity and specificity of the chemical cues produced in natural product extracts. If an effective chemical assay system were to be developed that could easily and reliably identify a compound with a specific functional group in an extract, the systematic exploration of the natural product chemical space would be greatly facilitated.
The “click reaction” is thermodynamically favorable and reliably leads to a single product under mild reaction conditions.8 We envisaged that the proper application of click chemistry would guarantee the specificity and reliability of a chemical assay system. Among various click reactions, the copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) reaction is the most popular and well-studied.9 It produces a 1,4-substituted triazole from an azide and a terminal alkyne in generally excellent yield. With this reaction, we designed a chemical assay system to assess the potential of click-based chemical assay-guided natural product isolation. Herein, we present a prototype system designed for terminal alkyne-containing substances, which exhibit a wide array of biological properties.10
Results and discussion
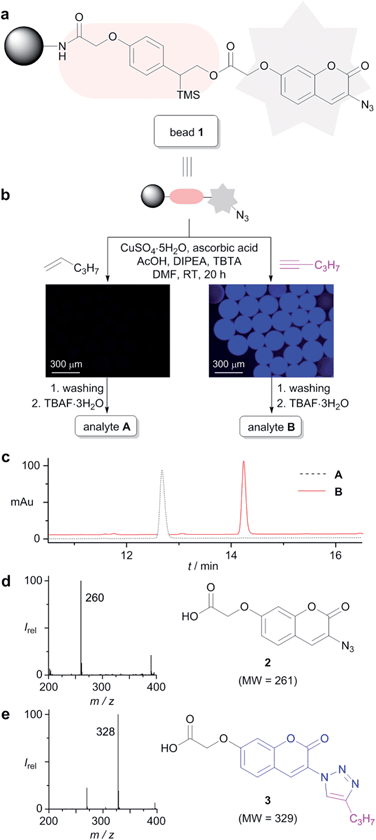
As shown in Fig. 1, the designed alkyne sensing chemical assay system consists of a fluorescent chemodosimetric probe, a linker, and a solid support. The fluorescent chemodosimetric probe11 is the key moiety of our system; it captures terminal alkyne groups through the CuAAC reaction and produces a fluorescence signal. Immobilization of the fluorescent probe on a solid support was adopted for easy handling and tracking. Consequently, the presence of compounds with a terminal alkyne group in the analyte would lead to fluorescent responses from the solid bead. In addition, via a cleavable linker, our sensing system allows the captured target component to be released from the bead as a CuAAC product for subsequent spectroscopic analysis.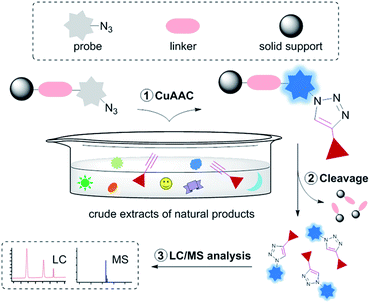 | ||
| Fig. 1 A solid-supported fluorogenic chemical assay strategy for the identification of terminal alkynes in natural product extracts. | ||
The proper choice of solid support and linker is critical to the success of any solid-phase chemistry. TentaGel™ MB amino resin was chosen as a solid support because of its high swelling capacity in various solvents and its relatively large particle size. The linker chosen was (2-phenyl-2-trimethylsilyl)ethyl-(PTMSEL)-linker (Fig. 2a), which can be readily cleaved by fluoride ion under mild conditions.12 The profluorophore 3-azidocoumarin was chosen as a fluorescent chemodosimetric probe because it is transformed into a highly fluorescent triazolylcoumarin after the CuAAC reaction with terminal alkynes.13 The probe was indirectly attached to the PTMSEL linker by binding through glycolic acid, allowing facile analysis by reverse-phase LC/MS after cleavage from the linker.
The alkyne sensing bead 1 was prepared with a loading level of 0.3 mmol g−1 using standard solid-phase synthesis techniques (see ESI†). With the alkyne sensing bead 1 in hand, we explored the optimal conditions for the CuAAC reaction. 1-Pentyne (0.9 µmol) was used as a model alkyne substrate. After investigating various sets of conditions, we observed that treatment of bead 1 (0.3 µmol, 1 mg) with CuSO4·5H2O (1.5 µmol), ascorbic acid (7.5 µmol), N,N-diisopropylethylamine (15.0 µmol), acetic acid (30.0 µmol), and tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (3.0 µmol) in DMF (ca. 50 µL) at room temperature generated the desired triazolylcoumarin product in nearly quantitative yield14 without undesirable side reactions.15,16 When 1-pentyne was reacted with bead 1 under the aforementioned conditions, fluorescence emission was observed from the beads under UV light. However, in a control experiment without the terminal alkyne, no fluorescence signal was observed. The fluorescence of these “reporter beads” was clearly distinguished by conventional fluorescence microscopy (Fig. 2b).
The resulting on-resin fluorescent product was cleaved from the solid support by treatment with TBAF·3H2O and subsequently analyzed by LC/MS. Because the detached compound had a UV-active functionality and a hydrophilic carboxylic acid group, as exemplified by compound 3 (Fig. 2e), the analysis was readily achieved using a conventional LC/MS system equipped with a diode-array UV detector and a reverse-phase column. The LC chromatogram of the analyte released from the fluorescent beads (analyte B) showed only one peak, which was identified as the molecular ion [M − H]− at m/z 328 in the ESI negative ionization mode. The molecular mass (329 Daltons) of this click product matched well with the theoretical value for triazolylcoumarin 3. The LC chromatogram for the control experiment (analyte A) also showed only one peak with a molecular ion at m/z 260 ([M − H]−), which matched well with the theoretical molecular weight of unreacted 3-azidocoumarin 2 (Fig. 2d).
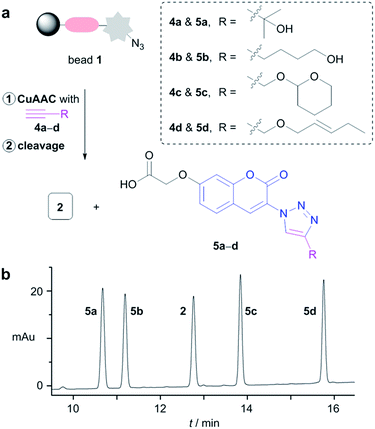
To further validate the system, a mixture of different terminal alkynes was assayed. A mixture of equimolar amounts of four terminal alkynes 4a–d (0.2 equiv. each with a maximum loading level of beads) was treated with bead 1 (Fig. 3a). The resulting fluorescent beads were treated with TBAF·3H2O, and the mixture of detached compounds was analyzed by LC/MS. As shown in Fig. 3b, five peaks were observed in the LC/MS chromatogram. The molecular masses of the peaks exactly matched the theoretical values of triazolylcoumarins 5a–d and 3-azidocoumarin 2. Furthermore, the integration values of the five peaks were similar, corresponding to the theoretical ratio. The chemical yields of each compound, estimated from the integration of peak areas, were nearly quantitative.14 This result revealed that the relative quantification of terminal alkynes is possible with this sensing system (see ESI†).
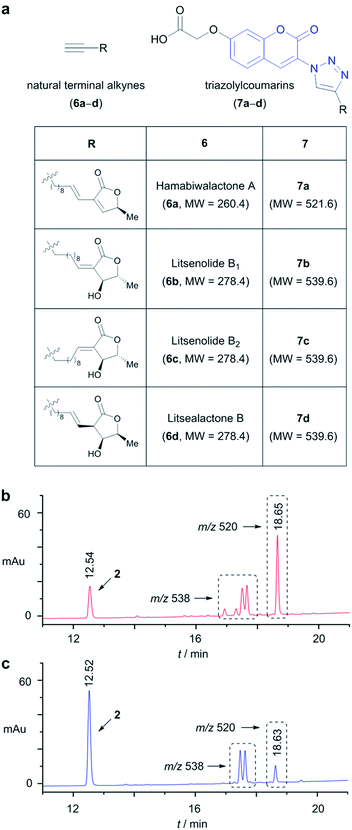
To demonstrate the utility of alkyne sensing bead 1 in the natural product isolation process, it was first used in the analysis of the methanol extract of the leaves of Litsea japonica (Lauraceae). A literature search revealed that four terminal alkyne compounds 6a–d have been isolated from the leaves of L. japonica by two independent groups (Fig. 4a).17 As expected, the leaf methanol extract (3 mg) exhibited fluorescence activation after the click reaction with our alkyne sensing bead 1 (0.5 mg). In the LC/MS analysis of the off-bead mixture, five peaks were observed in the LC/MS chromatogram in addition to the peak of 2 (Fig. 4b). The deduced molecular mass (521 Daltons) of the major peak (retention time: 18.65 min) matched well with the theoretical molecular weight of 7a, the triazolylcoumarin product resulting from the CuAAC reaction of 6a. The other four peaks exhibited the same m/z value of 538 ([M − H]−). The deduced molecular masses (539 Daltons) are in good agreement with those of triazolylcoumarins 7b–d. These results suggested the presence of four terminal alkyne compounds 6a–d and one additional, as yet unidentified terminal alkyne compound with a molecular weight of 278 in the leaves of L. japonica, although we could not exclude the possibility that the stereoisomers of 6a–d are the structures of natural terminal alkynes.
The methanol extract of the stem heartwood of the same plant (3 mg) also activated the fluorescence of the beads. However, the cleaved products showed a somewhat different pattern of peaks in the LC/MS chromatogram compared to the leaf extracts, suggesting that the levels of specific terminal alkyne natural products 6a–d varied among different parts of the plant (Fig. 4c). Because of the characteristic and intense UV absorption of the triazolylcoumarin moiety, the quantification of individual terminal alkyne compounds was possible using the standard curve method. For example, on the basis of the respective integration values, compound 6a is estimated to exist in the methanol extract of the leaves at a concentration of approximately 1.0 mg g−1, whereas it is present at a concentration of approximately 0.2 mg g−1 in the extract of the stem heartwood (see ESI†).
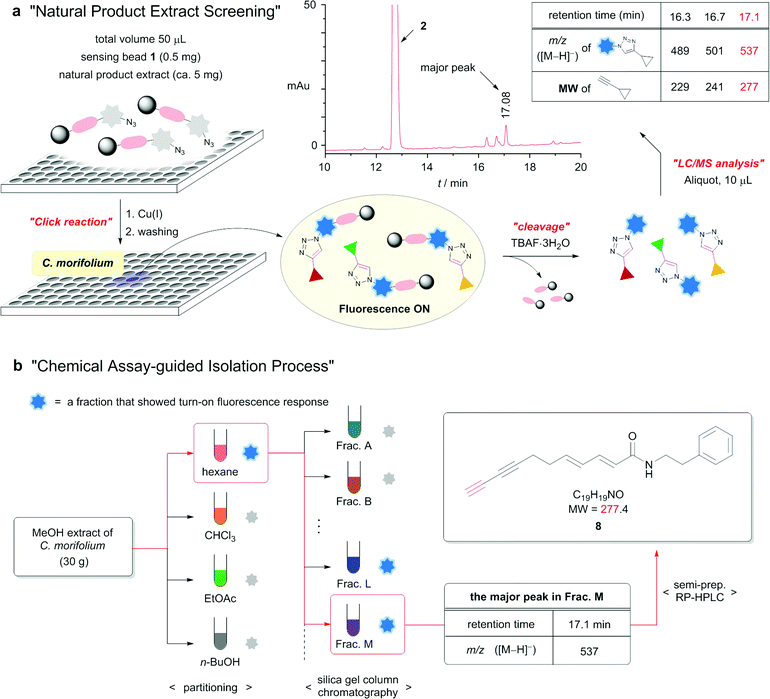
Encouraged by the successful results with the crude extract of L. japonica, we performed further investigations to isolate terminal alkyne compounds from the extracts of natural products using the alkyne sensing bead 1. Among the tested crude natural plant extracts of various origins, fluorescence activation was observed for the methanol extract of the whole plant of Chrysanthemum morifolium (Compositae) (Fig. 5a and ESI†), from which no terminal alkyne compounds have previously been isolated.
Analysis of the LC/MS chromatogram after resin cleavage indicated the presence of three terminal alkyne compounds. To isolate the terminal alkyne component corresponding to the major peak, 30 g of the methanol extract was suspended in water and successively extracted with hexane, chloroform, ethyl acetate, and n-butanol (Fig. 5b). A small portion of each fraction was subjected to the click reaction with alkyne sensing bead 1. Fluorescence activation was observed only in the hexane fraction. Fractionation of the hexane extract over silica gel produced fifteen different fractions (A–O). The chemical assay with bead 1 and LC/MS analysis indicated that the desired component was present primarily in fraction M. Purification of fraction M by semi-preparative reverse-phase HPLC with the guidance of the extracted ion chromatograms afforded a pure compound with an m/z value of 278 [M + H]+ as a white solid. Through analysis of the spectroscopic data, the isolated compound was determined to be terminal diyne 8.18 The isolated compound is a previously reported natural product.19 However, this is the first report of the known natural compound 8 being isolated from the extract of C. morifolium.
Conclusions
In conclusion, we have developed a novel prototype chemical assay-guided natural product isolation method. Click chemistry was used in the chemical assay system to reliably identify target compounds without interference from other components in the extracts. A click reaction-induced fluorescence sensing platform was employed to sensitively detect even low concentrations of the target compounds and to permit visualization. Immobilization of this “sensing and reporting” moiety on a solid support was adopted for easy handling and tracking. The presented chemical assay system was designed for the detection of terminal alkyne-containing natural products using CuAAC, the prototypical click chemistry reaction. Our newly designed alkyne sensing bead 1 can successfully and quantitatively identify terminal alkyne compounds on the basis of a fluorescence signal. Using this bead, we were able to identify, quantify, and isolate terminal alkyne natural products from plant extracts, demonstrating the prospect of chemical assay-guided natural product isolation. Further studies, including the extension of this chemical assay system to other functional groups through the use of other viable click chemistry reactions, are needed to robustly establish chemical assay-guided natural product isolation. We believe that our “click reaction/fluorescent chemodosimetric probe/solid support” chemical assay system could also be applicable to numerous other fields, such as metabolomics and food science, which require the analysis of complex mixtures of compounds.Acknowledgements
This work was supported by the Mid-Career Researcher Program (no. 2013R1A2A1A01015998) of the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP). This work was also supported by BK21 Plus Program in 2014. We thank Prof. Won Keun Oh (Seoul National University) for helpful advice about natural product isolation.Notes and references
- For recent reviews, see: (a) A. L. Harvey, Drug Discovery Today, 2008, 13, 894–901 CrossRef CAS PubMed; (b) J. W.-H. Li and J. C. Vederas, Science, 2009, 325, 161–165 CrossRef PubMed; (c) D. J. Newman and G. M. Cragg, J. Nat. Prod., 2012, 75, 311–335 CrossRef CAS PubMed.
- (a) A. M. Piggott and P. Karuso, Comb. Chem. High Throughput Screening, 2004, 7, 607–630 CrossRef CAS; (b) E. E. Carlson, ACS Chem. Biol., 2010, 5, 639–653 CrossRef CAS PubMed.
- (a) L. Bohlin and J. G. Bruhn, Bioassay Methods in Natural Product Research and Drug Development, Kluwer Academic Press, Dordrecht, 1999 Search PubMed; (b) A. A. Ströemstedt, J. Felth and L. Bohlin, Phytochem. Anal., 2014, 25, 13–28 CrossRef PubMed.
- For recent reviews, see: (a) F. E. Koehn and G. T. Carter, Nat. Rev. Drug Discovery, 2005, 4, 206–220 CrossRef CAS PubMed; (b) O. Sticher, Nat. Prod. Rep., 2008, 25, 517–554 RSC; (c) O. Potterat and M. Hamburger, Nat. Prod. Rep., 2013, 30, 546–564 RSC.
- F. E. Koehn, in Natural Compounds as Drugs Volume I, ed. F. Petersen and R. Amstutz, Birkhäuser, Basel, 2008, vol. 65, pp. 175–210 Search PubMed.
- (a) E. E. Carlson and B. F. Cravatt, Nat. Methods, 2007, 5, 429–435 Search PubMed; (b) S. S. Ebada, R. A. Edrada, W. Lin and P. Proksch, Nat. Protoc., 2008, 3, 1820–1831 CrossRef CAS PubMed.
- (a) N. R. Farnsworth, J. Pharm. Sci., 1966, 55, 225–276 CrossRef CAS; (b) S. Gibbons and A. I. Gray in Natural Products Isolation, ed. R. J. P. Cannell, Humana Press, New Jersey, 1988, vol. 4, ch. 4, pp. 209–245 Search PubMed.
- For reviews on click chemistry, see: (a) H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS; (b) H. C. Kolb and K. B. Sharpless, Drug Discovery Today, 2003, 8, 1128–1137 CrossRef CAS.
- (a) V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS; (b) E. Lallana, R. Riguera and E. Fernandez-Megia, Angew. Chem., Int. Ed., 2011, 50, 8794–8804 CrossRef CAS PubMed.
- (a) A. L. K. Shi Shun and R. R. Tykwinski, Angew. Chem., Int. Ed., 2006, 45, 1034–1057 CrossRef PubMed; (b) V. Dembitsky and A. Siddiq, Anti-Cancer Agents Med. Chem., 2008, 8, 132–170 CrossRef; (c) D. V. Kuklev, A. J. Domb and V. M. Dembitsky, Phytomedicine, 2013, 20, 1145–1159 CrossRef CAS PubMed.
- For reviews on fluorescent chemodosimeters, see: (a) D. G. Cho and J. L. Sessler, Chem. Soc. Rev., 2009, 38, 1647–1662 RSC; (b) C. Le Droumaguet, C. Wang and Q. Wang, Chem. Soc. Rev., 2010, 39, 1233–1239 RSC; (c) K. Kaur, R. Saini, A. Kumar, V. Luxami, N. Kaur, P. Singh and S. Kumar, Coord. Chem. Rev., 2012, 256, 1992–2028 CrossRef CAS PubMed; (d) J. Chan, S. C. Dodani and C. J. Chang, Nat. Chem., 2012, 4, 973–984 CrossRef CAS PubMed.
- M. Wagner and H. Kunz, Angew. Chem., Int. Ed., 2002, 41, 317–321 CrossRef CAS.
- K. Sivakumar, F. Xie, B. M. Cash, S. Long, H. N. Barnhill and Q. Wang, Org. Lett., 2004, 6, 4603–4606 CrossRef CAS PubMed.
- The product yield was calculated, after resin cleavage, according to the standard curve of triazolylcoumarin 3 (see ESI†).
- C. Shao, X. Wang, Q. Zhang, S. Luo, J. Zhao and Y. Hu, J. Org. Chem., 2011, 76, 6832–6836 CrossRef CAS PubMed.
- Y. Xia, W. Li, Z. Fan, X. Liu, C. Berro, E. Rauzy and L. Peng, Org. Biomol. Chem., 2007, 5, 1695–1701 CAS.
- (a) H. Tanaka, T. Nakamura, K. Ichino, K. Ito and T. Tanaka, Phytochemistry, 1990, 29, 857–859 CrossRef CAS; (b) B. S. Min, S. Y. Lee, J. H. Kim, O. K. Kwon, B. Y. Park, R. B. An, J. K. Lee, H. I. Moon, T. J. Kim, Y. H. Kim, H. Joung and H. K. Lee, J. Nat. Prod., 2003, 66, 1388–1390 CrossRef CAS PubMed.
- For previous reports of trapping terminal polyynes by CuAAC, see: (a) T. Luu, R. McDonald and R. R. Tykwinski, Org. Lett., 2006, 8, 6035–6038 CrossRef CAS PubMed; (b) C. Ross, K. Scherlach, F. Kloss and C. Hertweck, Angew. Chem., Int. Ed., 2014, 53, 7794–7798 CrossRef CAS PubMed.
- (a) R. Jente, P. H. Bonnet and F. Bohlmann, Chem. Ber., 1972, 105, 1694–1700 CrossRef CAS; (b) R. Martin and H. Becker, Phytochemistry, 1985, 24, 2295–2300 CrossRef CAS; (c) O. Hofer, H. Greger, W. Robien and A. Werner, Tetrahedron, 1986, 42, 2707–2716 CrossRef CAS; (d) G. P. Li, B. C. Shen, J. F. Zhao, X. D. Yang and L. Li, J. Integr. Plant Biol., 2007, 49, 1608–1610 CrossRef CAS PubMed; (e) V. Sharma, J. Boonen, B. D. Spiegeleer and V. K. Dixit, Phytother. Res., 2013, 27, 99–106 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5sc00360a |
| This journal is © The Royal Society of Chemistry 2015 |