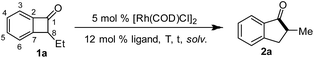Open Access Article
Open Access ArticleRh-catalyzed reagent-free ring expansion of cyclobutenones and benzocyclobutenones†
Peng-hao
Chen
a,
Joshua
Sieber
b,
Chris H.
Senanayake
b and
Guangbin
Dong
*a
aThe University of Texas at Austin, Department of Chemistry, Austin, TX 78712, USA
bDepartment of Chemical Development, Boehringer Ingelheim Pharmaceuticals, Inc., 900 Ridgebury Road/P.O. Box 368, Ridgefield, Connecticut 06877-0368, USA
First published on 10th July 2015
Abstract
Here we report a reagent-free rhodium-catalyzed ring-expansion reaction via C–C cleavage of cyclobutenones. A variety of poly-substituted cyclopentenones and 1-indanones can be synthesized from simple cyclobutenones and benzocyclobutenones. The reaction condition is near pH neutral without additional oxidants or reductants. The potential for developing a dynamic kinetic asymmetric transformation of this reaction has also been demonstrated. Further study supports the proposed pathway involving Rh-insertion into the cyclobutenone C–C bond, followed by β-hydrogen elimination, olefin insertion and reductive elimination.
Introduction
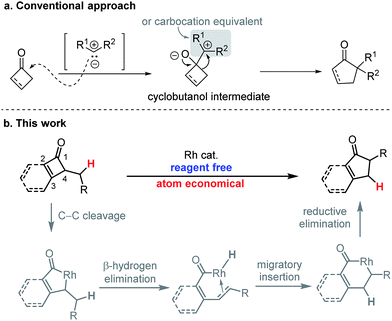
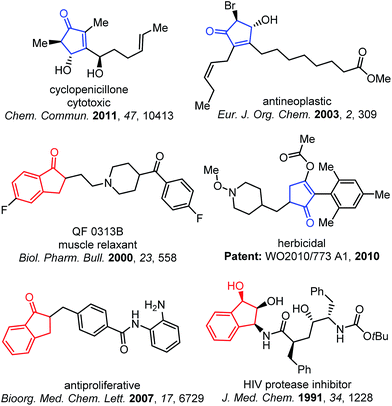
Ring expansion reactions, generally with cyclic ketones, are highly valuable transformations for constructing complex ring skeletons.1 Conventional approaches for direct one-carbon homologation of cyclic ketones primarily rely on addition of a carbene reagent or its equivalent (Scheme 1a).2 For example, diazoalkanes have been frequently employed for preparing cyclopentanones from cyclobutanones.3 In contrast, the corresponding transformations with unsaturated enones (e.g. cyclobutenones) are much rarer largely due to the competing reactions with the olefin moiety (e.g. conjugate addition and cyclopropanation)4 and lack of regioselectivity.5 Moreover, most existing methods for one-carbon ring expansion of four-membered ring ketones6 involve forming cyclobutanols7 (or cyclobutenols8) as a transient or isolatable intermediate (Scheme 1a). Considering that, as important classes of organic compounds, cyclopentenones and 1-indanones are frequently employed as building blocks and widely found in a number of bioactive molecules (Fig. 1), herein we describe a unique, simple, and atom-economical strategy for the direct catalytic ring expansion of alkyl cyclobutenones and benzocyclobutenones to five-membered unsaturated ketones.9Results and discussion
Research hypothesis
Our proposed strategy is described in Scheme 1b. Driven by strain release, cyclobutenones are known to undergo ring openings with transition metals (e.g. RhI) through cleavage of the C1–C4 bond.10 We hypothesized that subsequent β-hydrogen elimination with the resulting acyl metallacycle would lead to a metal hydride–olefin complex, which can undergo hydride re-insertion followed by reductive elimination to furnish the ring expanded product.While numerous elegant methods have been developed for synthesis of cyclopentenones and indanones,11 such as Pauson–Khand (PK)12 and Nazarov13 reactions, this approach nevertheless exhibits a number of complementary features. First, poly-substituted cyclobutenones and benzocyclobutenones are readily available through many approaches,14 including a [2 + 2] cycloaddition between an alkyne (or aryne) and a ketene equivalent (see ESI†). Second, it operates under near pH and redox-neutral conditions, which would tolerate many functional groups (Nazarov reactions generally require use of a strong acid). Third, this transformation shows a complete regioselectivity when forming α-substituted cyclopentenones (vide infra, Table 4); in contrast, it is non-trivial to control the regioselectivity for intramolecular PK reactions.
Optimization studies and substrate scope
To test this hypothesis, benzocyclobutenone 1a was employed as the model substrate, and the reaction was investigated by examining a number of parameters (Table 1). When Wilkinson's complex [RhCl(PPh3)3] was used as the catalyst, no desired product was observed (entry 1, Table 1), and 1a remained intact. It is known that bidentate ligands can facilitate migratory insertion and reductive elimination.15 Thus, a series of bisphosphine ligands were evaluated (entries 2–5, Table 1). To our delight, all these ligands provided the desired ring expansion product, whereas dppp proved to be the most efficient. Using dppp as the ligand, the reaction occurred smoothly at 80 °C albeit requiring a longer reaction time (entries 5–9, Table 1). When performed at 90 °C, the desired 1-indanone product was isolated in 93% yield (entry 9, Table 1). A survey of different solvents revealed that 1,4-dioxane was optimal, although THF and ethyl benzene worked almost equally well (entries 9–13, Table 1). Finally, control experiments showed that both the rhodium pre-catalyst and the phosphine ligand were essential for the success of this ring-expansion reaction (entries 14–15, Table 1).| Entry | Ligand | T (°C) | Solvent | Time | Yielda |
|---|---|---|---|---|---|
| a Unless otherwise noted, all yields were determined by 1H NMR using 1,1,2,2-tetrachloroethane as the internal standard. b Wilkinson's catalyst [RhCl(PPh3)3] was used. c Isolated yield. | |||||
| 1 | PPh3b | 110 | Dioxane | 24 h | 0 |
| 2 | dppe | 110 | Dioxane | 24 h | 75% |
| 3 | dppb | 110 | Dioxane | 24 h | 61% |
| 4 | dppf | 110 | Dioxane | 24 h | 64% |
| 5 | dppp | 110 | Dioxane | 24 h | 84% |
| 6 | dppp | 120 | Dioxane | 24 h | 68% |
| 7 | dppp | 100 | Dioxane | 24 h | 73% |
| 8 | dppp | 80 | Dioxane | 48 h | 77% |
| 9 | dppp | 90 | Dioxane | 48 h | 93% |
| 10 | dppp | 90 | THF | 48 h | 90% |
| 11 | dppp | 90 | PhEt | 48 h | 89% |
| 12 | dppp | 90 | Benzene | 48 h | 81% |
| 13 | dppp | 90 | Toluene | 48 h | 76% |
| 14 | dppp w/o [Rh] | 90 | Dioxane | 48 h | 0 |
| 15 | No ligand | 90 | Dioxane | 48 h | 0 |
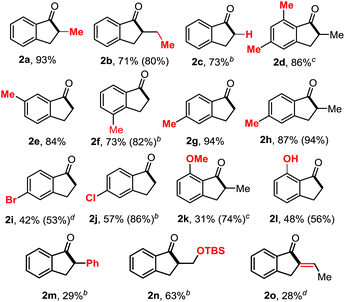
With the optimal condition established, we next investigated the substrate scope of the reaction (Table 2). Benzocyclobutenones bearing different substituents at the C8 position all afforded the desired products (2a–2d, 2m,162n). Substituents at all positions on the benzene ring can be tolerated (2d–2h). The 3,5-dimethyl substituted substrate required a higher temperature but still provided the desired product (2d) in a good yield. Functional groups, such as aryl bromides, chlorides, anisoles, free phenols and silyl ethers (2i–2l and 2n) were compatible under the reaction conditions, albeit giving moderate yields. Interestingly, olefin migration was observed when 8-allyl-substituted substrate (2o) was used.
| a All yields are isolated yields, and the numbers in parenthesis are yields based on recovered starting material. All reactions were carried out in a sealed vial under N2 atmosphere. b The reaction was run at 110 °C for 48 h. c The reaction was run at 150 °C for 48 h. d The reaction was run at 130 °C for 48 h. |
|---|
|
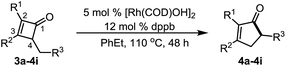
To explore whether the benzo-moiety is essential for the ring-expansion transformation, we next investigated simple cyclobutenone derivatives (Table 3). Compound 3a was employed as the model substrate. It was surprising to note that when the optimal conditions for the benzocyclobutenones (vide supra) were applied, no desired ring-expansion product was found even at elevated temperatures (entry 1, Table 3), suggesting a significant difference in reactivity between the two closely related compounds. A survey of other ligands also remained unfruitful, and unlike benzocyclobutenones, compound 3a remained inactive under these conditions (entries 2–4, Table 3). However, by switching the precatalyst from [Rh(COD)Cl]2 to [Rh(COD)OH]2 or [Rh(COD)OMe]2,17 cyclopentenone 4a could be isolated in moderate to good yields (entries 5 and 6, Table 3). The analogous [Ir(COD)OMe]2 failed to give any desired product (entry 7, Table 3). A number of bidentate phosphine ligands were subsequently evaluated (entries 8–11, Table 3), and dppb showed enhanced reactivity (entry 9, Table 3). Control experiments further revealed that both the metal and the ligand were required (entries 12–14, Table 3), and NaOH alone failed to catalyze the reaction, indicating that the transformation was not solely catalyzed by the hydroxy anion (entry 15, Table 3). It is worth noting that the reaction can occur at 80oC (entry 21, Table 3). Considering the overall reaction efficiency, 110 °C was chosen as the temperature for further optimization (entry 18, Table 3). Finally, the solvent effect was investigated, and ethyl benzene gave the best results (95%, entry 26, Table 3).
| Entry | Ligand | Precatalyst | T (°C) | Solvent | Time | Yielda |
|---|---|---|---|---|---|---|
| a Unless otherwise noted, all yields were determined by 1H NMR using 1,1,2,2-tetrachloroethane as the internal standard. Values in the parentheses are yields based on recovered starting material. b 30 mol% of PPh3 was used as the ligand. c Isolated yields. d 20 mol% of NaOH was used. | ||||||
| 1 | dppp | [Rh(COD)Cl]2 | 130 | Dioxane | 24 h | 0% |
| 2 | dppb | [Rh(COD)Cl]2 | 130 | Dioxane | 24 h | 0% |
| 3 | PPh3b | [Rh(COD)Cl]2 | 150 | Dioxane | 24 h | 0% |
| 4 | dppb | [Rh(COD)Cl]2 | 150 | Dioxane | 24 h | 0% |
| 5 | dppp | [Rh(COD)OH]2 | 150 | Dioxane | 24 h | 70%c |
| 6 | dppp | [Rh(COD)OMe]2 | 150 | Dioxane | 24 h | 56%c |
| 7 | dppp | [lr(COD)OMe]2 | 150 | Dioxane | 24 h | 0% |
| 8 | dppe | [Rh(COD)OH]2 | 150 | Dioxane | 24 h | 50% |
| 9 | dppb | [Rh(COD)OH]2 | 150 | Dioxane | 24 h | 86%c |
| 10 | dpppent | [Rh(COD)OH]2 | 150 | Dioxane | 24 h | 76% |
| 11 | dppb | [Rh(COD)OH]2 | 130 | Dioxane | 24 h | 81%c |
| 12 | None | None | 130 | Dioxane | 24 h | 0% |
| 13 | None | [Rh(COD)OH]2 | 130 | Dioxane | 24 h | 37% (67%) |
| 14 | dppp | None | 130 | Dioxane | 24 h | 0% |
| 15 | None | noned | 130 | Dioxane | 24 h | 0% |
| 18 | dppb | [Rh(COD)OH]2 | 110 | Dioxane | 48 h | 92% |
| 19 | dppb | [Rh(COD)OH]2 | 100 | Dioxane | 48 h | 74% (80%) |
| 20 | dppb | [Rh(COD)OH]2 | 90 | Dioxane | 48 h | 65% (82%) |
| 21 | dppb | [Rh(COD)OH]2 | 80 | Dioxane | 48 h | 42% (77%) |
| 22 | dppb | [Rh(COD)OH]2 | 110 | THF | 48 h | 80% (93%) |
| 23 | dppb | [Rh(COD)OH]2 | 110 | Toluene | 48 h | 90% |
| 24 | dppb | [Rh(COD)OH]2 | 110 | CH3CN | 48 h | 58% (73%) |
| 25 | dppb | [Rh(COD)OH]2 | 110 | nBu2O | 48 h | 30% (79%) |
| 26 | dppb | [Rh(COD)OH] 2 | 110 | PhEt | 48 h | 95% |
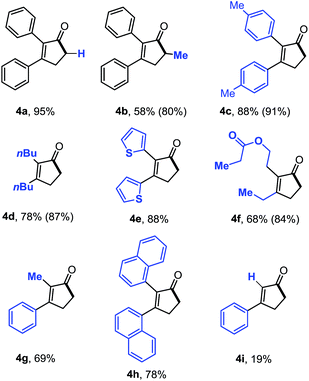
The substrate scope with simple cyclobutenones was then explored (Table 4). Substrates containing various aromatic or aliphatic substituents at the 2 and 3-positions all provided the desired cyclopentenones smoothly. Interestingly, simple α-methylated cyclopentenone 4b has not been synthesized previously. The more sensitive thiophene (4e) and naphthalene rings (4h) can be tolerated. Although the 3,4-disubstituted cyclobutenones (no substitution at the C2 position) are known to be highly unstable and prone to undergo olefin isomerization,18 the desired ring-expansion product (4i, Table 2) can still be obtained, suggesting the mildness of the reaction conditions.
| a All yields are isolated yields, and the numbers in parenthesis are yields based on recovered starting material. All reactions were carried out in a sealed vial under N2 atmosphere. |
|---|
|
Preliminary studies have revealed that chiral bidentate phosphine ligands, such as segphos, effect the dynamic kinetic asymmetric transformation (DYKAT) of benzocyclobutenone 1a with a promising level of enantioselectivity (eqn (1), 49% ee).19 This result suggests this reagent-free ring-expansion reaction is amenable to asymmetric catalysis, and work on this topic is ongoing.
 | (1) |
The utility of this method was demonstrated by converting ring-expansion product 4h to an interesting picene-derived ketone (5) using a light-mediated dehydrogenative cyclization (eqn (2)).20 Notably, picene 5 is nontrivial to prepare via a conventional approach. The application of picene 5 as an organic transistor material21 is under exploration.
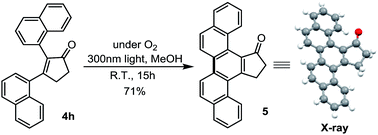 | (2) |
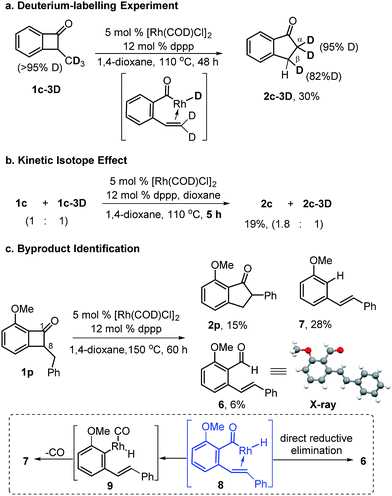
Mechanistic studies
To explore the proposed mechanistic pathway in Scheme 1b, we first conducted a deuterium labelling experiment (Scheme 2a). When the –CD3 substituted benzocyclobutenone (1c-3D) was used as the substrate, indeed, a near complete deuteration (95%) was found at the α-position of the 1-indanone product, and 82% deuterium incorporation was observed at one of the β-hydrogen. This result is consistent with the proposed β-hydrogen elimination and re-insertion pathway. Subsequently, a positive kinetic isotope effect (KIE) was observed when a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of 1c and 1c-3D were mixed and reacted under the standard conditions (Scheme 2b). While only a moderate KIE (1.8) was observed, this intermolecular competition experiment suggested the β-hydrogen elimination either occurs before the rate-limiting step or is the rate-limiting step.22
1 molar ratio of 1c and 1c-3D were mixed and reacted under the standard conditions (Scheme 2b). While only a moderate KIE (1.8) was observed, this intermolecular competition experiment suggested the β-hydrogen elimination either occurs before the rate-limiting step or is the rate-limiting step.22
Byproducts from a catalytic reaction often provide useful information about the reaction intermediates. Consequently, the byproducts of this ring-expansion reaction were investigated. Benzocyclobutenone 1p was subjected to the standard conditions at an elevated temperature. While the desired 2-phenyl-indanone product was afforded, two major byproducts, aldehyde 6 and stilbene 7, were isolated and characterized (Scheme 2c). The presence of these two ring-opening products supports the pathway of C1–C8 bond cleavage of benzocyclobutenone and Rh-hydride 8 as a possible intermediate. A direct acyl-hydrogen reductive elimination should lead to aldehyde 6, while decarbonylation of intermediate 8 followed by aryl-hydrogen reductive elimination should result in stilbene 7.23 Compared to the standard substrate (1a), the presence of significant side reactions with substrate 1p can be explained by an inefficient migratory insertion and/or reductive elimination with a trans-stilbene-like olefin (vide supra, Table 2, 2m).24
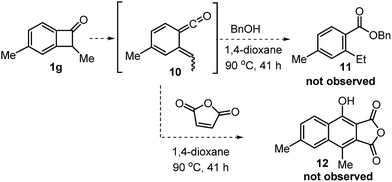
Finally, we investigated whether the cleavage of benzocyclobutenone C1–C8 bond is catalyzed by the Rh catalyst or simply triggered by thermal heat (Scheme 3). If the C–C cleavage was caused by the thermal heat alone, a vinyl ketene intermediate would be generated. Vinyl ketenes are highly reactive species, and are known to react with various nucleophiles or dienophiles.25 However, treatment of the benzocyclobutenone 1g with benzyl alcohol or maleic anhydride at the same reaction temperature did not yield any coupling products, which suggested that the rhodium catalyst played an important role in assisting the C–C cleavage.10
Conclusion
In summary, we have developed a unique Rh-catalyzed ring expansion of cyclobutenones and benzocyclobutenones via C–C bond cleavage. A range of poly-substituted cyclopentenones and 1-indanones can be prepared. This approach is featured by: (1) the substrates are relatively simple and do not require pre-installation of an additional reacting group; (2) no additional stoichiometric reagents are needed for the ring expansion, and the transformation is atom-economical; and (3) the reaction conditions are near pH and redox neutral allowing for tolerance of many functional groups. Finally, the preliminary mechanistic study supports the proposed pathway involving Rh-oxidative addition into the C–C bond, followed by β-hydrogen elimination, olefin migratory insertion and reductive elimination. Efforts on developing a highly enantioselective DYKAT of this reaction for asymmetric synthesis is currently undertaken in our laboratories.Acknowledgements
We thank CPRIT (R 1118), NIGMS (R01GM109054-01) and the Welch Foundation (F 1781) for research grants. GD is a Searle Scholar and a sloan fellow. Prof. Anslyn is acknowledged for advices on the kinetic isotope effect. Dr. Lynch is acknowledged for X-ray crystallography. We thank A. Spangenberg for her advice on NMR spectroscopy. We are also grateful to Johnson Matthey for a generous donation of the Rh salts.Notes and references
- (a) C. D. Gutsche and D. Redmore, in Carbocyclic Ring Expansion Reaction (Advances in Alicyclic Chemical Supplement), Academic Press, New York, 1968, p. 81 Search PubMed; (b) E. Vedejs, Acc. Chem. Res., 1984, 17, 358 CrossRef CAS; (c) P. Dowd and W. Zhang, Chem. Rev., 1993, 93, 2091 CrossRef CAS; (d) E. J. Kantorowski and M. J. Kurth, Tetrahedron, 2000, 56, 4317 CrossRef CAS; (e) H. Nemoto, Chem. Pharm. Bull., 2007, 55, 961 CrossRef CAS; (f) E. Leemans, M. D'hooghe and N. D. Kimpe, Chem. Rev., 2011, 111, 3268 CrossRef CAS PubMed.
- For reviews on diazo compound-mediated homologation reactions, see: (a) C. D. Gutsche, Org. React., 1954, 8, 364 Search PubMed; (b) T. Ye and M. A. McKervey, Chem. Rev., 1994, 94, 1091 CrossRef CAS; (c) Y. Zhang and J. Wang, Chem. Commun., 2009, 5350 RSC and 1d. For a book chapter, see: (d) D. C. Moebius, V. L. Rendina and J. S. Kingsbury, Top. Curr. Chem., 2014, 346, 111 CrossRef CAS.
- For reviews on homologations of four-membered ring ketones with diazo compounds, see: (a) D. Belluš and B. Ernst, Angew. Chem., Int. Ed. Engl., 1988, 27, 797 CrossRef PubMed; (b) T. Seiser, T. Saget, D. N. Tran and N. Cramer, Angew. Chem., Int. Ed., 2011, 50, 7740 CrossRef CAS PubMed; (c) J. C. Namyslo and D. E. Kaufmann, Chem. Rev., 2003, 103, 1485 CrossRef CAS PubMed.
- For selected examples of cyclopropanation of α,β unsaturated ketones with diazo-compounds, see: (a) H. E. Zimmerman and D. R. Diehl, J. Am. Chem. Soc., 1979, 101, 1841 CrossRef CAS; (b) H. E. Zimmerman and R. J. Pasteris, J. Org. Chem., 1980, 45, 4864 CrossRef CAS; (c) H. E. Zimmerman and R. J. Pasteris, J. Org. Chem., 1980, 45, 4876 CrossRef CAS; (d) H. E. Zimmerman and V. Suryanarayan, Eur. J. Org. Chem., 2007, 4091 CrossRef CAS PubMed.
- For a recent example, see: Y. Matsuya, N. Ohsawa and H. Nemoto, J. Am. Chem. Soc., 2006, 128, 13072 CrossRef CAS PubMed.
- For reviews on transition metal-catalyzed C–C bond activation of four-membered ring ketones, see: 3b, 3c and (a) A. Flores-Gaspar and R. Martin, Synthesis, 2013, 45, 563 CrossRef CAS; (b) M. Murakami and T. Matsuda, Chem. Commun., 2011, 47, 1100 RSC. For a book chapter, see: (c) T. Xu, A. Dermenci and G. Dong, Top. Curr. Chem., 2014, 346, 233 CrossRef CAS. For a seminal example of transition metal-catalyzed one-carbon ring expansion of cyclobutanone via 1,2-addition of an arylboron species, see: (d) T. Matsuda, M. Shigeno, M. Makino and M. Murakami, Org. Lett., 2006, 8, 3379 CrossRef CAS PubMed.
- For transition metal-catalyzed ring-expansion reactions of cyclobutanols, see: (a) G. R. Clark and S. Thiensathit, Tetrahedron Lett., 1985, 26, 2503 CrossRef CAS; (b) S. Kim, H. K. Uh, S. Lee and J. H. Park, Tetrahedron Lett., 1991, 32, 3395 CrossRef CAS; (c) S. Kim and K. H. Uh, Tetrahedron Lett., 1992, 33, 4325 CrossRef CAS; (d) B. B. Snider, N. H. Vo and B. M. Foxman, J. Org. Chem., 1993, 58, 7228 CrossRef CAS; (e) H. Nemoto, M. Yoshida and K. Fukumoto, J. Org. Chem., 1997, 62, 6450 CrossRef CAS; (f) H. Nemoto, J. Miyata, M. Yoshida, N. Raki and K. Fukumoto, J. Org. Chem., 1997, 62, 7850 CrossRef CAS; (g) M. Yoshida, H. Nemoto and M. Ihara, Tetrahedron Lett., 1999, 40, 8583 CrossRef CAS; (h) M. Yoshida, K. Sugimoto and M. Ihada, Tetrahedron Lett., 2000, 41, 5089 CrossRef CAS; (i) R. C. Larock and C. K. Reddy, Org. Lett., 2000, 2, 3325 CrossRef CAS PubMed; (j) M. Yoshida, K. Sugimoto and M. Ihara, Tetrahedron Lett., 2001, 42, 3877 CrossRef CAS; (k) B. M. Trost and T. Yasukata, J. Am. Chem. Soc., 2001, 123, 7162 CrossRef CAS; (l) R. C. Larock and C. K. Reddy, J. Org. Chem., 2002, 67, 2027 CrossRef CAS PubMed; (m) M. Yoshida, K. Sugimoto and M. Ihara, Tetrahedron, 2002, 58, 7839 CrossRef CAS; (n) B. M. Trost and J. Xie, J. Am. Chem. Soc., 2006, 128, 6044 CrossRef CAS PubMed; (o) B. M. Trost and J. Xie, J. Am. Chem. Soc., 2008, 130, 6231 CrossRef CAS PubMed; (p) T. Seiser, O. A. Roth and N. Cramer, Angew. Chem., Int. Ed., 2009, 48, 6320 CrossRef CAS PubMed; (q) M. Shigeno, T. Yamamoto and M. Murakami, Chem.–Eur. J., 2009, 15, 12929 CrossRef CAS PubMed; (r) T. Seiser and N. Cramer, Angew. Chem., Int. Ed., 2010, 49, 10163 CrossRef CAS PubMed; (s) A. Schweinitz, A. Chtchemelinine and A. Orellana, Org. Lett., 2011, 13, 232 CrossRef CAS PubMed; (t) A. Yada, S. Fujita and M. Murakami, J. Am. Chem. Soc., 2014, 136, 7217 CrossRef CAS PubMed.
- For transition metal-catalyzed ring expansion of cyclobutenols, see: (a) L. S. Liebeskind, D. Mitchell and B. S. Foster, J. Am. Chem. Soc., 1987, 109, 7908 CrossRef CAS; (b) L. S. Liebeskind and A. Bombrun, J. Org. Chem., 1994, 59, 1149 CrossRef CAS; (c) Y. Xia, Z. Liu, Z. Liu, R. Ge, F. Ye, M. Hossain, Y. Zhang and J. Wang, J. Am. Chem. Soc., 2014, 136, 3013 CrossRef CAS PubMed.
- For a single example of atom-economical ring expansion of a saturated cyclobutanone to a cyclopentanone via a β-oxygen-elimination pathway, see: (a) M. Murakami, T. Itahashi, H. Amii, K. Takahashi and Y. Ito, J. Am. Chem. Soc., 1998, 120, 9949 CrossRef CAS. For an example of atom-economical ring expansion of alkynylcyclopropanols to cyclopentenones, see: (b) B. M. Trost, J. Xia and N. Maulide, J. Am. Chem. Soc., 2008, 130, 17258 CrossRef CAS PubMed.
- For a seminal mechanistic study of Rh insertion into cyclobutenones, see: (a) M. A. Huffman, L. S. Liebeskind and W. T. Pennington, Organometallics, 1990, 9, 2194 CrossRef CAS; (b) M. A. Huffman, L. S. Liebeskind and W. T. Pennington, Organometallics, 1992, 11, 255 CrossRef CAS.
- For a recent review on synthesis of 2-cyclopentenones, see: (a) D. J. Aitken, H. Eijsberg, A. Frongia, J. Ollivier and P. P. Piras, Synthesis, 2014, 46, 1 CrossRef. For selected examples of synthesis of 1-indanone, see: (b) K. Kundu, J. V. McCullagh and A. T. Morehead, J. Am. Chem. Soc., 2005, 127, 16042 CrossRef CAS PubMed; (c) R. Shintani, K. Takatsu, T. Katoh, T. Nishimura and T. Hayashi, Angew. Chem., Int. Ed., 2008, 47, 1447 CrossRef CAS PubMed and references therein. (d) W. Zi and F. D. Toste, J. Am. Chem. Soc., 2013, 135, 12600 CrossRef CAS PubMed; (e) Y.-N. Yu and M.-H. Xu, J. Org. Chem., 2013, 78, 2736 CrossRef CAS PubMed; (f) J. Yang and N. Yoshikai, J. Am. Chem. Soc., 2014, 136, 16748 CrossRef CAS PubMed; (g) G. Yue, K. Lei, H. Hirao and J. Zhou, Angew. Chem., Int. Ed., 2015, 54, 6531 CrossRef CAS PubMed.
- For a recent review on Pauson–Khand reaction, see: T. Shibata, Adv. Synth. Catal., 2006, 348, 2328 CrossRef CAS PubMed.
- For a recent review on Nazarov cyclization, see: D. R. Wenz and J. R. de Alaniz, Eur. J. Org. Chem., 2014, 23 Search PubMed.
- For reviews on synthesis of cyclobutenones and benzocyclobutenones, see: ref. 3a and 6a. For a recent example of synthesis of benzocyclobutenones via [2 + 2] with ketene silyl acetals and benzyne, see: P.-H. Chen, N. A. Savage and G. Dong, Tetrahedron, 2014, 70, 4135 CrossRef CAS PubMed.
- For a kinetic study on the effect of bite angle on reductive elimination, see: J. E. Marcone and K. G. Moloy, J. Am. Chem. Soc., 1998, 120, 8527 CrossRef CAS.
- For substrate 1m, the ring-opening byproduct 2m′((E)-2-styrylbenzaldehyde) was isolated in 48% yield. For a discussion of the side reactions, see Scheme 2.
- The exact reason why [(COD)Rh(OH)]2 and [(COD)Rh(OMe)]2 are superior for ring expansion of simple cyclobutenones is unclear. These two catalysts were also found to be effective as the [Rh(COD)Cl]2 for the ring expansion of benzocyclobutenones. Cyclobutanone derivatives failed to give the ring-expansion product under the current conditions; work on this topic is still ongoing.
- For an example of olefin migration of 3,4-disubstituted cyclobutenones to give the more substituted isomers, see: R. L. Danheiser and S. Savariar, Tetrahedron Lett., 1987, 28, 3299 CrossRef CAS.
- Attempts to increase the enantioselectivity using (R)-MeO-biphep ligand resulted in a similar yield but 41% ee. Use of chiral phosphoramidite ligands did not afford any desired product.
- For a related procedure, see: Y. Ji, X. Verdaguer and A. Riera, Chem.–Eur. J., 2011, 17, 3942 CrossRef CAS PubMed.
- For a recent review on picenes as organic transistor material, see: Y. Kubozono, X. He, S. Hamao, K. Teranishi, H. Goto, R. Eguchi, T. Kambe, S. Gohda and Y. Nishihara, Eur. J. Inorg. Chem., 2014, 3806 CrossRef CAS PubMed.
- For an essay on the deuterium kinetic isotope effects, see: E. M. Simmons and J. F. Hartwig, Angew. Chem., Int. Ed., 2012, 51, 3066 CrossRef CAS PubMed.
- For recent examples of synthetic methods involving a decarbonylation/β-hydride elimination pathway, see: (a) X. Tao, N. A. Savage and G. Dong, Angew. Chem., Int. Ed., 2014, 53, 1891 CrossRef PubMed; (b) S. K. Murphy, J.-W. Park, F. A. Cruz and V. Dong, Science, 2015, 2, 56 CrossRef PubMed.
- The direct intramolecular hydroacylation with aldehyde substrates containing a trans-stilbene-like olefin, e.g.6 or 2m′, remains unreported.
- For selected examples, see: (a) M. P. Cava and R. J. Spangler, J. Am. Chem. Soc., 1967, 89, 4550 CrossRef CAS; (b) P. Schiess, M. Eberle, M. Huys-Francotte and J. Wirz, Tetrahedron Lett., 1984, 25, 2201 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1059514 and 1059515. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc01875g |
| This journal is © The Royal Society of Chemistry 2015 |