Open Access Article
Open Access ArticleExceptional time response, stability and selectivity in doubly-activated phenyl selenium-based glutathione-selective platform†
Youngsam
Kim‡
 ab,
Sandip V.
Mulay‡
ab,
Minsuk
Choi
c,
Seungyoon B.
Yu
c,
Sangyong
Jon
c and
David G.
Churchill
*ab
ab,
Sandip V.
Mulay‡
ab,
Minsuk
Choi
c,
Seungyoon B.
Yu
c,
Sangyong
Jon
c and
David G.
Churchill
*ab
aMolecular Logic Gate Laboratory, Department of Chemistry, Korea Advanced Institute of Science and Technology (KAIST), 373-1 Guseong-dong, Yuseong-gu, Daejeon, 305-701, Republic of Korea. E-mail: dchurchill@kaist.ac.kr
bCenter for Catalytic Hydrocarbon Functionalizations, Institute for Basic Science (IBS), 373-1 Guseong-dong, Yuseong-gu, Daejeon, 305-701, Republic of Korea
cDepartment of Biological Sciences, Korea Advanced Institute of Science and Technology (KAIST), 373-1 Guseong-dong, Yuseong-gu, Daejeon, 305-701, Republic of Korea
First published on 9th July 2015
Abstract
A phenyl-selenium-substituted coumarin probe was synthesized for the purpose of achieving highly selective and extremely rapid detection of glutathione (GSH) over cysteine (Cys)/homocysteine (Hcy) without background fluorescence. The fluorescence intensity of the probe with GSH shows a ∼100-fold fluorescent enhancement compared with the signal generated for other closely related amino acids, including Cys and Hcy. Importantly, the substitution reaction with the sulfhydryl group of GSH at the 4-position of the probe, which is doubly-activated by two carbonyl groups, occurs extremely fast, showing subsecond maximum fluorescence intensity attainment; equilibrium was reached within 100 ms (UV-vis). The probe selectivity for GSH was confirmed in Hep3B cells by confocal microscopy imaging.
Introduction
Biothiols, such as cysteine (Cys), homocysteine (Hcy) and glutathione (GSH), play crucial roles in biological systems as endogenous thiols. These biothiols exist in equilibrium between their oxidized disulfide and reduced free-thiol forms in biological systems.1 Each biothiol has its own role(s). GSH, an antioxidant enzyme, is the most abundant intracellular biothiol, composed from Cys, glutamine and glycine. GSH plays a central role in protecting the cell from oxidative damage and in maintaining biological homeostasis, which plays a significant role for cell growth and function among other biothiols (e.g. Cys, and Hcy).2 Although GSH is concentrated in the liver, it protects the whole body from various toxins produced by the body itself as a result of normal metabolic processes, and exposure to external toxins, such as environmental, illicit drug use, etc. Abnormal levels of GSH lead to oxidative stress responsible for, or observed in, premature aging and other conditions such as Alzheimer's disease (AD), Parkinson disease (PD), cancer, cystic fibrosis, AIDS, osteoporosis, cardiovascular disease and sickle cell anemia.3 Hence, numbers of research groups are involved in seeking to achieve the selective determination of GSH in biological systems; this is required for better understanding of its role in biological systems and in early diagnosis of disease.Due to the significant role of biothiols (Cys, Hcy and GSH), a number of reports have accounted for the detection of these species at the molecular level through the use of small synthetic probes.1e,f,4 Based on the unique reactivity of biothiols, numerous recent fluorescent probes have been developed and reported that can distinguish between biothiols and amino acids. The aldehyde group has been used in the selective detection of Cys and Hcy over GSH through forming thiazolidines and thiazinane, respectively.5
Aldehyde groups have also been exploited for their use in the detection of amino acids generally, through forming an iminium group, which shows a red-shifted absorption wavelength, compared to the aldehyde moiety.6 Acrylate (a 1,4-Michael acceptor) and beta-halide propionate (nucleophilic substitution) groups were used to discriminate Cys over Hcy and GSH by taking advantage of kinetic differences in intramolecular cyclization and concomitant release from the fluorophore moiety.7 Also, 1,4-Michael additions have been used for the detection of biothiols.8 However, it is still challenging to design a highly selective fluorescent probe for a particular biothiol because of the similarity in structure and reactivity between the species.
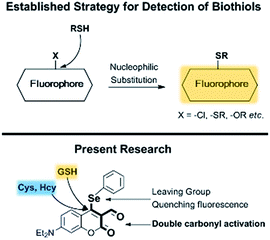
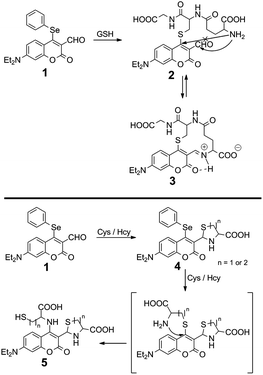
Recently, there have appeared reports for the selective sensing of GSH over Cys and Hcy.9–12 Nucleophilic substitution reactions by strong nucleophilic sulfhydryl groups, exploited for initial reactivity and for events, such as native chemical ligation,10 intramolecular displacement,11 and intramolecular cyclization,12 have allowed for the discrimination of GSH over Cys and Hcy. Although a few probes for the detection of thiols have been reported for real-time detection,13 to the best of our knowledge there is no probe for the selective detection of GSH demonstrating real-time detection as of yet. Considering the current state-of-the-art and these points of consideration, we have developed fluorescent probe 1, which has two independent potentially reactive sites, aldehyde and phenylselenide, for the selective detection of GSH (Fig. 1). Recently, our group has explored several fluorescent probes containing selenium as the reactive center to detect important biological analytes.14 Here, we chose to incorporate a phenylselenide group at the coumarin 4-position; this group is able to quench fluorescence via photo-induced electron transfer (PET) and is also able to behave as a leaving-group. The proximal aldehyde group plays a dual role: it enhances the electrophilicity of the 4-position as a Michael acceptor and enables a cyclization reaction with the sulfhydryl and primary amine groups of the biothiols (Scheme 1). Below, we discuss the preparation, screening, biological studies and general suitability of probe 1.
Results and discussion
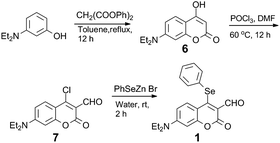
Probe 1 was synthesized from 4-chloro-7-(diethylamino)-2-oxo-2H-chromene-3-carbaldehyde (7)12b by treatment with PhSeZnBr15 (Scheme 2). The structure of 1 was characterized by spectroscopic techniques (multinuclear NMR spectral data, mass spectrometry; Fig. S3–S7†). Analyte screening was performed, which included various biothiols (Cys, Hcy, GSH) and amino acids (Pro, Thr, Val, Leu, Asn, Trp, Ile, Ala, His, Glu, Tyr, Lys, Met, Asp, Phe, Arg, Gly, Gln, Ser); the analytes were used to treat the solution (DMSO/10 mM PBS pH 7.4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
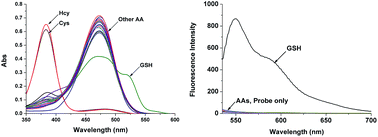
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) in the presence of 20 μM of 1. A new shoulder at ∼518 nm appeared, consistent with the addition of GSH to 1, with a simultaneous decrease of the absorption at ∼471 nm. On the other hand, upon treating Cys and Hcy, the changes in the absorption spectra were different. A new absorption peak at ∼380 nm was obtained, the original absorption peak for 1 simultaneously decreased; no change in the absorption at ∼518 nm was observed in the case of other amino acids (Fig. 2, left). A new absorption band (518 nm) and the absorption maxima for 1 (471 nm) were monitored. Only the solution of 1 with GSH led to enhanced fluorescence under λex = 471 nm (Fig. S14†) and 518 nm (Fig. 2, right) without background fluorescence. The solution of 1 under the same conditions with other amino acids, including Cys and Hcy, were non-emissive (λex = 471 and 518 nm).
3, v/v) in the presence of 20 μM of 1. A new shoulder at ∼518 nm appeared, consistent with the addition of GSH to 1, with a simultaneous decrease of the absorption at ∼471 nm. On the other hand, upon treating Cys and Hcy, the changes in the absorption spectra were different. A new absorption peak at ∼380 nm was obtained, the original absorption peak for 1 simultaneously decreased; no change in the absorption at ∼518 nm was observed in the case of other amino acids (Fig. 2, left). A new absorption band (518 nm) and the absorption maxima for 1 (471 nm) were monitored. Only the solution of 1 with GSH led to enhanced fluorescence under λex = 471 nm (Fig. S14†) and 518 nm (Fig. 2, right) without background fluorescence. The solution of 1 under the same conditions with other amino acids, including Cys and Hcy, were non-emissive (λex = 471 and 518 nm).
Titrations of 1 with GSH were then more carefully studied. Spectral determinations with compound 1 and various concentrations of GSH (0–40 μM) were performed (Fig. 3) in which both absorption and emission spectra were recorded (20 μM, DMSO/10 mM PBS pH 7.4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v). The absorption and fluorescence intensity, and concentration of GSH were linearly proportional and became saturated at 24 μM (1.2 equiv.). The detection limit was determined to be 2.7 × 10−7 M.
3, v/v). The absorption and fluorescence intensity, and concentration of GSH were linearly proportional and became saturated at 24 μM (1.2 equiv.). The detection limit was determined to be 2.7 × 10−7 M.
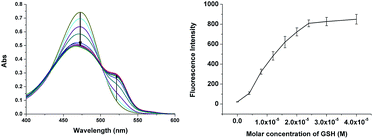 | ||
Fig. 3 Absorption and emission spectral changes of 1 (20 μM) with various concentrations of GSH (0–40 μM) in solution (DMSO/10 mM PBS pH 7.4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) incubated for 5 min at r.t. λex: 518 nm. 3, v/v) incubated for 5 min at r.t. λex: 518 nm. | ||
Our hypothesis, thus, is that the 4-position of 1, which is doubly-activated by two carbonyl groups, undergoes faster substitution with a strong nucleophilic sulfhydryl group such as that for GSH. Indeed, 1 shows very rapid analyte detection. The time-dependent fluorescent analysis was performed with 10 equiv. of GSH. The extremely fast emissive spectral change reached saturation involving a maximum intensity within 1 s (Fig. 4). Stopped-flow with UV-vis spectroscopy then allowed us to track two absorption wavelengths (471, 518 nm) at very short times, affected by the addition of GSH to 1. The equilibrium of absorption was reached within 100 ms (Fig. 5), suggesting that 1 undergoes an extremely fast reaction with GSH due to the doubly-activated α,β-unsaturated system.
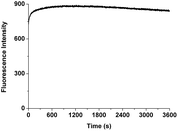 | ||
Fig. 4 Time-dependent emission spectral changes of 1 (20 μM) with 10 equiv. of GSH in solution (DMSO/10 mM PBS pH 7.4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v). λex: 518 nm. λem: 550 nm. 3, v/v). λex: 518 nm. λem: 550 nm. | ||
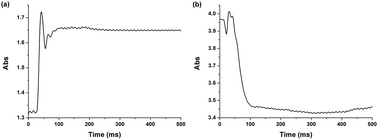 | ||
| Fig. 5 Absorption changes of 1 (200 μM) with 1.0 equiv. of GSH (200 μM) by stopped-flow spectroscopy (average of 4 experimental trials). (a) λ = 518 nm, (b) λ = 471 nm. | ||
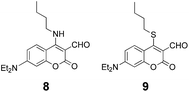
To help unravel the mechanism and structure of 1 with biothiols, model compounds 8 and 9 were synthesized (Fig. 6 and S8–S13†) and their reactivity studied. Compounds 8 and 9 have the same atoms at the 4-position of coumarin as would be expected through the reaction of Cys/Hcy and GSH with 1, respectively. The absorption and emission spectra of 8 were recorded under the same conditions as previously (DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 mM PBS, pH 7.4, v/v, 1
10 mM PBS, pH 7.4, v/v, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) (Fig. S15†). The spectra show very similar photophysical properties with the proposed structure 5, bearing a nitrogen atom at the 4-postion of the coumarin. Similar SAr substitution and replacement have also been reported.11 High resolution mass spectrometry (HRMS) data of 1 with Cys (1 and 10 equiv.) were recorded. Mass spectra of 1 with 1 equiv. of Cys showed a correct isotopic pattern for selenium and was in good agreement with the calculated mass value of 4 (Fig. S17†). The solution containing 10 equiv. of Cys indicated the formation of both 4 and 5, which does not have an isotopic pattern of selenium (Fig. S18†). Based on these results, the 4-position of coumarin is believed to undergo a substitution reaction and S,N-displacement. The aldehyde undergoes cyclization to form thiazolidine. Intermediates 4 and 5 would be the expected structures of 1 after reaction with Cys/Hcy.
3) (Fig. S15†). The spectra show very similar photophysical properties with the proposed structure 5, bearing a nitrogen atom at the 4-postion of the coumarin. Similar SAr substitution and replacement have also been reported.11 High resolution mass spectrometry (HRMS) data of 1 with Cys (1 and 10 equiv.) were recorded. Mass spectra of 1 with 1 equiv. of Cys showed a correct isotopic pattern for selenium and was in good agreement with the calculated mass value of 4 (Fig. S17†). The solution containing 10 equiv. of Cys indicated the formation of both 4 and 5, which does not have an isotopic pattern of selenium (Fig. S18†). Based on these results, the 4-position of coumarin is believed to undergo a substitution reaction and S,N-displacement. The aldehyde undergoes cyclization to form thiazolidine. Intermediates 4 and 5 would be the expected structures of 1 after reaction with Cys/Hcy.
In the case of the reaction of 1 with GSH, S,N-displacement would not be possible with GSH due to an unfavorable macrocyclic transition state.11b Structure 3 was proposed for the reaction of 1 with GSH; this motif is consistent with how coumarin-aldehydes react with amino acids to form iminium ions, previously reported by Glass et al.6 When coumarin-aldehyde forms an iminium group, it shows a red-shifted absorbance and emission (see pH-dependent studies below). This red-shifted wavelength was attributed to the hydrogen bond between the carbonyl and the iminium group. Based on these reports, compound 9 was synthesized and its photophysical properties examined because 9 was expected to show similar photophysical properties to 2. The absorption spectrum of 9 is very similar to that of 1. There was no absorption peak at ∼520 nm as shown in 1 with GSH. It is demonstrated that the absorption peak enhancement at ∼520 nm does not originate from the relevant features of structure 2, which undergoes a substitution reaction only. Compound 9 was treated with biothiols under the same conditions to help rationalize the absorption peak at ∼520 nm. When GSH is added to 9, the original maximum absorption peak (∼480 nm) decreased and simultaneous enhancement of ∼520 nm was observed, consistent with what is known for 1 and GSH (Fig. S16†). Furthermore, the observed mass spectra of the reaction mixture, 1 with GSH, supports that GSH underwent substitution and intramolecular cyclization (calc.: 533.1706, obtained: 533.1724 [3 + H]+); also, the selenium isotopic pattern has disappeared (Fig. S19†). These results strongly support that GSH undergoes nucleophilic substitution at the 4-position of the coumarin and intramolecular cyclization with aldehyde through forming an iminium-group.
Considering red-shifted wavelengths and hydrogen bonding between iminium and carbonyl oxygen, density functional theory (DFT) calculations (B3LYP/6-31g*,16 G09) were performed for 1, 2 and 3 to visualize the optimized structures and calculate molecular orbital energy levels. The results of the optimized structures show that 3 forms a stable hydrogen bond between the carbonyl group and iminium, which induces a red-shifted wavelength (Fig. S20†). The calculated frontier orbital energy difference for 3 is also smaller than that for 1 and 2 (Fig. S21†).
To demonstrate the effects of pH on 1, the fluorescence emission of 1 was checked with a wide range of pH under λex = 518 nm. It shows that pH does not affect the fluorescence of 1. Furthermore, GSH (10 equiv.) was added to solutions at various pH values in the presence of 20 μM of 1 (Fig. 7). In the pH range of 4–8, 1 showed a strong response for GSH. The results indicated that 1 is stable within the overall range of pHs tested and works properly under physiological conditions.
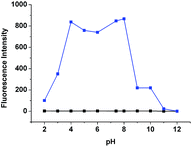 | ||
| Fig. 7 Fluorescence intensity changes by 1 (20 μM, black) and 1 (20 μM) with 10 equiv. of GSH (blue) under various different pH environments. λex: 518 nm. λem: 550 nm. | ||
To confirm that 1 has passed through the cellular membrane and is interacting with GSH in living cells, several biological studies were carried out. Human liver carcinoma Hep3B cells were treated with various concentrations of 1 (0 μM, 2 μM, 5 μM, and 10 μM) for 30 min to determine the optimum concentration for probe treatment (Fig. S22†). From the photophysical test results (Fig. 2 and S14†), a 488 nm argon laser was chosen for the excitation to demonstrate the reaction between 1 and intracellular GSH. Fluorescence emission was detected at a concentration of 2 μM, but 10 μM was considered optimal as the signal was valid within the major population of cells. Upon treatment of 1 (10 μM), a strong green fluorescence emission was observed, indicating that the reaction of 1 with intracellular GSH proceeds (Fig. S22,† 10 μM). Using live cell imaging techniques, it was shown that the fluorescence signal became noticeable within 5 min, confirming the rapid interaction between 1 and GSH (ESI, Movie file†). The results demonstrated that 1 permeates into living cells and reacts with intracellular GSH.
Additional experiments were performed to better support the conclusion that fluorescence signals were due to the interaction between 1 and GSH in the cells. A thiol-specific reagent N-ethylmaleimide (NEM) at a concentration of 1 mM was pre-treated in Hep3B cells for 30 min; the cells were further incubated with 1 for another 30 min. The confocal microscopy image of NEM-treated cells showed a significant decrease in the fluorescence signal, suggesting the specific reactivity of 1 with intracellular GSH (Fig. 8).
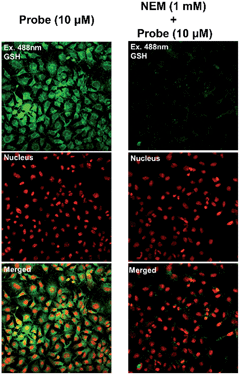 | ||
| Fig. 8 Confocal microscopy image of Hep3B cells pre-treated with 1 mM of NEM for 30 min and then treated with 1 for 30 min. | ||
In order to evaluate the biocompatibility of the probe, cell viability testing was carried out. Hep3B cells were pre-incubated with various concentrations of 1 for 1 h followed by WST-1 cell proliferation assays (Fig. 9). This assay resulted in no significant decrease in viability of the cells incubated with 50 μM of 1, compared with untreated cells; thus, the probe has low cytotoxicity and may be appropriate for real biological applications.
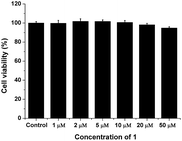 | ||
| Fig. 9 Concentration-dependent cell viability assays. Hep3B cells were pre-incubated with various concentrations of 1; their proliferation rates were measured with a WST-1 assay kit. | ||
Conclusions
In conclusion, a new probe has been developed for the highly selective and rapid detection of GSH in live cells; selectivity over Cys/Hcy without background fluorescence was demonstrated. The fluorescence intensity was ∼100-fold greater, compared with other amino acids, including Cys and Hcy. Importantly, the probe reached maximum fluorescence intensity within ∼1 s and reached equilibrium of absorption within ∼100 ms. Confocal microscopy imaging of living cell systems indicate the probe detects GSH in Hep3B cells rapidly and specifically. Furthermore, cell viability testing showed low cytotoxicity of the probe and its potential for biological uses.Acknowledgements
The Molecular Logic Gate Laboratory operated by Prof. D. G. Churchill acknowledges support from (i) Mid-Career Researcher Program through the NRF (National Research Foundation) of Korea (NRF-2014R1A2A1A11052980) funded by MEST and (ii) Institute for Basic Science (IBS) for financial support. Mr Youngsam Kim and Dr Sandip V. Mulay acknowledge IBS research Fellowships and KAIST for providing research facilities. Also the research support staff at KAIST facilitating the acquisition of MS data.Notes and references
- (a) C. Yin, F. Huo, J. Zhang, R. Martinez-Manez, Y. Yang, H. Lv and S. Li, Chem. Soc. Rev., 2013, 42, 6032 RSC; (b) Z. A. Wood, E. S. Der, J. R. Harris and L. B. Poole, Trends Biochem. Sci., 2003, 28, 32 CrossRef CAS; (c) X. Li, X. Gao, W. Shi and H. Ma, Chem. Rev., 2014, 114, 590 CrossRef CAS PubMed; (d) M. H. Lee, Z. Yang, C. W. Lim, Y. H. Lee, S. Dongbang, C. Kang and J. S. Kim, Chem. Rev., 2013, 113, 5071 CrossRef CAS PubMed; (e) H. S. Jung, X. Chen, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2013, 42, 6019 RSC; (f) X. Chen, Y. Zhou, X. Peng and J. Yoon, Chem. Soc. Rev., 2010, 39, 2120 RSC.
- (a) L. M. Hyman and K. J. Franz, Coord. Chem. Rev., 2012, 256, 2333 CrossRef CAS PubMed; (b) C. Hwang, A. J. Sinskey and H. F. Lodish, Science, 1992, 257, 1496 CAS; (c) Y. Feng, J. Cheng, L. Zhou, X. Zhou and H. Xiang, Analyst, 2012, 137, 4885 RSC; (d) T. P. Dalton, H. G. Shertzer and A. Puga, Annu. Rev. Pharmacol. Toxicol., 1999, 39, 67 CrossRef CAS PubMed.
- (a) D. M. Townsend, K. D. Tew and H. Tapiero, Biomed. Pharmacother., 2003, 57, 145 CrossRef CAS; (b) B. Helbling, J. V. Overbeck and B. H. Lauterburg, Eur. J. Clin. Invest., 1996, 26, 38 CrossRef CAS.
- (a) H. Peng, W. Chen, Y. Cheng, L. Hakuna, R. Strongin and B. Wang, Sensors, 2012, 12, 15907 CrossRef CAS PubMed; (b) L. Y. Niu, Y. Z. Chen, H. R. Zheng, L. Z. Wu, C. H. Tung and Q. Z. Yang, Chem. Soc. Rev., 2015 10.1039/C5CS00152H.
- (a) Z. Yang, N. Zhao, Y. Sun, F. Miao, Y. Liu, X. Liu, Y. Zhang, W. Ai, G. Song, X. Shen, X. Yu, J. Sun and W. Y. Wong, Chem. Commun., 2012, 48, 3442 RSC; (b) W. Wang, O. Rusin, X. Xu, K. K. Kim, J. O. Escobedo, S. O. Fakayode, K. A. Fletcher, M. Lowry, C. M. Schowalter, C. M. Lawrence, F. R. Fronczek, I. M. Warner and R. M. Strongin, J. Am. Chem. Soc., 2005, 127, 15949 CrossRef CAS PubMed; (c) K. S. Lee, T. K. Kim, J. H. Lee, H. J. Kim and J. I. Hong, Chem. Commun., 2008, 6173 RSC; (d) H. Chen, Q. Zhao, Y. Wu, F. Li, H. Yang, T. Yi and C. Huang, Inorg. Chem., 2007, 46, 11075 CrossRef CAS PubMed; (e) O. Rusin, N. N. S. Luce, R. A. Agbaria, J. O. Escobedo, S. Jiang, I. M. Warner, F. B. Dawan, K. Lian and R. M. Strongin, J. Am. Chem. Soc., 2004, 126, 438 CrossRef CAS PubMed; (f) W. Wang, O. Rusin, X. Xu, K. K. Kim, J. O. Escobedo, S. O. Fakayode, K. A. Fletcher, M. Lowry, C. M. Schowalter, C. M. Lawrence, F. R. Fronczek, I. M. Warner and R. M. Strongin, J. Am. Chem. Soc., 2005, 127, 15950 Search PubMed.
- (a) K. E. Secor and T. E. Glass, Org. Lett., 2004, 6, 3727 CrossRef CAS PubMed; (b) J. L. Klockow and T. E. Glass, Org. Lett., 2013, 15, 235 CrossRef CAS PubMed; (c) E. K. Feuster and T. E. Glass, J. Am. Chem. Soc., 2003, 125, 16174 CrossRef CAS PubMed; (d) T. M. Tran, Y. Alan and T. E. Glass, Chem. Commun., 2015, 51, 7915 RSC; (e) K. Secor, J. Plante, C. Avetta and T. E. Glass, J. Mater. Chem., 2005, 15, 4073 RSC.
- (a) X. Yang, Y. Guo and R. M. Strongin, Angew. Chem., Int. Ed., 2011, 50, 10690 CrossRef CAS PubMed; (b) H. Wang, G. Zhou, H. Gai and X. Chen, Chem. Commun., 2012, 48, 8341 RSC; (c) D. P. Murale, H. Kim, W. S. Choi, Y. Kim and D. G. Churchill, RSC Adv., 2014, 4, 46513 RSC; (d) Y. Kim, M. Choi, S. Seo, S. T. Manjare, S. Jon and D. G. Churchill, RSC Adv., 2014, 4, 64183 RSC; (e) Z. Guo, S. Nam, S. Park and J. Yoon, Chem. Sci., 2012, 3, 2760 RSC.
- (a) T. Matsumoto, Y. Urano, T. Shoda, H. Kojima and T. Nagano, Org. Lett., 2007, 9, 3375 CrossRef CAS PubMed; (b) H. S. Jung, K. C. Ko, G.-H. Kim, A.-R. Lee, Y.-C. Na, C. Kang, J. Y. Lee and J. S. Kim, Org. Lett., 2011, 13, 1498 CrossRef CAS PubMed; (c) H. S. Jung, T. Pradhan, J. H. Han, K. J. Heo, J. H. Lee, C. Kang and J. S. Kim, Biomaterials, 2012, 33, 8495 CrossRef CAS PubMed.
- J. Yin, Y. Kwon, D. Kim, D. Lee, G. Kim, Y. Hu, J. H. Ryu and J. Yoon, J. Am. Chem. Soc., 2014, 136, 5351 CrossRef CAS PubMed.
- X. F. Yang, Q. Huang, Y. Zhong, Z. Li, H. Li, M. Lowry, J. O. Escobedo and R. M. Strongin, Chem. Sci., 2014, 5, 2177 RSC.
- (a) Y. Zhang, X. Shao, Y. Wang, F. Pan, R. Kang, F. Peng, Z. Huang, W. Zhang and W. Zhao, Chem. Commun., 2015, 51, 4245 RSC; (b) L. Y. Niu, Y. S. Guan, Y. Z. Chen, L. Z. Wu, C. H. Tung and Q. Z. Yang, J. Am. Chem. Soc., 2012, 134, 18928 CrossRef CAS PubMed; (c) Y. Liu, X. Lv, J. Liu, Y. Q. Sun and W. Guo, Chem.–Eur. J., 2015, 21, 4747 CrossRef CAS PubMed; (d) J. Liu, Y.-Q. Sun, H. Zhang, Y. Huo, Y. Shi and W. Guo, Chem. Sci., 2014, 5, 3183 RSC; (e) S. Y. Lim, K. H. Hong, D. I. Kim, H. Kwon and H. J. Kim, J. Am. Chem. Soc., 2014, 136, 7018 CrossRef CAS PubMed; (f) D. Lee, G. Kim, J. Yin and J. Yoon, Chem. Commun., 2015, 51, 6518 RSC.
- (a) F. Wang, L. Zhou, C. Zhao, R. Wang, Q. Fei, S. Luo, Z. Guo, H. Tian and W.-H. Zhu, Chem. Sci., 2015, 6, 2584 RSC; (b) J. Liu, Y. Q. Sun, Y. Huo, H. Zhang, L. Wang, P. Zhang, D. Song, Y. Shi and W. Guo, J. Am. Chem. Soc., 2014, 136, 574 CrossRef CAS PubMed.
- (a) Z. Lou, P. Li, X. Sun, S. Yang, B. Wang and K. Han, Chem. Commun., 2013, 49, 391 RSC; (b) L. Yi, H. Li, L. Sun, L. Liu, C. Zhang and Z. Xi, Angew. Chem., Int. Ed., 2009, 48, 4034 CrossRef CAS PubMed.
- (a) D. P. Murale, S. T. Manjare, Y. S. Lee and D. G. Churchill, Chem. Commun., 2014, 50, 359 RSC; (b) S. T. Manjare, S. Kim, W. D. Heo and D. G. Churchill, Org. Lett., 2014, 16, 410 CrossRef CAS PubMed.
- (a) S. Santoro, B. Battistelli, L. Testaferri, M. Tiecco and C. Santi, Eur. J. Org. Chem., 2009, 4921 CrossRef CAS PubMed; (b) C. Santi, S. Santoro, B. Battistelli, L. Testaferri and M. Tiecco, Eur. J. Org. Chem., 2008, 5387 CrossRef CAS PubMed.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: 1H, 13C, 77Se and 2D NMR, HRMS spectra of compounds, details of the synthetic procedure, additional fluorescent spectra, confocal microscopy image, DFT calculation data, live imaging movie. See DOI: 10.1039/c5sc02090e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |