Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Practical and economic lithiations of functionalized arenes and heteroarenes using Cy2NLi in the presence of Mg, Zn or La halides in a continuous flow†‡
Matthias R.
Becker
,
Maximilian A.
Ganiek
and
Paul
Knochel
*
Ludwig-Maximilians-Universität München, Department Chemie, Butenandtstrasse 5-13 (Haus F), 81377 München, Germany. E-mail: paul.knochel@cup.uni-muenchen.de
First published on 10th August 2015
Abstract
The economic amide base lithium dicyclohexylamide (Cy2NLi) allows fast and convenient (40 s, 0 °C) in situ trapping flow metalations of a broad range of functionalized arenes, heteroarenes and acrylate derivatives in the presence of various metal salts (ZnCl2·2LiCl, MgCl2, LaCl3·2LiCl). The resulting Zn-, Mg- or La-organometallic intermediates are trapped with various electrophiles in high yields. These flow metalations are easily scaled-up without further optimization.
Introduction
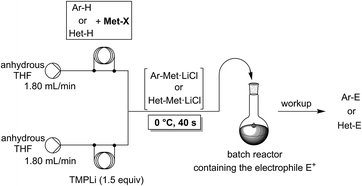
The lithiation of arenes and heteroarenes is a common method for the functionalization of unsaturated molecules.1 Pioneering work of Snieckus2 and others3 have demonstrated the utility of aromatic lithiations for the preparation of pharmaceutical and agrochemical targets. Nevertheless, the use of powerful lithium bases has some drawbacks such as low metalation temperatures and a moderate functional group tolerance. Also, it requires a careful choice of the lithium base used for the metalation step.Recently, we have shown that an in situ trapping metalation sequence using TMPLi (TMP = 2,2,6,6-tetramethylpiperidyl) allows the performance of selective lithiations of various arenes and heteroarenes at 0 °C within 40 s if conducted in a continuous flow system (Scheme 1).4,5 Under conventional batch conditions, these in situ trapping metalations require cryogenic temperatures (−78 °C) in order to avoid unwanted side reactions or decomposition of the organometallic intermediate. Furthermore, the scale-up of these batch metalations proved to be difficult, requiring much optimization. Despite the convenient reaction conditions in flow mode, the use of stoichiometric amounts of TMPLi makes this lithiation still expensive (TMPH = ca. 630 $ mol−1).6 The steric hindrance of the TMP-moiety was required in order to avoid side-reactions. Due to the fast mixing of the reaction components and the prevention of hot spot formation,7 such highly sterically hindered bases may no longer be mandatory when using the flow methodology.8 Preliminary experiments attempting to perform metalations of various aromatics using cheaper readily available lithium or other metallic amides R2NMet (R = iPr (isopropyl), Cy (cyclohexyl), TMS (trimethylsilyl); Met = Li, MgHal, ZnHal) were disappointing either due to insufficient reactivity or unwanted side-reactions. The in situ trapping methodology developed in our laboratory, in which we mix the aromatic substrate with a metallic salt and add TMPLi proves to be compatible with the replacement of TMPLi with much cheaper bases, since this Barbier-type lithiation minimizes the contact time of the lithium base with the aromatic substrate. The replacement of TMPLi by Cy2NLi is of special importance since the price of the corresponding amine Cy2NH (ca. 6.40 $ mol−1) is only ca. 1% of TMPH.6,9
Herein we wish to report the use of the economic amide base lithium dicyclohexylamide (Cy2NLi) instead of TMPLi for in situ trapping metalations under continuous flow conditions. Cy2NLi has – to the best of our knowledge – not yet been used for extensive lithiations of functionalized arenes and heteroarenes.10
Results and discussion
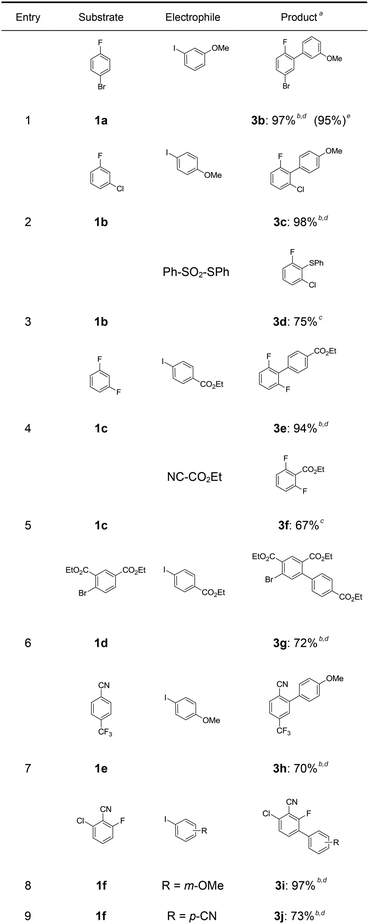
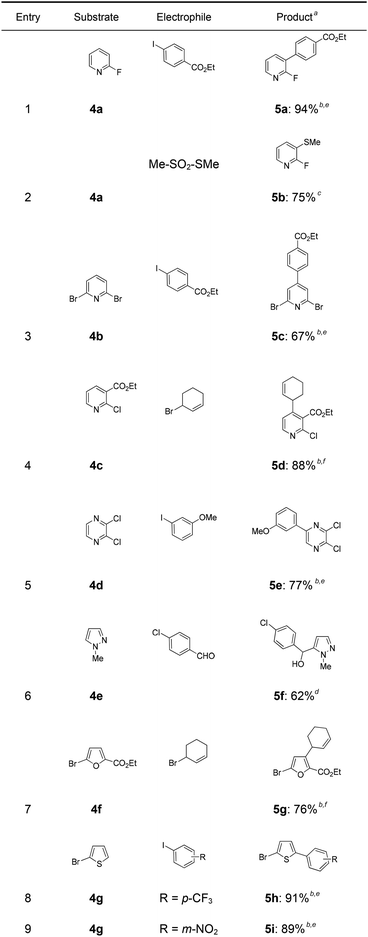
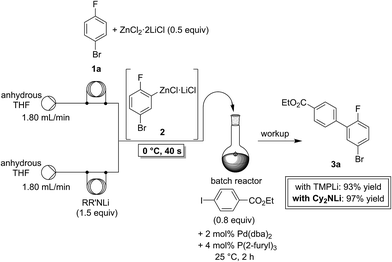
In a first experiment, we have metalated 1-bromo-4-fluorobenzene (1a) under flow conditions (Scheme 2). Thus, 1a (1.0 equiv.) was mixed with ZnCl2·2LiCl (0.5 equiv.) and submitted to flow metalation11 (0 °C, 40 s) using respectively TMPLi and Cy2NLi. The corresponding arylzinc intermediate (2) was quenched via a Pd-catalyzed Negishi cross-coupling12 in a batch reactor containing ethyl 4-iodobenzoate (0.8 equiv.) and a standard Pd-catalytic system (2 mol% Pd(dba)2; dba = dibenzylideneacetone and 4 mol% P(2-furyl)3)13 providing the expected biphenyl (3a) in 93% (using TMPLi) and 97% (using Cy2NLi) yield. Like for reactions with TMPLi, in situ trapping metalations with Cy2NLi can be simply scaled up without further optimization just by running the reaction for a longer time. Therefore, the reaction of 2 with 3-iodoanisole (0.8 equiv.) affords after a Negishi cross-coupling the expected product 3b in 97% yield on a 1.7 mmol scale and in 95% yield on a 11 mmol scale (Table 1, entry 1). Using Cy2NLi for the ortho-lithiation of 1,3-dihaloarenes (1b, c) abstracts under our standard reaction conditions (0 °C, 40 s) the most acidic hydrogen at position 2. In situ transmetalations with ZnCl2·2LiCl or MgCl2 (0.5 equiv., respectively) generate the corresponding aryl-zinc and -magnesium species, which are quenched in subsequent batch reactions with aryl iodides (0.8 equiv.), S-phenyl benzenethiosulfonate (0.8 equiv.) and ethyl cyanoformate (0.8 equiv.) leading to the trisubstituted arenes (3c–f) in 67–98% yield (entries 2–5). The in situ metalations with Cy2NLi are not limited to haloarenes, and sensitive functionalities such as esters and nitriles are tolerated as well. Thus, diethyl 4-bromoisophthalate (1d) is smoothly flow-zincated in position 6 and a Negishi cross-coupling with ethyl 4-iodobenzoate (0.8 equiv.) produces the expected triester (3g) in 72% yield (entry 6). Similarly, substituted nitriles such as 1e and 1f are in situ metalated in the presence of ZnCl2·2LiCl (0.5 equiv.) within 40 s at 0 °C, and subsequent quenching reactions with aryl iodides (0.8 equiv.) having either electron-donating or electron-withdrawing substituents lead to the cyano-substituted biphenyls (3h–j) in 70–97% yield (entries 7–9).This in situ trapping methodology with Cy2NLi in a flow reactor is not limited to functionalized arenes. In fact, it can be readily extended to a broad range of sensitive, electron-deficient heteroarenes (Table 2). Thus, 2-fluoropyridine (4a), which is notoriously difficult to metalate,14 undergoes a smooth zincation or magnesiation in position 3 in the presence of ZnCl2·2LiCl or MgCl2 and quenching with ethyl 4-iodobenzoate (0.8 equiv.) or S-methyl methanethiosulfonate (0.8 equiv.) produces the disubstituted pyridines (5a, b) in 75–94% yield (entries 1 and 2). However, using our standard conditions, 2,6-dibromopyridine (4b) is in situ metalated in position 4 and a subsequent Negishi cross-coupling with ethyl 4-iodobenzoate (0.8 equiv.) affords the desired pyridine (5c) in 67% yield (entry 3). Similarly, ethyl 2-chloronicotinate (4c) is flow-zincated within 40 s at 0 °C in position 4 affording the trisubstituted pyridine (5d) in 88% yield after a Cu-mediated allylation with 3-bromocyclohexene (0.8 equiv.; entry 4). The sensitive 2,3-dichloropyrazine (4d) is smoothly flow-metalated (0 °C, 40 s) in the presence of ZnCl2·2LiCl (0.5 equiv.) and quenching with 3-iodoanisole (0.8 equiv.) leads to the pyrazine (5e) in 77% yield (entry 5). The in situ trapping metalations with Cy2NLi can also be used for the functionalization of a broad range of substituted 5-membered ring heterocycles. Thus, the lanthanation of 1-methylpyrazole (4e) in the presence of LaCl3·2LiCl (0.5 equiv.) under standard conditions (0 °C, 40 s) produces the desired alcohol (5f) in 62% yield after addition to p-chlorobenzaldehyde (0.8 equiv.; entry 6). Ethyl 5-bromo-2-furoate (4f) is regioselectively flow metalated in position 3 and a subsequent Cu-catalyzed reaction with 3-bromocyclohexene (0.8 equiv.) leads to the trisubstituted furan (5g) in 76% yield (entry 7). The in situ trapping zincation of 2-bromothiophene (4g) within 40 s at 0 °C abstracts the most acidic hydrogen at position 5 affording the 2,5-disubstituted thiophenes (5h, i) in 89–91% yield after Negishi cross-couplings with 4-iodobenzotrifluoride (0.8 equiv.) and 1-iodo-3-nitrobenzene (0.8 equiv.; entries 8 and 9).
To demonstrate the broad practicability of the in situ trapping metalations with Cy2NLi, we investigated the functionalization of acyclic acrylate derivatives, which are prone to polymerize. However, submitting a mixture of (E)-methyl 3-methoxyacrylate (6) with MgCl2 (0.5 equiv.) to the flow metalation with Cy2NLi (1.5 equiv.) for 40 s at 0 °C leads to the formation of the magnesium intermediate 7 in high conversion (Scheme 3). Subsequent reaction of 7 with 2,6-dichlorobenzaldehyde (0.8 equiv.) produces the lactone 8 in 65% yield. Similarly, (E)-ethyl 3-(dimethylamino)acrylate (9) is in situ metalated (0 °C, 40 s) in the presence of MgCl2 or ZnCl2·2LiCl (Scheme 4). The corresponding magnesium (10) and zinc (12) organometallic intermediates undergo various quenching reactions, such as an addition to 4-(trifluoromethyl)benzaldehyde (0.8 equiv.) and a Negishi cross-coupling with 4-iodobenzotrifluoride (0.8 equiv.), providing the corresponding lactone (11) and the ester 13 in 62–70% yield.
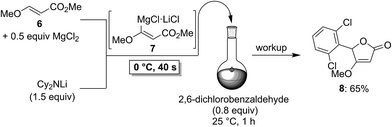 | ||
| Scheme 3 In situ trapping magnesiation of (E)-methyl 3-methoxyacrylate (6) using Cy2NLi in a flow reactor. | ||
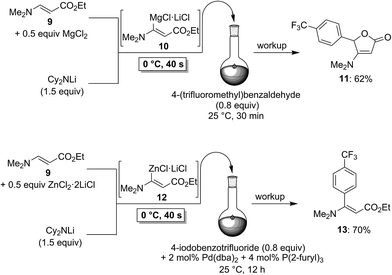 | ||
| Scheme 4 In situ trapping magnesiation and zincation of (E)-ethyl 3-(dimethylamino)acrylate (9) using Cy2NLi in a flow reactor. | ||
Conclusions
In summary, the economic amide base lithium dicyclohexylamide (Cy2NLi) undergoes fast and convenient (40 s, 0 °C) in situ trapping flow metalations of a broad range of functionalized arenes, heteroarenes and acrylate derivatives in the presence of various metal salts (ZnCl2·2LiCl, MgCl2, LaCl3·2LiCl). The resulting Zn-, Mg- or La-organometallic intermediates are trapped with numerous electrophiles in high yields. These flow-metalations are easily scaled-up without further optimization simply by running the reaction for a longer time. Further applications and extensions of this method are currently underway.Notes and references
- (a) J. Clayden, Organolithiums: Selectivity for Synthesis, Elsevier, Oxford, 2002 Search PubMed; (b) J. Clayden, F. E. Knowles and I. R. Baldwin, J. Am. Chem. Soc., 2005, 127, 2412 CrossRef CAS; (c) J. Clayden, J. Dufour, D. M. Grainger and M. Helliwell, J. Am. Chem. Soc., 2007, 129, 7488 CrossRef CAS PubMed; (d) Z. Rappoport, I. Marek, The Chemistry of Organolithium Compounds, Wiley-VCH, Chichester, 2004 Search PubMed; (e) P. García-Álvarez, R. E. Mulvey and J. A. Parkinson, Angew. Chem., Int. Ed., 2011, 50, 9668 CrossRef PubMed; (f) R. E. Mulvey and S. D. Robertson, Angew. Chem., Int. Ed., 2013, 52, 11470 CrossRef CAS PubMed; (g) D. R. Armstrong, E. Crosbie, E. Hevia, R. E. Mulvey, D. L. Ramsay and S. D. Robertson, Chem. Sci., 2014, 5, 3031 RSC; (h) C. Unkelbach, D. F. O'Shea and C. Strohmann, Angew. Chem., Int. Ed., 2014, 53, 553 CrossRef CAS PubMed; (i) A. Salomone, F. M. Perna, A. Falcicchio, S. O. N. Lill, A. Moliterni, R. Michel, S. Florio, D. Stalke and V. Capriati, Chem. Sci., 2014, 5, 528 RSC; (j) V. Capriati, F. M. Perna and A. Salomone, Dalton Trans., 2014, 43, 14204 RSC; (k) D. R. Armstrong, J. A. Garden, A. R. Kennedy, S. M. Leenhouts, R. E. Mulvey, P. O'Keefe, C. T. O'Hara and A. Steven, Chem.–Eur. J., 2013, 19, 13492 CrossRef CAS PubMed.
- (a) P. Beak and V. Snieckus, Acc. Chem. Res., 1982, 15, 306 CrossRef CAS; (b) V. Snieckus, Chem. Rev., 1990, 90, 879 CrossRef CAS; (c) M. C. Whisler, S. MacNeil, V. Snieckus and P. Beak, Angew. Chem., Int. Ed., 2004, 43, 2206 CrossRef CAS PubMed.
- (a) M. Schlosser, Angew. Chem., Int. Ed., 2005, 44, 376 CrossRef CAS PubMed; (b) R. Chinchilla, C. Nájera and M. Yus, Chem. Rev., 2004, 104, 2667 CrossRef CAS; (c) F. Foubelo and M. Yus, Chem. Soc. Rev., 2008, 37, 2620 RSC; (d) R. E. Mulvey, F. Mongin, M. Uchiyama and Y. Kondo, Angew. Chem., Int. Ed., 2007, 46, 3802 CrossRef CAS PubMed.
- In situ trapping metalations in batch: A. Frischmuth, M. Fernández, N. M. Barl, F. Achrainer, H. Zipse, G. Berionni, H. Mayr, K. Karaghiosoff and P. Knochel, Angew. Chem., Int. Ed., 2014, 53, 7928 CrossRef CAS PubMed ; In situ trapping metalations in a continuous flow system: M. R. Becker and P. Knochel, Angew. Chem., Int. Ed., 2015 DOI:10.1002/anie.201502393.
- For lithiations in flow mode, see: (a) A. Nagaki, Y. Takahashi and J.-i. Yoshida, Chem.–Eur. J., 2014, 20, 7931 CrossRef CAS PubMed; (b) A. Nagaki, D. Ichinari and J.-i. Yoshida, J. Am. Chem. Soc., 2014, 136, 12245 CrossRef CAS PubMed; (c) A. Nagaki, K. Imai, S. Ishiuchi and J.-i. Yoshida, Angew. Chem., Int. Ed., 2015, 54, 1914 CrossRef CAS PubMed; (d) L. Kupracz and A. Kirschning, Adv. Synth. Catal., 2013, 355, 3375 CrossRef CAS PubMed; (e) J. Wu, X. Yang, Z. He, X. Mao, T. A. Hatton and T. F. Jamison, Angew. Chem., Int. Ed., 2014, 53, 8416 CrossRef CAS PubMed; (f) T. Fukuyama, T. Totoki and I. Ryu, Org. Lett., 2014, 16, 5632 CrossRef CAS PubMed; (g) H. Kim, H.-J. Lee and D.-P. Kim, Angew. Chem., Int. Ed., 2015, 54, 1877 CrossRef CAS.
- The price of the corresponding amine TMPH from Sigma-Aldrich is ca. 630 $ mol−1 for the largest package.
- J.-i. Yoshida, Flash Chemistry: Fast Organic Synthesis in Microsystems, John Wiley & Sons Ltd, West Sussex, 2008 Search PubMed.
- For recent advances in flow chemistry, see: (a) T. Noёl and S. L. Buchwald, Chem. Soc. Rev., 2011, 40, 5010 RSC; (b) M. Chen and S. L. Buchwald, Angew. Chem., Int. Ed., 2013, 52, 4247 CrossRef CAS PubMed; (c) M. Chen, S. Ichikawa and S. L. Buchwald, Angew. Chem., Int. Ed., 2015, 54, 263 CrossRef CAS PubMed; (d) Y. Zhang, S. C. Born and K. F. Jensen, Org. Process Res. Dev., 2014, 18, 1476 CrossRef CAS; (e) T. P. Petersen, M. R. Becker and P. Knochel, Angew. Chem., Int. Ed., 2014, 53, 7933 CrossRef CAS PubMed; (f) R. J. Ingham, C. Battilocchio, D. E. Fitzpatrick, E. Sliwinski, J. M. Hawkins and S. V. Ley, Angew. Chem., Int. Ed., 2015, 54, 144 CrossRef CAS PubMed; (g) D. N. Tran, C. Battilocchio, S.-B. Lou, J. M. Hawkins and S. V. Ley, Chem. Sci., 2015, 6, 1120 RSC; (h) S. V. Ley, D. E. Fitzpatrick, R. J. Ingham and R. M. Myers, Angew. Chem., Int. Ed., 2015, 54, 3449 CrossRef CAS PubMed; (i) J. M. Sauks, D. Mallik, Y. Lawryshyn, T. Bender and M. G. Organ, Org. Process Res. Dev., 2014, 18, 1310 CrossRef CAS; (j) K. S. Nalivela, M. Tilley, M. A. McGuire and M. G. Organ, Chem.–Eur. J., 2014, 20, 6603 CrossRef CAS PubMed; (k) K. Somerville, M. Tilley, G. Li, D. Mallik and M. G. Organ, Org. Process Res. Dev., 2014, 18, 1315 CrossRef CAS; (l) K. Gilmore, D. Kopetzki, J. W. Lee, Z. Horváth, D. T. McQuade, A. Seidel-Morgenstern and P. H. Seeberger, Chem. Commun., 2014, 50, 12652 RSC; (m) D. B. Ushakov, K. Gilmore, D. Kopetzki, D. T. McQuade and P. H. Seeberger, Angew. Chem., Int. Ed., 2014, 53, 557 CrossRef CAS PubMed; (n) D. Ghislieri, K. Gilmore and P. H. Seeberger, Angew. Chem., Int. Ed., 2015, 54, 678 CAS.
- The price of the corresponding amine Cy2NH from Sigma-Aldrich is ca. 6.40 $ mol−1 for the largest package.
- For the use of Cy2N-bases for metalations, see: (a) R. N. McDonald, H. E. Petty, N. L. Wolfe and J. V. Paukstelis, J. Org. Chem., 1974, 39, 1877 CrossRef CAS; (b) M. Jørgensen, S. Lee, X. Liu, J. P. Wolkowski and J. F. Hartwig, J. Am. Chem. Soc., 2002, 124, 12557 CrossRef PubMed; (c) A. Renaudat, L. Jean-Gérard, R. Jazzar, C. E. Kefalidis, E. Clot and O. Baudoin, Angew. Chem., Int. Ed., 2010, 49, 7261 CrossRef CAS PubMed; (d) T. Truong, J. Alvarado, L. D. Tran and O. Daugulis, Org. Lett., 2010, 12, 1200 CrossRef CAS PubMed.
- Flow reactions were performed with commercially available equipment from Uniqsis Ltd (FlowSyn; http://www.uniqsis.com).
- (a) E. Negishi, L. F. Valente and M. Kobayashi, J. Am. Chem. Soc., 1980, 102, 3298 CrossRef CAS; (b) E. Negishi, Acc. Chem. Res., 1982, 15, 340 CrossRef CAS.
- V. Farina and B. Krishnan, J. Am. Chem. Soc., 1991, 113, 9585 CrossRef CAS.
- The metalation of 2-fluoropyridine using LDA (−78 °C, 4 h) and iodolysis afforded 2-fluoro-3-iodopyridine in 85% yield: L. Estel, F. Marsais and G. Quéguiner, J. Org. Chem., 1988, 53, 2740 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5sc02558c |
| ‡ We thank the SFB 749 (DFG) for support and financial contributions to this project. We also thank Rockwood Lithium GmbH (Frankfurt) and BASF AG (Ludwigshafen) for the generous gift of chemicals. |
| This journal is © The Royal Society of Chemistry 2015 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yield of isolated product.
Yield of isolated product.