Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Efficient synthesis of oligofluoranthene nanorods with tunable functionalities†
Xin-Gui
Li‡
*ab,
Yaozu
Liao‡
abc,
Mei-Rong
Huang
*ab and
Richard B.
Kaner
*bd
aState Key Laboratory of Pollution Control and Resource Reuse, College of Environmental Science and Engineering, Tongji University, Shanghai 200092, China. E-mail: adamxgli@yahoo.com; huangmeirong@tongji.edu.cn; Fax: +86-21-65983869; Tel: +86-21-65983869
bDepartment of Chemistry & Biochemistry, California NanoSystems Institute, University of California, Los Angeles, California 90095, USA. E-mail: kaner@chem.ucla.edu; Fax: +1 310 206 4038; Tel: +1 310 825 5346
cState Key Laboratory for Modification of Chemical Fibers & Polymer Materials, College of Materials Science and Engineering, Donghua University, Shanghai 201620, China
dDepartment of Materials Science & Engineering, University of California, Los Angeles, California 90095, USA
First published on 17th September 2015
Abstract
Strongly fluorescence-emitting oligofluoranthene (OFA) nanorods are readily synthesized by a direct template-free chemical oxidative oligomerization of fluoranthene in nitromethane containing ferric chloride as an oxidant. The OFAs likely consist of five fluoranthene units containing cyclic pentamers with crystalline order and tunable electrical conductivity across 12 orders of magnitude. The OFA nanorods are heat-resistant materials and efficient precursors for macroporous carbon materials with high carbon yield in argon at 1100 °C. In particular, the optimal ring-like pentamer shows 12.2 times stronger cyan fluorescence-emission than recognized highly fluorescent fluoranthene under the same conditions, which makes the OFAs into ideal strong fluorescent emitters, tunable conductors, and high carbon-yield precursors for the preparation of sensors and carbon materials. These findings demonstrate an advance in the direct synthesis of oligomers from fused-ring aromatic hydrocarbons and provide a potential direction to optimize the synthesis and functionalities of wholly aromatic nanomaterials.
Introduction
Since conjugated polymers demonstrate versatile functionalities including unique energy migration across metal ions as a result of variations in absorption, emission, and redox properties, they have great potential for applications in highly sensitive sensors1,2 based on extraordinarily efficient energy transfer quenching and in polymer light emitting diodes. In comparison to conventional small molecule-based sensors, conjugated polymer chemosensors exhibit some important advantages due to signal amplification when subjected to external stimuli.3,4 As one of the important polycyclic aromatic hydrocarbons, fluoranthene (FA) has been used as a dopant for molecular crystals used in laser spectroscopy because of its strong fluorescent emission.5 Fluoranthene-based oligomers represent a new type of conjugated polymer that exhibits a low band gap, and novel electronic, optical and photovoltaic properties attributed to its unique ladder-type topology and highly delocalized π-conjugation.6 It is believed that oligofluoranthene (OFA) possesses better electronic and optoelectronic properties than that of the fluoranthene monomer which by itself is suitable for chemosensors.7 Recently we reported that OFA can emit strong fluorescence that gives rise to a unique superamplified quenching effect for the fabrication of advanced chemosensors for Fe(III) and explosives such as picric acid.8 However, a systematic investigation on the scalable synthesis of functional OFA has been yet been carried out. To the best of our knowledge, only two papers are concerned with making OFA films and these were carried out by electropolymerization.9,10 Unfortunately, the OFA films prepared electrochemically have low electrical conductivity, limited synthetic yield, and poor thermal stability because of their irregular structures and low degree of conjugation.On the other hand, porous carbon materials have attracted more attention because they hold great potential for important applications in fuel cells,11 lithium ion batteries,12,13 electronic capacitors,14,15 and adsorbents for environmental protection16,17 due to their low density, high thermal and electrical conductivity, high chemical and mechanical stability, and large specific surface area. A variety of methods such as template-synthesis,18 ultrasonic spray pyrolysis,19 solvent-assisting synthesis,20 and flash heating21 have been applied to prepare porous carbon materials. OFA as a highly aromatic oligomer has a theoretical carbon content as high as 96%. The thermal stability of OFA is high enough to prepare porous carbon because of its highly aromatic rings without any thermally unstable structures. Therefore, a new synthetic method for producing high-quality OFA nanomaterials for developing their applications as porous carbon precursors also holds great interest.
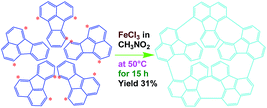
In this work, we explore a simple scalable method to synthesize OFA nanorods by using a direct chemical oxidative oligomerization of fluoranthene without any templates. The effect of oxidant species, oxidant/monomer ratio, polymerization temperature, and reaction time on the oligomerization yield, structure, morphology, properties, and functionalities of the OFA is explored. The as-synthesized OFA nanorods as precursors for macroporous carbon materials having widely tunable conductivities, ranging from 10−9 S cm−1 for virgin OFA to 10−4 S cm−1 upon iodine-doping and up to 100 S cm−1 upon carbonization, is systematically investigated. The fluorescence emission of the OFAs is optimized and controlled by adjusting the oxidant/monomer ratio and the OFA solution concentration. Chemical oxidative synthesis of the OFA nanorods directly from FA monomer is considered as a simple and effective route to make highly fluorescent organic substances and high carbon-yield precursors to porous carbon materials.
Experimental section
Synthesis of oligofluoranthene (OFA) nanorods
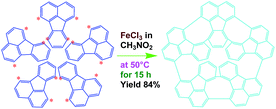
As a typical synthetic procedure for the OFA nanorods, 1 mmol (0.21 g) of FA monomer and 9 mmol of anhydrous FeCl3 oxidant were dissolved in 5 mL of CH3NO2, respectively. The two solutions were kept at 50 °C in a water bath for 30 min and then the FeCl3 solution was slowly added into the FA solution during magnetic stirring. The mixture was further magnetically stirred for 15 h at 50 °C to complete the oligomerization. Note that upon adding the first drop of the oxidant solution into the monomer solution, the light-yellow monomer solution quickly turned orange. The color then gradually became darker as more oxidant solution was added and turned completely black after finishing the addition of the FeCl3 solution. Virgin nanorods were obtained after purification with ethanol and DI water by centrifugation. Dedoped nanorods were prepared by further washing successively with 1.0 M HCl, 1.0 M NaOH and DI water several times at 50 °C in order to remove the residual monomer, oxidant and their HCl/NaOH aqueous soluble byproducts. Finally, the pure dedoped OFA particles were obtained after drying at 80 °C for 48 h. Dedoped OFA particles were doped by iodine vapor as follows: OFA/iodine (3/97) particles were kept in a sealed vessel at 80 °C under atmospheric pressure for two days, during which the OFA and iodine particles in the vessel were not allowed to contact each other.Measurements
UV-vis spectra of dedoped OFA particles in N-methylpyrrolidone (NMP) and concentrated H2SO4 were recorded on a Perkin-Elmer Lambda 25 UV-vis spectrophotometer. ATR-FTIR spectra were obtained with a Nicolet Magna-IRTM 550. Raman spectra of the solid powder of the OFA and PFA were taken using a British Renishaw inVia Raman Microscope (Bert) with a 785 nm red laser pumped with a solid state diode. The molecular weight of the soluble part of the OFA nanorods was obtained on a matrix-assisted laser desorption time-of-flight mass spectrometer (MALDI-TOF-MS) spectrum using a Voyager DE STR MALDI-TOF mass spectrometer with 2,5-dihydroxybenzoic acid as the matrix. 1H-NMR spectra were taken on a Bruker ARX-500 and 400 spectrometer using dimethyl sulfoxide (DMSO)-D6 and CDCl3 as solvents. X-ray diffraction (XRD) patterns were scanned on a Philips X'pert Pro powder diffractometer using copper-monochromatized CuKα radiation (λ = 0.154178 nm). Morphologies of OFA and carbon-based materials were observed on a JEOL JSM-6700 field emission scanning electron microscopy (SEM), whose samples were prepared by dropping the ethanol dispersion of the particles onto silicon wafers and then gold sputtering. Fluorescence spectra of FA and OFA solutions in NMP were acquired on a QM-6SE PTI fluorescence spectrometer. Thermogravimetric (TG) analysis was carried out on a Perkin Elmer TGA Pyris 1 in argon at a heating rate of 15 °C min−1 from room temperature to 1100 °C. The carbon materials made from TG measurements were imaged by a JEOL JSM-6700 SEM without gold sputtering. The bulk electrical conductivity of an approximately 0.5 mm-thick pressed disk of OFA particles was measured using a two-probe method with an area of 0.785 cm2 at room temperature.Results and discussion
Synthesis of OFA particles
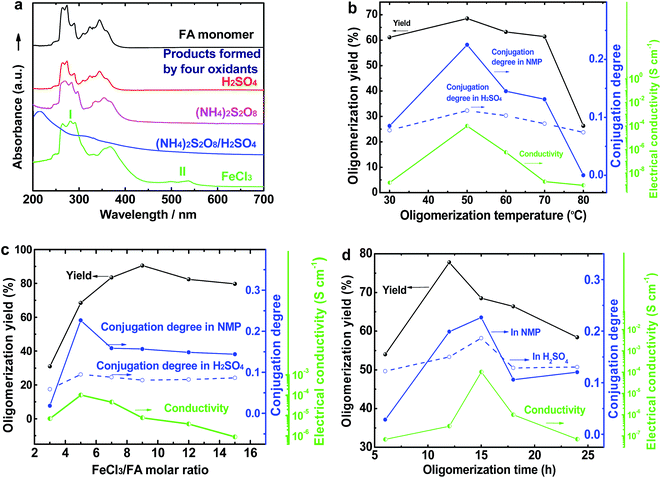
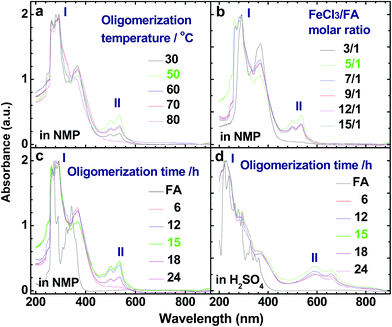
The UV-vis spectrum of an FA solution in NMP does not display any significant absorption at wavelengths above 400 nm (Fig. 1a). The reaction products obtained using only H2SO4 or ammonium persulfate present very similar UV-vis absorptions to that of the monomer, indicating that these two oxidants do not lead to a successful synthesis of OFA because of their too low or too high standard RPs, respectively. A combination of H2SO4 and ammonium persulfate results in some kind of oxidative oligomerization of FA monomer because the product exhibits a completely different UV-vis spectrum with a weak absorbance tailing at long wavelengths up to 600 nm. It is interesting to note that when the solution FeCl3/CH3NO2 was applied as an oxidant, the reaction instantly turned black, finally leading to solid dark particles. By continually adding FeCl3 solution, the reaction color became darker with black flocculates deposited on the bottom of the reaction vessel. Brownish red precipitates were obtained after washing with alcohol and water. The product prepared by FeCl3 shows new UV-vis absorptions at 499 and 537 nm associated with large π-conjugation among different FA units, signifying the occurrence of the oxidative oligomerization of FA.8,22 Furthermore, the conjugation degree of the OFA can be quantitatively evaluated by defining the relative UV-vis absorbance ratio of Band II and Band I, as shown in Fig. 1b–d. Clearly, FeCl3 is the best oxidant because OFA synthesized with FeCl3 possesses the highest degree of conjugation. In fact, FeCl3 is not only an oxidant, but also a Lewis acid. This chemical has been successfully used as an oxidant for C–C coupling reactions with other conjugated molecules including benzene, pyrene, pyrrole, and triphenylene.7,23,24 Therefore, FeCl3 has been chosen as an optimal oxidant for the following investigation.
Structure of the oligofluoranthenes
Moreover, these molecular structures have been substantially confirmed by their MALDI/TOF MASS spectra shown in Fig. 5. The OFA obtained at the FeCl3/FA molar ratio of 3/1 in Fig. 5a probably consists of a mixture of two isomers as given in Table 1 since each of its characteristic peaks is composed of a few substances with a very close mass, while the OFA obtained at the FeCl3/FA molar ratios of 7/1 and 9/1 in Fig. 5b and c may contain some minor impurities besides the major 5-FA unit-containing oligomers likely because more oxidants can lead to over-oxidation of the OFA as discussed earlier. These imply that the molecular structure of the OFAs produced depends on the synthetic conditions.
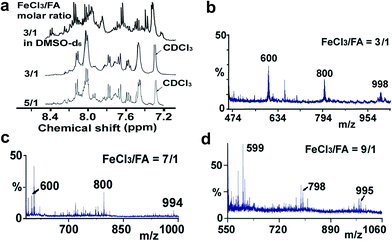 | ||
| Fig. 5 (a) 1H-NMR spectra in DMSO-d6 at 500 MHz and CDCl3 at 400 MHz and (b–d) MALDI/TOF MS spectra of the OFAs synthesized with three representative FeCl3/FA molar ratios. | ||
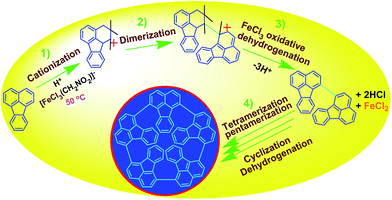
Oxidative pentamerization mechanism of FA. The possible mechanism for the direct cationic chemical oxidative pentamerization of FA in CH3NO2 was proposed in Scheme 1.
FeCl3 can react with CH3NO2 to generate in situ the H+[FeCl3(CH2NO2)]− complex, which initiates the cationic oxidative pentamerization of FA just like the polymerization of benzene and pyrene by FeCl3.7,23 The FA pentamerization likely consists of the following steps: (1) the cationization of FA monomers and the formation of FA carbonium ions by H+, (2) the dimerization of FA carbonium ions with concomitant formation of a dimeric cation, (3) the oxidative dehydrogenation of FA dimeric carbonium ions by FeCl3, (4) the tetramerization/pentamerization, cyclization, and dehydrogenation among active FA dimer, trimer, and pentamer carbonium ions. The constant decline of both the open-circuit potential and the pH of the FA oligomerization solution containing FeCl3 oxidant with reaction time supports this pentamerization mechanism because of a fast consumption of the oxidant to produce cation oligomerization and also HCl as a byproduct. In short, FA monomers likely undergo cationization, coupling, dehydrogenation, and cyclizing pentamerization to give the resulting OFAs.
Morphology
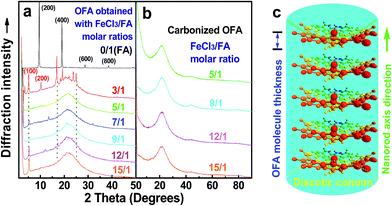
Fig. 7 shows SEM images of the OFA nanorods synthesized with four different oxidant/monomer ratios. When an FeCl3/FA molar ratio of 3/1 is used, the OFAs formed look like large flakes and needles with smooth surfaces. By increasing the oxidant/monomer molar ratio to 5/1, 9/1, and up to 12/1, the obtained OFAs mainly appear as rod-like nanostructures with diameters around 35, 38, and 40 nm, respectively, and lengths greater than 300 nm. It appears that the morphology of the OFAs significantly depends on the FeCl3/FA ratio, which can be confirmed by five more sharp diffraction peaks for the OFA formed at the FeCl3/FA molar ratio of 3/1 when compared to the other OFAs at FeCl3/FA molar ratios between 5/1 and 12/1. It has been reported that many electrically conducting polymers or oligomers such as poly(o-anisidine),34 poly(azomethine),35 poly(3-hexylthiophene),36 and thiophene/phenylene co-oligomer37 also self-assemble into one-dimensional nanostructures through strong π–π interactions between the packed molecules. Analogously, the π–π stacking in the OFAs with conical configurations could be one of the reasons for the formation of one-dimensional nanorods.8 A significant red-shift of 192 nm in the UV-vis and fluorescence spectra of the OFAs can be attributed to the strong π–π stacking.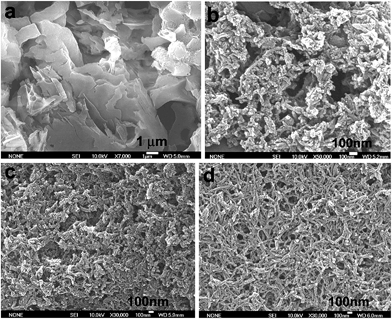 | ||
| Fig. 7 SEM images of OFA particles and nanorods synthesized with FeCl3/FA molar ratios: (a) 3/1, (b) 5/1, (c) 9/1, and (d) 12/1. | ||
Solubility and solvatochromism
Table 2 shows the solubility and solvatochromism of FA and OFAs synthesized with various FeCl3/FA ratios. Although the FA is soluble in most organic solvents including HCOOH, NMP, DMSO, dimethyl formamide (DMF), CH3CN, tetrahydrofuran (THF), and n-hexane, most of the OFAs are soluble in highly polar NMP with high polarity index, but partly or slightly soluble in DMSO, DMF, H2SO4 and HCOOH, which is in accordance with other conducting polymers reported earlier.38–41 Meanwhile, the obtained OFAs are insoluble, but dispersible in CH3CN, THF, and n-hexane. This signifies that the OFAs have higher chemical resistance than FA likely due to their higher molecular weight and thus larger π-conjugated structures. The OFAs synthesized under optimal conditions show the lowest solubility and the darkest color in various solvents due to the highest degree of π-conjugation. Furthermore, the optimal OFA displays a unique solvatochromism including yellow, green, red, dark green, and gray in different solvents associated with the variation of the conformations of OFA molecules. The OFAs synthesized with different FeCl3/FA ratios all demonstrate longer wavelength UV-vis absorptions at 660–663 nm in H2SO4 when compared to 535–537 nm in NMP, which explains that the unique solvatochromism is due to their different absorptions.| FeCl3/FA molar ratio | Solubilitya (solution colorb, UV-vis maximum absorption wavelength [nm]) | Thermal degradation parameters | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hexane | THF | CH3CN | DMF | DMSO | NMP | HCOOH | H2SO4 | T d (°C) | T dm (°C) | (dα/dt)m (% min−1) | Carbon yield at 1100 °C (%) | |
| a ID = insoluble but dispersible; MS = mainly soluble; PS = partially soluble; S = soluble; SS = slightly soluble. b br = brownish red; c = colorless; dg = dark green; g = green; gr = gray; p = pink; r = red; y = yellow. c The temperature corresponding to 2% weight loss. d The temperature corresponding to the maximal weight-loss rate. | ||||||||||||
| 0/1(FA) | S(y) | S(c) | S(y) | S(c) | S(c) | S(c, 345) | S(y) | PS(g, 362) | 211 | 344 | 32.4 | 0 |
| 3/1 | ID(g) | SS(y) | SS(br) | MS(y) | MS(r) | S(r, 535) | SS(gr) | PS(dg, 660) | NA | NA | NA | NA |
| 5/1 | ID(br) | ID(r) | ID(br) | PS(r) | PS(r) | S(r, 537) | SS(gr) | PS(dg, 663) | 384 | 497 | 5.9 | 23.2 |
| 7/1 | ID(br) | ID(r) | ID(br) | PS(r) | PS(p) | S(r, 536) | SS(gr) | PS(dg, 662) | 419 | 528 | 1.6 | 66.8 |
| 9/1 | ID(br) | ID(r) | ID(br) | PS(r) | PS(p) | S(r, 536) | SS(gr) | PS(dg, 662) | 446 | 633 | 1.2 | 77.6 |
| 12/1 | ID(br) | ID(r) | ID(br) | PS(r) | PS(p) | S(r, 536) | SS(gr) | PS(dg, 662) | 445 | 628 | 2.9 | 28.7 |
| 15/1 | ID(br) | ID(r) | ID(br) | PS(r) | PS(p) | S(r, 536) | SS(gr) | PS(dg, 662) | 425 | 604 | 3.3 | 29.2 |
Electrical conductivity
As compared with electrically insulating FA, the virgin OFAs are powders having conductivities ranging from 10−11 and 10−7 S cm−1. The conductivity can be enhanced by 3 orders of magnitude (to 10−4 S cm−1) by iodine doping. The effect of oligomerization temperature, FeCl3/FA molar ratios, and oligomerization time on the conductivity of iodine-doping OFA is summarized in Fig. 1b–d. With increasing FeCl3/FA molar ratio from 3/1 to 15/1, the conductivity increases first and then declines, exhibiting a maximum of 1.0 × 10−4 S cm−1 at 5/1, likely because too high an FeCl3/FA ratio severely impairs the conductivity due to over-oxidation of the OFA. Meanwhile, it can be seen that the maximum conductivity occurs at the optimal oligomerization temperature and time of 50 °C and 15 h, respectively. This is due to the maximum doping levels and the maximum degree of π-conjugation that is confirmed by the UV-vis spectra shown in Fig. 2. Thus, it is concluded that iodine vapor doping is an effective method to significantly enhance the conductivity of the OFAs, which is associated with enhancement of the charge carrier concentration.7,23Thermal degradation of the OFAs for preparation of porous carbon
The virgin OFA powders do not exhibit optical birefringence at room temperature under a polarized light microscope, implying that they are optically isotropic. The OFA powders melt at 300 °C and the melt exhibits optically anisotropic order because optical birefringence appears, as shown in Fig. 8a and b. This optical birefringence disappears after the OFA sample thoroughly melts or decomposes at higher temperatures. It appears that the OFAs are thermotropic liquid crystals because they can be considered as wholly aromatic discotic molecules as schematized in Table 1 and Fig. 6c. The thermal degradation of the OFAs synthesized with different oxidant/monomer ratios was quantitatively studied by TG as presented in Fig. 8c and d and Table 2. The FA monomer loses its entire weight over a temperature range from 200 to 346 °C, but the virgin OFAs in argon do not demonstrate any significant weight loss below 355 °C, further confirming that the synthesized OFAs have much higher molecular weights than the FA monomer. Moreover, by increasing the FeCl3/FA molar ratio from 5/1 to 15/1, the thermal degradation temperatures (Td and Tdm) and char yield at 1100 °C in argon first increase and then decrease, demonstrating maximum degradation temperatures and char yield at the FeCl3/FA ratio of 9/1. In contrast, the maximum weight-loss rate, (dα/dt)m, first decreases and then increases with increasing FeCl3/FA ratio from 5/1 to 15/1, demonstrating a minimum (dα/dt)m at the FeCl3/FA molar ratio of 9/1. These results signify that the OFA synthesized at the FeCl3/FA ratio of 9/1 has the maximum thermal stability likely due to its highest aromaticity or C/H ratio. Note that the OFA possesses the highest char yield of up to 77.6 wt% at 1100 °C in argon, which supports the discotic molecular structure of OFA with high thermal stability and high carbonized yield, as opposed to a linear chain structure with relatively low thermal stability and low carbonized yield. Note that the thermal stability of the OFAs synthesized in this study is much higher than that of electropolymerized FA.10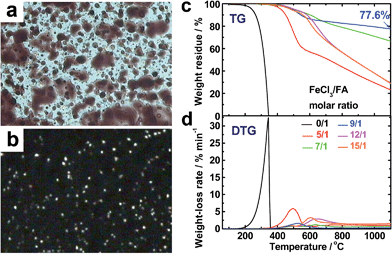 | ||
| Fig. 8 Optical microscope images of OFAs (after heating to 300 °C) under (a) sunlight and (b) polarized light. (c) TG and (d) DTG curves of FA and the OFAs synthesized with different FeCl3/FA ratios. | ||
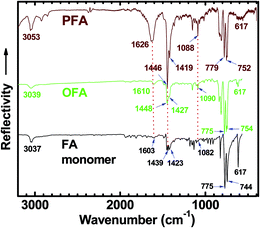
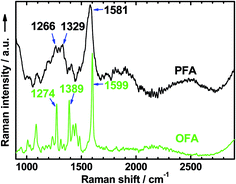
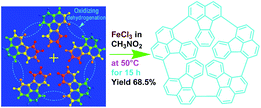
The high char yield of the OFAs provides a new route to prepare carbon materials at low cost. Fig. 6b shows the XRD curves of four OFAs-based carbon materials made in argon at 1100 °C, demonstrating only one broad diffraction centered at 2θ = 23.5° corresponding to the (002) reflection of turbostratic graphite. Meanwhile, a new weak diffraction centered at 2θ = 43.5° emerges that can be assigned to the (101) Bragg reflection of graphite.42 The carbonized material possesses completely different XRD characteristics from virgin OFA powder when used as a carbon precursor. This implies that the OFAs have been converted into graphite-like carbon materials after thermal pyrolysis in argon at 1100 °C. This result has been confirmed by two other methods: (1) the Raman spectrum of carbonized polyfluoranthene (PFA) at 1100 °C under an inert atmosphere exhibits the two Raman bands expected for carbon: these are the strongest and sharpest bands at 1306 and 1584 cm−1 assigned to D- and G-bands, respectively31 and (2) much higher electrical conductivity of up to 100 S cm−1 of this carbonized OFA when compared to virgin OFA and I2-doped OFA powders, as discussed below. SEM images reveal that the obtained carbon materials contain 3D interconnected macropores with diameters ranging from 400 to 1200 nm (Fig. 9).
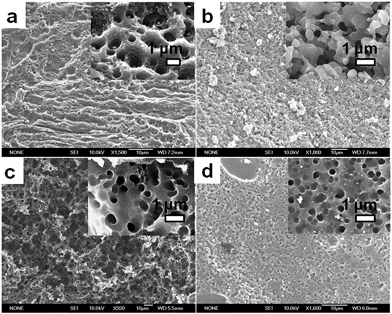 | ||
| Fig. 9 SEM images of porous carbon materials based on the OFAs synthesized with the four FeCl3/FA molar ratios: (a) 5/1, (b) 9/1, (c) 12/1, and (d) 15/1. | ||
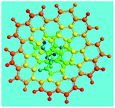
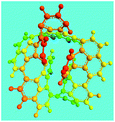
The OFA possesses a special molecular structure consisting of one naphthalene and benzene bridged by a penta-carbocyclic ring, possibly resulting in some gaps in its supramolecular aggregation, thus leading to porous structures after carbonization. In particular, the macroporous carbon materials have electrical conductivities up to 100 S cm−1, which is much higher than that of virgin OFA and I2-doped OFA powders. Such a large enhancement of the conductivity can also be attributed to the satisfactory conversion of the OFA into graphitic carbon.
Fluorescence
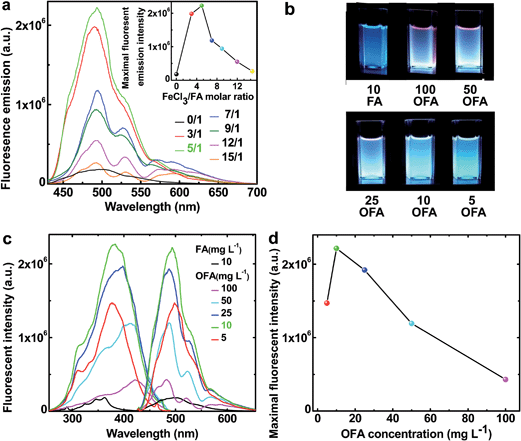
The fluorescence emission spectra in NMP of FA and OFAs synthesized with different FeCl3/FA ratios are illustrated in Fig. 10a.8 By changing the FeCl3/FA molar ratio from 0/1 to 15/1, the fluorescence emission first quickly increases and then decreases, as shown in the inset to Fig. 9a, i.e., the fluorescence emission can be adjusted by regulating the FeCl3/FA ratio. Most significantly, the OFA synthesized with an FeCl3/FA ratio of 5/1 displays the maximum emission fluorescence at 494 nm, possibly due to its symmetric molecular structure as shown in Table 1 and thus the optimal π-conjugation shown in Fig. 1c. The fluorescence excitation/emission intensity can also be tuned by adjusting the OFA concentration. It is found from Fig. 10b–d that 10 mg L−1 OFA in NMP demonstrates both the strongest excitation and emission fluorescence at 383 and 494 nm, respectively. Apparently, the OFAs demonstrate different characteristics in their excitation and emission spectra from FA. The bandwidth of the emission spectrum of the OFA is about 70 nm and basically displays a single cyan color at 10 mg L−1 as shown in Fig. 10b instead of the blue color of FA. More importantly, the intensity of both the emission and excitation fluorescence is dramatically enhanced by 12.2 times upon oligomerization of FA (Fig. 10c) under exactly the same conditions. Besides the OFA solution, a freestanding robust composite film containing 0.76 wt% OFA in a non-fluorescent polysulfone matrix emits very strong fluorescence when exposed to 365 nm UV light and displays a 4 nm redshift in its emission spectrum similar to the OFA solution.8 Strong and controllable fluorescence emission is an important reason why optimized OFA has been shown to be an ultra-sensitive fluorescent emitter for use in amplified and reversible quenching fluorescence chemosensors.8,43–50Conclusions
Dispersible OFA nanorods with diameters of 35–40 nm and lengths of >300 nm have been successfully synthesized by a simple template-free oxidative oligomerization of FA in CH3NO2 using FeCl3 as the oxidant. The pentamerization is thought to proceed via a cationic oxidative mechanism. The proper oligomerization conditions including oxidant species, oxidant/monomer ratio, polymerization temperature and time have been elucidated for the productive synthesis of OFA with optimal molecular and large π-conjugated structures, morphology, solvatochromism, electrical conductivity, fluorescence, and thermal stability. The OFAs are most likely a five FA-unit-containing cyclic pentameric oligomer that possesses reversible and controllable variation of the electrical conductivity from 10−11 S cm−1 to 10−4 S cm−1 by I2 doping or dedoping. The OFA nanorods are also excellent precursors to make macroporous carbon materials with pore diameters of 400–1200 nm, carbon yield of 77.6% at 1100 °C, and conductivities up to 100 S cm−1. These features make the OFAs of interest as conductivity controllable materials and as high carbon-yield precursors for carbon nanomaterials. Since the emitting fluorescence of the optimized OFA nanorods is 12.2 times stronger than that of recognized highly fluorescent FA of the low molecular organic fluorescent substances, OFAs may have potential as ultrasensitive fluorescent emitters.Acknowledgements
We thank (1) the National Natural Science Foundations of China (51273148 and 50873077), (2) the Foundation of State Key Laboratory of Pollution Control and Resource Reuse (Tongji University), China (PCRRY14003), (3) a Scholarship from the China Scholarship Council (201308310009), and (4) Dr. Wen-Bo Xin from UCLA, USA for the Raman measurements.Notes and references
- S. W. Thomas III, G. D. Joly and T. M. Swager, Chem. Rev., 2007, 107, 1339–1386 CrossRef PubMed.
- L. Basabe-Desmonts, D. N. Reinhoudt and M. Crego-Calama, Chem. Soc. Rev., 2007, 36, 993–1017 RSC.
- D. J. Ahn and J. M. Kim, Acc. Chem. Res., 2008, 41, 805–816 CrossRef CAS PubMed.
- L. J. Fan, Y. Zhang, C. B. Murphy, S. E. Angell, M. F. L. Parker, B. R. Flynn and W. E. Jones Jr, Coord. Chem. Rev., 2009, 253, 410–422 CrossRef CAS PubMed.
- B. Marciniak, J. Cryst. Growth, 2002, 236, 333–346 CrossRef CAS.
- M. Kertesz and A. Ashertehrani, Macromolecules, 1996, 29, 940–945 CrossRef CAS.
- X. G. Li, Y. W. Liu, M. R. Huang, S. Peng, L. Z. Gong and M. G. Moloney, Chem.–Eur. J., 2010, 16, 4803–4813 CrossRef CAS PubMed.
- X. G. Li, Y. Z. Liao, M. R. Huang, V. Strong and R. B. Kaner, Chem. Sci., 2013, 4, 1970–1978 RSC.
- R. J. Waltmann and A. F. Diaz, J. Electrochem. Soc., 1985, 132, 631–634 CrossRef PubMed.
- J. Xu, J. Hou, S. Zhang, H. Y. Xia and S. Z. Pu, J. Phys. Chem. B, 2006, 110, 2643–2648 CrossRef CAS PubMed.
- J. H. Bang, K. Han, S. E. Skrabalak, H. Kim and K. S. Suslick, J. Phys. Chem. C, 2007, 111, 10959–10964 CAS.
- H. S. Zhou, S. Zhu, M. Hibino, I. Honma and M. Ichihara, Adv. Mater., 2003, 15, 2107–2111 CrossRef CAS PubMed.
- J. H. Hou, C. B. Cao, F. Idrees and X. L. Ma, ACS Nano, 2015, 9, 2556–2564 CrossRef CAS PubMed.
- M. Sevilla and A. B. Fuertes, ACS Nano, 2014, 8, 5069–5078 CrossRef CAS PubMed.
- X. Li, X. Zang, Z. Li, X. Li, P. Li, P. Sun, X. Lee, R. Zhang, Z. Huang, K. Wang, D. Wu, F. Kang and H. Zhu, Adv. Funct. Mater., 2013, 23, 4862–4869 CAS.
- P. Branton, A. H. Lu and F. Schüth, Carbon, 2009, 47, 1005–1011 CrossRef CAS PubMed.
- G. P. Hao, W. C. Li, D. Qian, G. H. Wang, W. P. Zhang, T. Zhang, A. Q. Wang, F. Schüth, H. J. Bongard and A. H. Lu, J. Am. Chem. Soc., 2011, 133, 11378–11388 CrossRef CAS PubMed.
- J. Lee, J. Kim and T. Hyeon, Adv. Mater., 2006, 18, 2073–2094 CrossRef CAS PubMed.
- S. E. Skrabalak and K. S. Suslick, J. Am. Chem. Soc., 2006, 128, 12642–12643 CrossRef CAS PubMed.
- H. S. Peng and X. M. Sun, Chem. Commun., 2009, 1058–1060 RSC.
- F. Kurosaki, H. Koyanaka, T. Hata and Y. Imamura, Carbon, 2007, 45, 671–673 CrossRef CAS PubMed.
- G. G. Wallace, G. M. Spinks, L. A. P. Kane-Maguire and P. R. Teasdale, Conductive electroactive polymers: Intelligent materials systems, Lancaster, Pennsylvania, CRC Press, 2003, pp. 170–172 Search PubMed.
- P. Kovacic and F. W. Koch, J. Org. Chem., 1963, 28, 1864–1868 CrossRef CAS.
- Y. Z. Liao, V. Strong, Y. Wang, X. G. Li, X. Wang and R. B. Kaner, Adv. Funct. Mater., 2012, 22, 726–735 CrossRef CAS PubMed.
- P. Kovacic and J. Oziomek, J. Org. Chem., 1964, 29, 100–104 CrossRef CAS.
- S. A. Chen and G. W. Hwang, J. Am. Chem. Soc., 1995, 117, 10055–10062 CrossRef CAS.
- X. G. Li, A. Li and M. R. Huang, Chem.–Eur. J., 2008, 14, 10309–10317 CrossRef CAS PubMed.
- N. D. Marsh, C. J. Mikolajczak and M. J. Wornat, Spectrochim. Acta, Part A, 2000, 56, 1499–1511 CrossRef.
- S. Hino, K. Iwasaki and K. Matsumoto, Synth. Met., 1994, 64, 259–264 CrossRef CAS.
- G. W. Lu, L. T. Qu and G. Q. Shi, Electrochim. Acta, 2005, 51, 340–346 CrossRef CAS PubMed.
- X. G. Li, Y. Z. Liao, M. R. Huang and R. B. Kaner, Chem. Sci., 2015, 6, 2087–2101 RSC.
- W. H. Geng, T. Nakajima, H. Takanashi and A. Ohki, Fuel, 2009, 88, 139–144 CrossRef CAS PubMed.
- L. L. Qu, Y.-T. Li, D.-W. Li, J.-Q. Xue, J. S. Fosseyab and Y.-T. Long, Analyst, 2013, 138, 1523–1528 RSC.
- Y. Wang, H. D. Tran and R. B. Kaner, J. Phys. Chem. C, 2009, 113, 10346–10349 CAS.
- J. Miyake, Y. Tsuji, A. Nagai and Y. Chujo, Chem. Commun., 2009, 2183–2185 RSC.
- B. S. Ong, Y. Wu, P. Liu and S. Gardner, J. Am. Chem. Soc., 2004, 126, 3378–3379 CrossRef CAS PubMed.
- M. Schiek, F. Balzer, K. Al-Shamery, A. Lützen and H. G. Rubahn, J. Phys. Chem. C, 2009, 113, 9601–9608 CAS.
- X. G. Li, M. R. Huang, W. Duan and Y. L. Yang, Chem. Rev., 2002, 102, 2925–3030 CrossRef CAS PubMed.
- M. R. Huang, H. J. Lu and X. G. Li, J. Mater. Chem., 2012, 22, 17685–17699 RSC.
- X. G. Li, H. Feng, M. R. Huang, G. L. Gu and M. G. Moloney, Anal. Chem., 2012, 84, 134–140 CrossRef CAS PubMed.
- Z. Q. Li, C. J. Lu, Z. P. Xia, Y. Zhou and Z. Luo, Carbon, 2007, 45, 1686–1695 CrossRef CAS PubMed.
- E. Ahmed, S. W. Morton, P. T. Hammond and T. M. Swager, Adv. Mater., 2013, 25, 4504–4510 CrossRef CAS PubMed.
- A. Rose, Z. G. Zhu, C. F. Madigan, T. M. Swager and V. Bulovic, Nature, 2005, 434, 876–879 CrossRef CAS PubMed.
- J. Kim, D. Tyler McQuade, S. K. McHugh and T. M. Swager, Angew. Chem., Int. Ed., 2000, 39, 3868–3872 CrossRef CAS.
- L. Chen, D. W. McBranch, H. Wang, R. Helgeson, F. Wudl and D. G. Whitten, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 12287–12292 CrossRef CAS.
- Z. J. Zhao, Z. F. Chang, B. R. He, B. Chen, C. M. Deng, P. Lu, H. Y. Qiu and B. Z. Tang, Chem.–Eur. J., 2013, 19, 11512–11517 CrossRef CAS PubMed.
- D. W. Domaille, E. L. Que and C. J. Chang, Nat. Chem. Biol., 2008, 4, 168–175 CrossRef CAS PubMed.
- C. Y. K. Chan, Z. J. Zhao, J. W. Y. Lam, J. Z. Liu, S. M. Chen, P. Lu, F. Mahtab, X. J. Chen, H. H. Y. Sung, H. S. Kwok, Y. G. Ma, I. D. Williams, K. S. Wong and B. Z. Tang, Adv. Funct. Mater., 2012, 22, 378–389 CrossRef CAS PubMed.
- A. Vu, J. Phillips, P. Bühlmann and A. Stein, Chem. Mater., 2013, 25, 711–722 CrossRef CAS.
- Y. Y. Bao, H. D. Keersmaecker, S. Corneillie, F. Yu, H. Mizuno, G. F. Zhang, J. Hofkens, B. Mendrek, A. Kowalczuk and M. Smet, Chem. Mater., 2015, 27, 3450–3455 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: SEM images of OFA nanorods. See DOI: 10.1039/c5sc03041b |
| ‡ X.-G. Li and Y. Z. Liao contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |