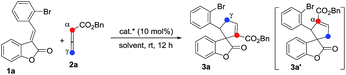Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chiral phosphine-catalyzed tunable cycloaddition reactions of allenoates with benzofuranone-derived olefins for a highly regio-, diastereo- and enantioselective synthesis of spiro-benzofuranones†
De
Wang
a,
Guo-Peng
Wang
b,
Yao-Liang
Sun
a,
Shou-Fei
Zhu
b,
Yin
Wei
*a,
Qi-Lin
Zhou
*b and
Min
Shi
*a
aState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, P. R. China. E-mail: mshi@mail.sioc.ac.cn
bState Key Laboratory and Institute of Element-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071, China. E-mail: qlzhou@nankai.edu.cn
First published on 15th September 2015
Abstract
The first regioselective catalytic asymmetric [3 + 2] cycloaddition of benzofuranone-derived olefins with allenoates and substituted allenoates has been developed in the presence of (R)-SITCP, affording different functionalized 3-spirocyclopentene benzofuran-2-ones in good yields with high enantioselectivities under mild conditions. The substrate scope has also been examined. The regioselective outcomes for this phosphine-catalyzed [3 + 2] cycloaddition reaction can be rationalized using DFT calculations.
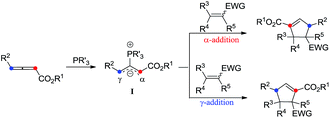
Phosphine-catalyzed [3 + 2] cycloaddition of electron-deficient olefins with allenoates, which provides alternative access to a variety of useful carbocycles, was first reported by Zhang and Lu in 1995.1,2 Pioneering work on the catalytic asymmetric Lu's [3 + 2] cycloaddition of allenoates with olefins was disclosed by Zhang in 1997.3 No further progress was made on the development of this enantioselective [3 + 2] cyclization for about a decade after Zhang's promising results, until Fu and co-workers recently developed a series of axially chiral binaphthyl frameworks containing phosphines that catalyzed the asymmetric cycloaddition of allenoates with electron-deficient olefins, affording the corresponding cycloadducts in good yields with excellent diastereo- and enantioselectivities.4 Moreover, Marinetti and co-workers have also discovered that chiral phosphines based on a planar chiral 2-phospha[3]ferrocenophane scaffold were efficient catalysts for this type of asymmetric reaction as well.5 A variety of multifunctional chiral phosphines derived from natural amino acids have also emerged as powerful catalysts to promote the [3 + 2] cycloaddition of allenoates with electron-deficient olefins or imines, affording a variety of cyclopentene or pyrrolidine derivatives in good yields with high diastereo- and enantioselectivities under mild conditions.6 For example, Miller and co-workers achieved the enantioselective cyclization of allenoates and enones using phosphines containing α-amino acids.6a Jacobsen and co-workers utilized phosphine–thiourea catalysts for enantioselective annulations of allenes and imines.6b Zhao6c and Lu6d–s developed a series of multifunctional phosphine catalysts based on different types of amino acids, and applied these functional phosphine-containing catalysts to different types of cycloadditions. Recently, Kwon's group developed a new class of rigid chiral bicyclic phosphines and applied them to the asymmetric synthesis of multi-substituted pyrrolines.6t In addition, some commercially available bidentate chiral phosphine-promoted [3 + 2] cycloadditions have also been reported.7
The phosphine-catalyzed [3 + 2] cycloaddition of electron-deficient olefins with allenoates was commonly considered to start from the formation of the corresponding zwitterionic intermediate I between PR3 and the allenoate. The nature of this zwitterion shown in Scheme 1 may be depicted in two ways, which include anion localization at the α-carbon or γ-carbon, thus two regioisomers derived from the α-addition and γ-addition could be produced at the same time (Scheme 1). Therefore, the selective synthesis of highly regio-, diastereo- and enantioselective products becomes a big challenge. Previous reports mainly focus on how to obtain a single highly regioselective product, however, few people have made efforts to obtain both the α-addition and γ-addition isomers in a controllable way with high regio-, diastereo- and enantioselective values, not to mention the mechanistic study of the regioselectivity.8
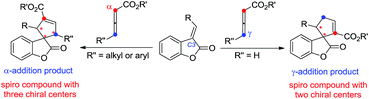
Benzofuranones as one of the important building blocks exist in a variety of natural products9 and potential medicines.10 The enantioselective synthesis of chiral benzofuranones remains a considerable challenge,11 especially in the field of construction of a chiral spiro-quaternary center at the C3 position of benzofuranones.12 As part of our ongoing investigation on phosphine-catalyzed asymmetric cycloaddition,13 we wish to report a spiro phosphine (R)-SITCP14 catalyzed asymmetric [3 + 2] cycloaddition of allenic esters with benzofuranones, furnishing the spiro cycloadducts in good yields with excellent regio-, diastereo- and enantioselectivities, by adjusting the substituents of the allenic esters to obtain both the α-addition and γ-addition products, and using rational DFT calculations to reveal the reason for the regioselectivity. This asymmetric [3 + 2] cycloaddition catalyzed by a chiral phosphine features the simultaneous formation of spiro-quaternary and tertiary stereocenters (two or three chiral centers) in a single step (Scheme 2). In addition, this type of reaction is also suitable for substrates such as arylideneoxindole and alkylidene azlactone, which makes this type of reaction have promising applications.
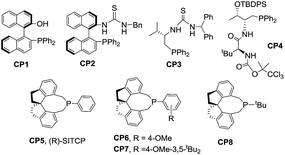
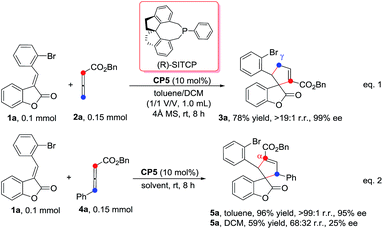
We initially screened a variety of chiral phosphines CP1–CP8 using (E)-3-(2-bromobenzylidene)benzofuran-2(3H)-one 1a and benzyl 2,3-butadienoate 2a as the model substrates in toluene. The results are summarized in Table 1. We found the γ-addition product 3a as the main product and the α-addition product 3a′ as the minor product, which were obtained in 26–92% total yields, with the regioselectivity ratios (r.r.) of 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a′ from 86
3a′ from 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 to 95
14 to 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
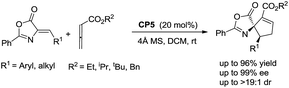
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, and excellent diastereoselectivities (the minor diastereomer almost could not be detected by 1H NMR); the ee value of the main product 3a is obtained from 8% to 88% (Table 1, entries 1–8). The catalyst CP5 gave the highest yield, regio- and enantioselectivity compared to other catalysts (Table 1, entry 5). Having identified the best catalyst in this reaction, we next attempted to further optimize the reaction conditions by screening of the solvent and reaction temperature (Table 1, entries 8–14). The reaction outcomes revealed that using 10 mol% of CP5 as the catalyst and carrying out the reaction in dichloromethane (DCM) and toluene as the mixing solvents (1
5, and excellent diastereoselectivities (the minor diastereomer almost could not be detected by 1H NMR); the ee value of the main product 3a is obtained from 8% to 88% (Table 1, entries 1–8). The catalyst CP5 gave the highest yield, regio- and enantioselectivity compared to other catalysts (Table 1, entry 5). Having identified the best catalyst in this reaction, we next attempted to further optimize the reaction conditions by screening of the solvent and reaction temperature (Table 1, entries 8–14). The reaction outcomes revealed that using 10 mol% of CP5 as the catalyst and carrying out the reaction in dichloromethane (DCM) and toluene as the mixing solvents (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with 4 Å MS (30 mg) as the additive affords 3a at room temperature for 12 h in 78% yield with >19
1) with 4 Å MS (30 mg) as the additive affords 3a at room temperature for 12 h in 78% yield with >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 r.r. and 99% ee value, which served as the best reaction conditions for this reaction (Scheme 3, eqn(1)). Using γ-phenyl allenoate 4a as the Michael acceptor, the reaction proceeded smoothly to give the α-addition product as the major product in 96% yield, with >19
1 r.r. and 99% ee value, which served as the best reaction conditions for this reaction (Scheme 3, eqn(1)). Using γ-phenyl allenoate 4a as the Michael acceptor, the reaction proceeded smoothly to give the α-addition product as the major product in 96% yield, with >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 r.r. and 95% ee value in toluene, however, the reaction proceeded in DCM, diminishing the yield, r.r. and ee value significantly (Scheme 3, eqn (2)).
1 r.r. and 95% ee value in toluene, however, the reaction proceeded in DCM, diminishing the yield, r.r. and ee value significantly (Scheme 3, eqn (2)).
| Entrya | Cat.* | Solvent | T (°C) | Yieldb (%) | r.r.c (3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a′) 3a′) |
eec (%) |
|---|---|---|---|---|---|---|
a All reactions were carried out with 1a (0.1 mmol), 2a (0.15 mmol), and catalyst (10 mol%) in solvent (1.0 mL).
b Isolated yield.
c Determined using 1H NMR of the crude product; determined using HPLC.
d Disordered.
e Toluene/DCM = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
f Toluene/DCM = 1 1.
f Toluene/DCM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
g Toluene/DCM = 1 1.
g Toluene/DCM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4 Å MS (30 mg) was added as the additive. 1, 4 Å MS (30 mg) was added as the additive.
|
||||||
| 1 | CP1 | Toluene | 25 | 37 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
8 |
| 2 | CP2 | Toluene | 25 | 32 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
20 |
| 3 | CP3 | Toluene | 25 | 26 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
14 |
| 4d | CP4 | Toluene | 25 | — | — | — |
| 5 | CP5 | Toluene | 25 | 92 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
88 |
| 6 | CP6 | Toluene | 25 | 72 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
83 |
| 7 | CP7 | Toluene | 25 | 74 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
88 |
| 8 | CP8 | Toluene | 25 | Trace | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
13 |
| 9 | CP5 | DCM | 25 | 58 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
>99 |
| 10 | CP5 | THF | 25 | 47 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
93 |
| 11 | CP5 | CH3CN | 25 | 22 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
94 |
| 12 | CP5 | Toluene/DCMe | 25 | 85 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
91 |
| 13 | CP5 | Toluene/DCMf | 25 | 64 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98 |
| 14 | CP5 | Toluene/DCMg | 25 | 78 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 15 | CP5 | Toluene/DCMg | 0 | 53 | >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
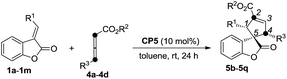
Having identified the optimal reaction conditions, the generality of this (R)-SITCP (CP5) catalyzed asymmetric γ-addition [3 + 2] cycloaddition was examined using a variety of aryl or alkyl-substituted benzofuranones 1 and allenic esters 2. The results are summarized in Table 2. Whether R1 is an electron-rich or -deficient aromatic ring, the reactions proceeded smoothly to give the corresponding spiro-cycloadducts 3b–3j in moderate to good yields with 87–96% ee values and 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 to >99
12 to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 r.r. (Table 2, entries 1–9). In the case of 4-CF3C6H4 benzofuranone 1e, the regioselectivity ratio decreased to 88
1 r.r. (Table 2, entries 1–9). In the case of 4-CF3C6H4 benzofuranone 1e, the regioselectivity ratio decreased to 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 (Table 2, entry 4). Using 4-CNC6H4 benzofuranone 1g as the substrate, the corresponding adduct was obtained in 57% yield along with a relatively lower ee value (87% ee) (Table 2, entry 6). When R1 is a heteroaromatic group (R1 = 2-furyl, 2-thienyl) or a sterically hindered 1-naphthyl moiety, the reactions also proceed efficiently to afford the corresponding products 3k–3m in 48–99% yields with 93–99% ee values and good regioselectivities (Table 2, entries 10–12). Changing R1 from the aromatic group to an aliphatic group provided the corresponding product 3n in 68% yield with 95% ee and a 98
12 (Table 2, entry 4). Using 4-CNC6H4 benzofuranone 1g as the substrate, the corresponding adduct was obtained in 57% yield along with a relatively lower ee value (87% ee) (Table 2, entry 6). When R1 is a heteroaromatic group (R1 = 2-furyl, 2-thienyl) or a sterically hindered 1-naphthyl moiety, the reactions also proceed efficiently to afford the corresponding products 3k–3m in 48–99% yields with 93–99% ee values and good regioselectivities (Table 2, entries 10–12). Changing R1 from the aromatic group to an aliphatic group provided the corresponding product 3n in 68% yield with 95% ee and a 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 regioselectivity ratio (Table 2, entry 13). Other electron deficient allenes such as ethyl-2,3-butadienoate and penta-3,4-dien-2-one are also suitable for this asymmetric [3 + 2] cycloaddition, giving the corresponding products in 94% and 83% yields with 99% and 96% ee values as well as excellent regioselectivities, respectively (Table 2, entries 14 and 15). The absolute configuration of 3m has been assigned using X-ray diffraction as 1S, 5R. The ORTEP drawing and the CIF data are summarized in the ESI.†19
2 regioselectivity ratio (Table 2, entry 13). Other electron deficient allenes such as ethyl-2,3-butadienoate and penta-3,4-dien-2-one are also suitable for this asymmetric [3 + 2] cycloaddition, giving the corresponding products in 94% and 83% yields with 99% and 96% ee values as well as excellent regioselectivities, respectively (Table 2, entries 14 and 15). The absolute configuration of 3m has been assigned using X-ray diffraction as 1S, 5R. The ORTEP drawing and the CIF data are summarized in the ESI.†19
| Entrya | 1 (R1) | 2 (R2) | Yieldb (%) | r.r.c | ee (%)d |
|---|---|---|---|---|---|
| a The reactions were carried out with 1 (0.1 mmol), 2a (0.15 mmol), CP5 (0.01 mmol) and 4 Å MS (30 mg) in DCM (0.5 mL) and toluene (0.5 mL) at rt for 12 h. Unless otherwise mentioned, the compounds 1 were E-isomers. b Isolated yield using column chromatography. c Regioselectivity ratios determined using crude 1H NMR spectroscopy; r.r. = regioselectivity ratio. d Determined using chiral HPLC analysis. e The absolute configuration of 3m has been determined using X-ray diffraction as (1S, 5R). f Compound 1n was the mixture of Z and E isomers, Z/E = 1/1 based on 1H NMR analysis. | |||||
| 1 | 1b (4-BrC6H4) | 2a (OBn) | 3b: 92 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95 |
| 2 | 1c (4-CH3C6H4) | 2a (OBn) | 3c: 76 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
91 |
| 3 | 1d (4-CH3OC6H4) | 2a (OBn) | 3d: 72 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
96 |
| 4 | 1e (4-CF3C6H4) | 2a (OBn) | 3e: 87 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
91 |
| 5 | 1f (4-FC6H4) | 2a (OBn) | 3f: 67 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
94 |
| 6 | 1g (4-CNC6H4) | 2a (OBn) | 3g: 57 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
87 |
| 7 | 1h (3,4-Cl2C6H3) | 2a (OBn) | 3h: 82 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
90 |
| 8 | 1i (C6H5) | 2a (OBn) | 3i: 79 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
94 |
| 9 | 1j (4-PhC6H4) | 2a (OBn) | 3j: 76 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96 |
| 10 | 1k (2-furyl) | 2a (OBn) | 3k: 48 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
96 |
| 11 | 1l (2-thienyl) | 2a (OBn) | 3l: 67 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
93 |
| 12e | 1m (1-naphthyl) | 2a (OBn) | 3m: 99 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
99 |
| 13 | 1n (cyclohexyl)f | 2a (OBn) | 3n: 68 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
95 |
| 14 | 1a (2-BrC6H4) | 2b (OEt) | 3o: 94 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 15 | 1a (2-BrC6H4) | 2c (Me) | 3p: 83 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96 |
We next attempted to examine the asymmetric α-addition [3 + 2] cycloaddition reactions of the benzofuranones 1 and the γ-substituted allenoates 4 (Table 3). As for substrate 1b, product 5b was obtained in 91% yield, along with 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 r.r. and an 85% ee value (Table 3, entry 2). For these substrates with electron-rich substituents on their aromatic rings, spiro-cycloadducts 5c–5d were obtained in relatively moderate yields but with high ee values and regioselectivities (Table 3, entries 3–4). The substrates 1e–1m with various electron-poor substituents on their aromatic rings were more suitable for this reaction, affording the corresponding cycloadducts in good yields with 91%–99% ee values and 92
16 r.r. and an 85% ee value (Table 3, entry 2). For these substrates with electron-rich substituents on their aromatic rings, spiro-cycloadducts 5c–5d were obtained in relatively moderate yields but with high ee values and regioselectivities (Table 3, entries 3–4). The substrates 1e–1m with various electron-poor substituents on their aromatic rings were more suitable for this reaction, affording the corresponding cycloadducts in good yields with 91%–99% ee values and 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 to >99
8 to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 regioselectivity ratios (Table 3, entries 5–12). The aliphatic group is also suitable for this reaction (Table 3, entry 13). Some other allenic esters such as ethyl-, tert-butyl 4-phenylbuta-2,3-dienoates or benzyl penta-2,3-dienoate are also suitable for this asymmetric [3 + 2] cycloaddition, giving the corresponding products in 67–83% yields with 90–97% ee values and 95
1 regioselectivity ratios (Table 3, entries 5–12). The aliphatic group is also suitable for this reaction (Table 3, entry 13). Some other allenic esters such as ethyl-, tert-butyl 4-phenylbuta-2,3-dienoates or benzyl penta-2,3-dienoate are also suitable for this asymmetric [3 + 2] cycloaddition, giving the corresponding products in 67–83% yields with 90–97% ee values and 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to >99
5 to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 regioselectivities (Table 3, entries 14–16). The absolute configuration of 5j has been assigned using X-ray diffraction as 1R, 4R, 5R. The ORTEP drawing and the CIF data are summarized in the ESI.†19
1 regioselectivities (Table 3, entries 14–16). The absolute configuration of 5j has been assigned using X-ray diffraction as 1R, 4R, 5R. The ORTEP drawing and the CIF data are summarized in the ESI.†19
| Entrya | 1 (R1) | 4 (R2/R3) | Yieldb (%) | r.r.c | eed (%) |
|---|---|---|---|---|---|
| a The reactions were carried out with 1a (0.1 mmol), 2a (0.12 mmol), and CP5 (0.01 mmol) in toluene (1.0 mL) at rt for 24 h. Unless otherwise mentioned, the compounds 1 were E-isomers. b Isolated yield using column chromatography. c Regioselectivity ratios determined using crude 1H NMR spectroscopy; r.r. = regioselectivity ratios. d Determined using chiral HPLC analysis. e The absolute configuration of 5j has been determined using X-ray diffraction as (1R, 4R, 5R). f Compound 1n was a mixture of Z and E isomers, Z/E = 1/1 based on 1H NMR analysis. | |||||
| 1 | 1a (2-BrC6H4) | 4a (Bn/Ph) | 5a: 96 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95 |
| 2 | 1b (4-BrC6H4) | 4a (Bn/Ph) | 5b: 91 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
85 |
| 3 | 1c (4-CH3C6H4) | 4a (Bn/Ph) | 5c: 72 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
99 |
| 4 | 1d (4-CH3OC6H4) | 4a (Bn/Ph) | 5d: 68 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
96 |
| 5 | 1e (4-F3C6H4) | 4a (Bn/Ph) | 5e: 92 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92 |
| 6 | 1f (4-FC6H4) | 4a (Bn/Ph) | 5f: 78 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
99 |
| 7 | 1g (4-CNC6H4) | 4a (Bn/Ph) | 5g: 75 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 8 | 1h (3,4-Cl2C6H3) | 4a (Bn/Ph) | 5h: 82 | >92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
99 |
| 9 | 1i (C6H5) | 4a (Bn/Ph) | 5i: 86 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 10e | 1j (4-PhC6H4) | 4a (Bn/Ph) | 5j: 83 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 11 | 1k (2-furyl) | 4a (Bn/Ph) | 5k: 77 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 12 | 1m (1-naphthyl) | 4a (Bt/Ph) | 5l: 73 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 |
| 13 | 1n (cyclohexyl)f | 4a (Bt/Ph) | 5m: 92 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 |
| 14 | 1c (4-CH3C6H4) | 4b (Et/Ph) | 5n: 67 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
90 |
| 15 | 1c (4-CH3C6H4) | 4c (tBu/Ph) | 5o: 83 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
97 |
| 16 | 1c (4-CH3C6H4) | 4d (Bn/Me) | 5p: 62 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
94 |
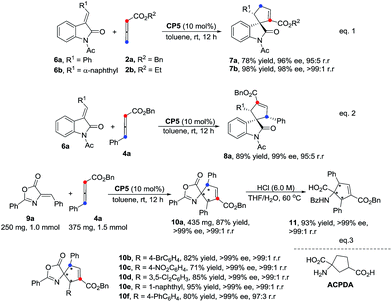
It is noteworthy that this catalytic system can also be applied in the regioselective construction of spiroindolines5h,8a,15 in good yields, with high ee values and high regioselectivities (Scheme 4, eqn (1) and eqn (2)). The γ-addition [3 + 2] cycloadducts 7a and 7b were obtained in 78% and 98% yields, 96% and 98% ee values and 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and >99
5 and >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 r.r., respectively. The α-addition [3 + 2] cycloadduct 8a was formed in 89% yield, 99% ee value and 95
1 r.r., respectively. The α-addition [3 + 2] cycloadduct 8a was formed in 89% yield, 99% ee value and 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 r.r. The enantioselective approach for the construction of spirocyclic oxindolic cyclopentanes based on a phosphine-mediated γ-addition has been reported by Marinetti's group.5h Furthermore, the preparations of carbocyclic amino acids have received great attention in medicinal chemistry recently due to their unique biological activities.13e,16 As illustrated in Scheme 4 (eqn (3)), the spiro-cycloadduct 10a was obtained in 87% yield with a >99% ee value and a high regioselectivity using alkylidene azlactone 9a (1.0 mmol) and the substituted allenoate 4a (1.5 mmol) as the substrates. The reactions of other substrates with different aromatic rings also proceeded smoothly, affording the corresponding cycloadducts 10b–10f in good yields with high ee values (>99% ee) and excellent regioselectivities. The ring-opened α-amino acid product 11 was easily obtained via treatment with 6 M HCl in high yield without the ee value diminishing (Scheme 4, eqn(3)).
5 r.r. The enantioselective approach for the construction of spirocyclic oxindolic cyclopentanes based on a phosphine-mediated γ-addition has been reported by Marinetti's group.5h Furthermore, the preparations of carbocyclic amino acids have received great attention in medicinal chemistry recently due to their unique biological activities.13e,16 As illustrated in Scheme 4 (eqn (3)), the spiro-cycloadduct 10a was obtained in 87% yield with a >99% ee value and a high regioselectivity using alkylidene azlactone 9a (1.0 mmol) and the substituted allenoate 4a (1.5 mmol) as the substrates. The reactions of other substrates with different aromatic rings also proceeded smoothly, affording the corresponding cycloadducts 10b–10f in good yields with high ee values (>99% ee) and excellent regioselectivities. The ring-opened α-amino acid product 11 was easily obtained via treatment with 6 M HCl in high yield without the ee value diminishing (Scheme 4, eqn(3)).
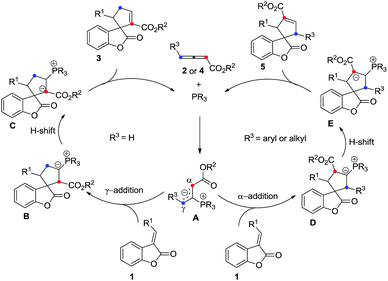
The plausible mechanisms for this phosphine-catalyzed [3 + 2] cycloaddition have been proposed in Scheme 5 on the basis of our experiments and previous literature.1,2 The reaction starts from the formation of a zwitterionic intermediate A between the allenoate (2 or 4) and phosphine. Intermediate A acts as a 1,3-dipole and undergoes a [3 + 2] cycloaddition with benzofuranone 1 to give a phosphrous ylide Bvia γ-addition or Dvia α-addition. For allenoate 2 (R3 = H), γ-addition is the main pathway. In contrast, allenoate 4 (R3 = aryl or alkyl group) mainly undergoes α-addition. Then, an intramolecular1,2 proton transfer is speculated to convert the phosphorus ylide B or D to another zwitterionic intermediate C or E, which, upon elimination of the phosphine catalyst, gives rise to the final cycloadduct 3 or 5.
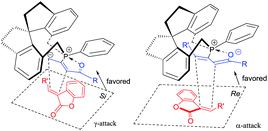
The possible transition state of this asymmetric [3 + 2] cycloaddition is illustrated in Scheme 6 and may account for the stereochemical outcomes. The zwitterionic intermediate2s,17 derived from the chiral phosphine and allenoate could approach the benzofuranone 1 through either the Re face or Si face. Presumably, due to steric reasons, the zwitterionic intermediate (R3 = H) is more favored to attack the benzofuranone 1 from the Si face to give the corresponding product (Scheme 6, left), however, the zwitterionic intermediate (R3 = Ph or Me) is more favored to attack the benzofuranone 1 from the Re face to afford the corresponding product (Scheme 6, right).
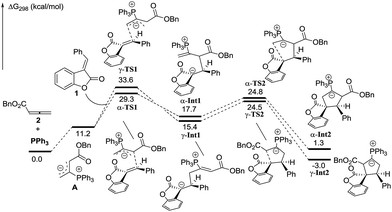
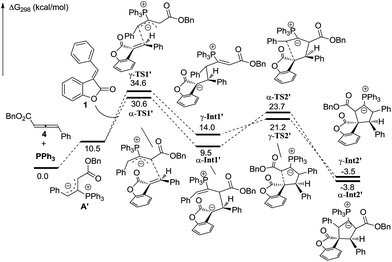
In order to understand the regiochemical outcome of this reaction, we have done theoretical investigations on this [3 + 2] cycloaddition. All calculations have been performed at the mPW1K/6-31G(d) level with the Gaussian 09 program (see the ESI†). The calculation results indicated that the cycloaddition process is stepwise, which agrees with the previous theoretical studies by Yu’s group.17 For allenoate 2 (R3 = H), two intermediates, γ-INT1 and γ-INT2, in the γ-addition mode are thermodynamically more favorable than those intermediates in the α-addition mode, which may account for why the γ-addition adducts were experimentally obtained as the major products. In contrast, using allenoate 4 (R3 = Ph) as a substrate, the energies of the intermediates γ-INT1′ and γ-INT2′ in the γ-addition mode are higher than those of α-INT1′ and α-INT2′ in the α-addition mode, probably due to the steric hindrance between the R3 substituents and benzofuranone in the intermediates γ-INT1′ and γ-INT2′. Thus, the α-addition mode is more favorable in this case (see Schemes 7 and 8). All of these DFT calculations have been summarized in the ESI.†
In summary, we have reported the first example of the successful asymmetric and regioselective construction of 3,3’-spirocyclopentenebenzofuranones catalyzed by a chiral phosphine (R-SITCP) by employing benzofuranone and two types of allenic esters. Under the present catalytic system, γ-addition products and α-addition products can be obtained in 48–99% yields with 87–99% ee values and 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 to >19
12 to >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 regioselectivity ratios and in 62–96% yields with 85–99% ee values and 84
1 regioselectivity ratios and in 62–96% yields with 85–99% ee values and 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 to >19
16 to >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 regioselectivity ratios, respectively. Moreover, this catalytic asymmetric [3 + 2] system can be also applied in the regioselective construction of spiro-oxindoles 7 and 8 as well as spiro-azlactone 10 which can be easily transformed to aspartic acid analogues.18 The DFT studies disclosed the origins of the regioselective outcomes for this phosphine-catalyzed [3 + 2] reaction. Further application of this type of reaction for the synthesis of more natural and natural-like spiro-compounds is ongoing.
1 regioselectivity ratios, respectively. Moreover, this catalytic asymmetric [3 + 2] system can be also applied in the regioselective construction of spiro-oxindoles 7 and 8 as well as spiro-azlactone 10 which can be easily transformed to aspartic acid analogues.18 The DFT studies disclosed the origins of the regioselective outcomes for this phosphine-catalyzed [3 + 2] reaction. Further application of this type of reaction for the synthesis of more natural and natural-like spiro-compounds is ongoing.
Acknowledgements
We are grateful for the financial support from the National Basic Research Program of China (973)-2015CB856603-2015CB856603, and the National Natural Science Foundation of China (20472096, 21372241, 21361140350, 20672127, 21421091, 21372250, 21121062, 21302203, 20732008, and 21572052).Notes and references
- C. Zhang and X. Lu, J. Org. Chem., 1995, 60, 2906 CrossRef CAS.
- (a) P. I. Dalko, Comprehensive Enantioselective Organocatalysis: Catalysts, Reactions, and Applications, Wiley-VCH, 2013, For reviews on phosphine-catalyzed reactions, see: Search PubMed; (b) X. Lu, C. Zhang and Z. Xu, Acc. Chem. Res., 2001, 34, 535 CrossRef CAS PubMed; (c) J. L. Methot and W. R. Roush, Adv. Synth. Catal., 2004, 346, 1035 CrossRef CAS PubMed; (d) L.-W. Ye, J. Zhou and Y. Tang, Chem. Soc. Rev., 2008, 37, 1140 RSC; (e) B. J. Cowen and S. J. Miller, Chem. Soc. Rev., 2009, 38, 3102 RSC; (f) A. Marinetti and A. Voituriez, Synlett, 2010, 174 CrossRef CAS; (g) Y. Wei and M. Shi, Acc. Chem. Res., 2010, 43, 1005 CrossRef CAS PubMed; (h) S.-X. Wang, X. Han, F. Zhong and Y. Lu, Synlett, 2011, 19, 2766 Search PubMed; (i) Q.-Y. Zhao, Z. Lian, Y. Wei and M. Shi, Chem. Commun., 2012, 48, 1724 RSC; (j) C. Nising and S. Bräse, Chem. Soc. Rev., 2008, 37, 1218 RSC; (k) Y.-C. Fan and O. Kwon, Chem. Commun., 2013, 49, 11588 RSC; (l) Z. Wang, X. Xu and O. Kwon, Chem. Soc. Rev., 2014, 43, 2927 RSC, Selected papers on the phosphine-catalyzed cyclization of allenoates: (m) G.-L. Zhao and M. Shi, J. Org. Chem., 2005, 70, 9975 CrossRef CAS PubMed; (n) J. L. García Ruano, A. Núñez, Jr., M. R. Martín and A. Fraile, J. Org. Chem., 2008, 73, 9366 CrossRef PubMed; (o) C. E. Henry and O. Kwon, Org. Lett., 2007, 9, 3069 CrossRef CAS PubMed; (p) S. G. Pyne, K. Schafer, B. W. Skelton and A. H. White, Chem. Commun., 1997, 33, 2267 RSC; (q) S. Xu, L. Zhou, R. Ma, H. Song and Z. He, Chem.–Eur. J., 2009, 15, 8698 CrossRef CAS PubMed; (r) X.-F. Zhu, C. E. Henry, J. Wang, T. Dudding and O. Kwon, Org. Lett., 2005, 7, 387 CrossRef PubMed; (s) X.-F. Zhu, A.-P. Schaffner, R. C. Li and O. Kwon, Org. Lett., 2005, 7, 2977 CrossRef CAS PubMed; (t) T. Dudding, O. Kwon and E. Mercier, Org. Lett., 2006, 8, 3643 CrossRef CAS PubMed; (u) G. S. Creech and O. Kwon, Org. Lett., 2008, 10, 429 CrossRef CAS PubMed; (v) X.-F. Zhu, C.-E. Henry and O. Kwon, J. Am. Chem. Soc., 2007, 129, 6722 CrossRef CAS PubMed; (w) Y. S. Tran and O. Kwon, J. Am. Chem. Soc., 2007, 129, 12632 CrossRef CAS PubMed; (x) R. Na, C. Jing, Q. Xu, H. Jiang, X. Wu, J. Shi, J. Zhong, M. Wang, D. Benitez, E. Tkatchouk, W. A. Goddard III, H. Guo and O. Kwon, J. Am. Chem. Soc., 2011, 133, 13337 CrossRef CAS PubMed.
- G. Zhu, Z. Chen, Q. Jiang, D. Xiao, P. Cao and X. Zhang, J. Am. Chem. Soc., 1997, 119, 3836 CrossRef CAS.
- (a) J. E. Wilson and G. C. Fu, Angew. Chem., Int. Ed., 2006, 45, 1426 CrossRef CAS PubMed; (b) Y. K. Chung and G. C. Fu, Angew. Chem., Int. Ed., 2009, 48, 2225 CrossRef CAS PubMed; (c) R. P. Wurz and G. C. Fu, J. Am. Chem. Soc., 2005, 127, 12234 CrossRef CAS PubMed; (d) Y. Fujiwara and G. C. Fu, J. Am. Chem. Soc., 2011, 133, 12293 CrossRef CAS PubMed; (e) S. Y. Lee, Y. Fujiwara, A. Nishiguchi, M. Kalek and G. C. Fu, J. Am. Chem. Soc., 2015, 137, 4587 CrossRef CAS PubMed; (f) S. Kramer and G. C. Fu, J. Am. Chem. Soc., 2015, 137, 3803 CrossRef CAS PubMed.
- (a) L. Jean and A. Marinetti, Tetrahedron Lett., 2006, 47, 2141 CrossRef CAS PubMed; (b) N. Fleury-Brégeot, L. Jean, P. Retailleau and A. Marinetti, Tetrahedron, 2007, 63, 11920 CrossRef PubMed; (c) A. Panossian, N. Fleury-Brégeot and A. Marinetti, Eur. J. Org. Chem., 2008, 3826 CrossRef CAS PubMed; (d) N. Pinto, N. Fleury-Brégeot and A. Marinetti, Eur. J. Org. Chem., 2009, 146 CAS; (e) A. Voituriez, A. Panossian, N. Fleury-Brégeot, P. Retailleau and A. Marinetti, J. Am. Chem. Soc., 2008, 130, 14030 CrossRef CAS PubMed; (f) A. Voituriez, A. Panossian, N. Fleury-Brégeot, P. Retailleau and A. Marinetti, Adv. Synth. Catal., 2009, 351, 1968 CrossRef CAS PubMed; (g) N. Pinto, M. Neel, A. Panossian, P. Retailleau, G. Frison, A. Voituriez and A. Marinetti, Chem.–Eur. J., 2010, 16, 1033 CrossRef CAS PubMed; (h) A. Voituriez, N. Pinto, M. Neel, P. Retailleau and A. Marinetti, Chem.–Eur. J., 2010, 16, 12541 CrossRef CAS PubMed; (i) M. Schuler, A. Voituriez and A. Marinetti, Tetrahedron: Asymmetry, 2010, 21, 1569 CrossRef CAS PubMed; (j) N. Pinto, P. Retailleau, A. Voituriez and A. Marinetti, Chem. Commun., 2011, 47, 1015 RSC; (k) M. Neel, J. Gouin, A. Voituriez and A. Marinetti, Synthesis, 2011, 12, 2003 Search PubMed; (l) D. Duvvuru, N. Pinto, C. Gomez, J.-F. Betzer, P. Retailleau, A. Voituriez and A. Marinetti, Adv. Synth. Catal., 2012, 354, 408 CrossRef CAS PubMed; (m) M. Gicquel, Y. Zhang, P. Aillard, P. Retailleau, A. Voituriez and A. Marinetti, Angew. Chem., Int. Ed., 2015, 54, 5470 CrossRef CAS PubMed.
- Selected papers on the chiral amino acid derived phosphine catalyzed asymmetric cyclization reactions: (a) B. J. Cowen and S. J. Miller, J. Am. Chem. Soc., 2007, 129, 10988 CrossRef CAS PubMed; (b) Y.-Q. Fang and E. N. Jacobsen, J. Am. Chem. Soc., 2008, 130, 5660 CrossRef CAS PubMed; (c) H. Xiao, Z. Chai, C.-W. Zheng, Y.-Q. Yang, W. Liu, J.-K. Zhang and G. Zhao, Angew. Chem., Int. Ed., 2010, 49, 4467 CrossRef CAS PubMed; (d) X. Han, Y. Wang, F. Zhong and Y. Lu, J. Am. Chem. Soc., 2011, 133, 1726 CrossRef CAS PubMed; (e) X. Han, S.-X. Wang, F. Zhong and Y. Lu, Synthesis, 2011, 12, 1859 Search PubMed; (f) F. Zhong, X. Han, Y. Wang and Y. Lu, Angew. Chem., Int. Ed., 2011, 50, 7837 CrossRef CAS PubMed; (g) F. Zhong, Y. Wang, X. Han, K.-W. Huang and Y. Lu, Org. Lett., 2011, 13, 1310 CrossRef CAS PubMed; (h) X. Han, Y. Wang, F. Zhong and Y. Lu, Org. Biomol. Chem., 2011, 9, 6734 RSC; (i) X. Han, F. Zhong, Y. Wang and Y. Lu, Angew. Chem., Int. Ed., 2012, 51, 767 CrossRef CAS PubMed; (j) Q.-Y. Zhao, X. Han, Y. Wei, M. Shi and Y. Lu, Chem. Commun., 2012, 48, 970 RSC; (k) F. Zhong, X. Han, Y. Wang and Y. Lu, Chem. Sci., 2012, 3, 1231 RSC; (l) F. Zhong, J. Luo, G.-Y. Chen, X. Dou and Y. Lu, J. Am. Chem. Soc., 2012, 134, 10222 CrossRef CAS PubMed; (m) F. Zhong, G.-Y. Chen, X. Han, W. Yao and Y. Lu, Org. Lett., 2012, 14, 3764 CrossRef CAS PubMed; (n) F. Zhong, X. Dou, X. Han, W. Yao, Q. Zhu, Y. Meng and Y. Lu, Angew. Chem., Int. Ed., 2013, 52, 943 CrossRef CAS PubMed; (o) R. Lee, F. Zhong, B. Zhen, Y. Meng, Y. Lu and K.-W. Huang, Org. Biomol. Chem., 2013, 11, 4818 RSC; (p) T. Wang, W. Yao, F. Zhong, G. H. Pang and Y. Lu, Angew. Chem., Int. Ed., 2014, 53, 2964 CrossRef CAS PubMed; (q) X. Han, W. Yao, T. Wang, Y. R. Tan, Z. Yan, J. Kwiatkowski and Y. Lu, Angew. Chem., Int. Ed., 2014, 53, 5643 CrossRef CAS PubMed; (r) W. Yao, X. Dou and Y. Lu, J. Am. Chem. Soc., 2015, 137, 54 CrossRef CAS PubMed; (s) T. Wang, D. L. Hoon and Y. Lu, Chem. Commun., 2015, 51, 10186 RSC; (t) C. E. Henry, Q. Xu, Y. C. Fan, T. J. Martin, L. Belding, T. Dudding and O. Kwon, J. Am. Chem. Soc., 2014, 136, 11890 CrossRef CAS PubMed.
- (a) D. J. Wallace, R. L. Sidda and R. A. Reamer, J. Org. Chem., 2007, 72, 1051 CrossRef CAS PubMed; (b) M. Sampath and T.-P. Loh, Chem. Commun., 2009, 45, 1568 RSC; (c) M. Sampath and T.-P. Loh, Chem. Sci., 2010, 1, 739 RSC.
- (a) C. Gomez, M. Gicquel, J.-C. Carry, L. Schio, P. Retailleau, A. Voituriez and A. Marinetti, J. Org. Chem., 2013, 78, 1488 CrossRef CAS PubMed; (b) S. S. Bruna and M. V. D. P. Tetesa, Eur. J. Org. Chem., 2013, 3901 Search PubMed.
- Selected examples: (a) N. Lindquist, W. Fenical, G. D. van Duyne and J. Clardy, J. Am. Chem. Soc., 1991, 113, 2303 CrossRef CAS; (b) B. Sontag, M. Rüth, P. Spiteller, N. Arnold, W. Steglich, M. Reichert and G. Bringmann, Eur. J. Org. Chem., 2006, 1023 CrossRef CAS PubMed; (c) Y.-J. Kwon, M.-J. Sohn, C.-J. Zheng and W.-G. Kim, Org. Lett., 2007, 9, 2449 CrossRef CAS PubMed; (d) H. M. Ge, C. H. Zhu, D. H. Shi, L. D. Zhang, D. Q. Xie, J. Yang, S. W. Ng and R. X. Tan, Chem.–Eur. J., 2008, 14, 376 CrossRef CAS PubMed; (e) K. C. Nicolaou, Q. Kang, T. R. Wu, C. S. Lim and D. Y.-K. Chen, J. Am. Chem. Soc., 2010, 132, 7540 CrossRef CAS PubMed.
- (a) S. A. Adediran, D. Vabaret, B. Drouillat, R. F. Pratt and M. Wakselman, Bioorg. Med. Chem., 2001, 9, 1175 CrossRef CAS; (b) E. K. Panisheva, L. M. Alekseeva, M. I. Evstratova, S. S. Kiselev and V. G. Granik, Pharm. Chem. J., 2007, 41, 549 CrossRef CAS.
- For the construction of the chiral quaternary center of benzofuranones: (a) X. Li, Z. G. Xi, S. Z. Luo and J.-P. Cheng, Adv. Synth. Catal., 2010, 352, 1097 CrossRef CAS PubMed; (b) X. Li, S. S. Hu, Z. G. Xi, L. Zhang, S. Z. Luo and J.-P. Cheng, J. Org. Chem., 2010, 75, 8697 CrossRef CAS PubMed; (c) C.-L. Zhu, F.-G. Zhang, W. Meng, J. Nie, D. Cahard and J.-A. Ma, Angew. Chem., Int. Ed., 2011, 50, 5869 CrossRef CAS PubMed; (d) X. Li, Y.-Y. Zhang, X.-S. Xue, J.-L. Jin, B.-X. Tan, C. Liu, N. Dong and J.-P. Cheng, Eur. J. Org. Chem., 2012, 1774 CrossRef CAS PubMed; (e) C. Liu, B.-X. Tan, J.-L. Jin, Y.-Y. Zhang, N. Dong, X. Lin and J.-P. Cheng, J. Org. Chem., 2011, 76, 5838 CrossRef CAS PubMed; (f) E. Vedejs and J. Wang, Org. Lett., 2000, 2, 1031 CrossRef CAS; (g) I. D. Hills and G. C. Fu, Angew. Chem., Int. Ed., 2003, 42, 3921 CrossRef CAS PubMed; (h) S. A. Shaw, P. Aleman and E. Vedejs, J. Am. Chem. Soc., 2003, 125, 13368 CrossRef CAS PubMed; (i) S. A. Shaw, P. Aleman, J. Christy, J. W. Kampf, P. Va and E. Vedejs, J. Am. Chem. Soc., 2006, 128, 925 CrossRef CAS PubMed; (j) B. M. Trost, N. Cramer and S. M. Silverman, J. Am. Chem. Soc., 2007, 129, 12396 CrossRef CAS PubMed; (k) G. Bergonzini and P. Melchiorre, Angew. Chem., Int. Ed., 2012, 51, 971 CrossRef CAS PubMed; (l) K. Ohmatsu, M. Ito, T. Kunieda and T. Ooi, J. Am. Chem. Soc., 2013, 135, 590 CrossRef CAS PubMed; (m) X.-F. Cheng, Y. Li, Y.-M. Su, F. Yin, J.-Y. Wang, J. Sheng, H. U. Vora, X.-S. Wang and J.-Q. Yu, J. Am. Chem. Soc., 2013, 135, 1236 CrossRef CAS PubMed.
- For the construction of the chiral spiro quaternary center of benzofuranones: (a) X. Companyό, A. Zea, A. N. R. ACPa, A. Mazzanti, A. Moyano and R. Rios, Chem. Commun., 2010, 46, 6953 RSC; (b) C. Cassani, X. Tian, E. C. Escudero-Adan and P. Melchiorre, Chem. Commun., 2011, 47, 233 RSC; (c) X. Li, F. Wang, N. Dong and J.-P. Cheng, Org. Biomol. Chem., 2013, 11, 1451 RSC; (d) X. Li, C. Yang, J. L. Jin, X. S. Xue and J.-P. Cheng, Chem.–Asian. J., 2013, 8, 997 CrossRef CAS PubMed.
- (a) G.-L. Zhao, J.-W. Huang and M. Shi, Org. Lett., 2003, 5, 4737 CrossRef CAS PubMed; (b) Y.-L. Shi and M. Shi, Org. Lett., 2005, 7, 3057 CrossRef CAS PubMed; (c) X.-C. Zhang, S.-H. Cao, Y. Wei and M. Shi, Chem. Commun., 2011, 47, 1548 RSC; (d) X.-C. Zhang, S.-H. Cao, Y. Wei and M. Shi, Org. Lett., 2011, 13, 1142 CrossRef CAS PubMed; (e) D. Wang, Y. Wei and M. Shi, Chem. Commun., 2012, 48, 2764 RSC; (f) Q.-Y. Zhao, C.-K. Pei, X.-Y. Guan and M. Shi, Adv. Synth. Catal., 2011, 353, 1973 CrossRef CAS PubMed; (g) C.-K. Pei, Y. Jiang, Y. Wei and M. Shi, Angew. Chem., Int. Ed., 2012, 51, 11328 CrossRef CAS PubMed.
- (a) S.-F. Zhu, Y. Yang, L.-X. Wang, B. Liu and Q.-L. Zhou, Org. Lett., 2005, 7, 2333 CrossRef CAS PubMed; (b) J.-H. Xie and Q.-L. Zhou, Acc. Chem. Res., 2008, 41, 581 CrossRef CAS PubMed.
- M. Gicquel, C. Gomez, P. Retailleau, A. Voituriez and A. Marinetti, Org. Lett., 2013, 15, 4002 CrossRef CAS PubMed.
- For selected examples see:
(a) B. M. Trost and P. J. Morris, Angew. Chem., Int. Ed., 2011, 50, 6167 CrossRef CAS PubMed;
(b) D. Uraguchi, K. Yoshika, Y. Ueki and T. Ooi, J. Am. Chem. Soc., 2012, 134, 19370 CrossRef CAS PubMed As for alkylidene azlactone, the γ-attack that initiated the asymmetric [3 + 2] cycloaddition with CP5 has been reported before (see ref. 13e).
. - For mechanistic investigations, see: (a) Y. Liang, S. Liu, Y. Xia, Y. Li and Z.-X. Yu, Chem.–Eur. J., 2008, 14, 4361 CrossRef CAS PubMed; (b) Y. Xia, Y. Liang, Y. Chen, M. Wang, L. Jiao, F. Huang, S. Liu, Y. Li and Z.-X. Yu, J. Am. Chem. Soc., 2007, 129, 3470 CrossRef CAS PubMed; (c) E. Mercier, B. Fonovic, C. Henry, O. Kwon and T. Dudding, Tetrahedron Lett., 2007, 48, 3617 CrossRef CAS PubMed; (d) Y. Liang, S. Liu and Z.-X. Yu, Synlett, 2009, 905 CAS; (e) W. Meng, H.-T. Zhao, J. Nie, Y. Zheng, A. Fu and J.-A. Ma, Chem. Sci., 2012, 3, 3053 RSC; (f) T. Wang, Z. Yu, D. L. Hoon, K.-W. Huang, Y. Lan and Y. Lu, Chem. Sci., 2015, 6, 4912 RSC; (g) G.-T. Huang, T. Lankau and C.-H. Yu, J. Org. Chem., 2014, 79, 1700 CrossRef CAS PubMed; (h) G.-T. Huang, T. Lankau and C.-H. Yu, Org. Biomol. Chem., 2014, 12, 7297 RSC.
- (a) H. O. Bertrand, A. S. Bessis, J. P. Pin and F. C. Acher, J. Med. Chem., 2002, 45, 3171 CrossRef CAS PubMed; (b) V. Jayaraman, R. Keesey and D. R. Madden, Biochemistry, 2000, 39, 8693 CrossRef CAS PubMed.
- ESI†.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data of new compounds. CCDC 961159, 967550 and 1010550. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc03135d |
| This journal is © The Royal Society of Chemistry 2015 |