Synthesis of conjugated microporous polymer nanotubes with large surface areas as absorbents for iodine and CO2 uptake†
Yingfan
Chen
a,
Hanxue
Sun
a,
Ruixia
Yang
b,
Tingting
Wang
b,
Chunjuan
Pei
a,
Zhentao
Xiang
a,
Zhaoqi
Zhu
a,
Weidong
Liang
a,
An
Li
*a and
Weiqiao
Deng
*b
aCollege of Petrochemical Technology, Lanzhou University of Technology, Lanzhou 730050, P. R. China. E-mail: lian2010@lut.cn; Tel: +86-931-5-2973305
bState Key Laboratory of Molecular Reaction Dynamics, Dalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, P. R. China. E-mail: dengwq@dicp.ac.cn
First published on 6th November 2014
Abstract
Conjugated microporous polymer nanotubes (CMPNs) with a surface area of up to 1368 m2 g−1 were synthesized by a simple one-step crosscoupling reaction and employed as a platform for investigation of CO2 and I2 adsorption. A high adsorption capacity of up to 208 wt% for reversible I2 capture was achieved.
Conjugated microporous polymers (CMPs) are synthesized using metal-catalysed crosscoupling chemistry to form networks with extended π-conjugation and have been the subject of much interest in a wide range of applications including gas storage,1–3 hydrogen production,4 catalysis,5–8 supercapacitors,9 light harvesting,10 absorption, separation11 and so on. Compared with conventional porous materials, the porous characters of CMPs could be finely tuned by varying the strut length of the monomers in the CMP network which have been well-studied by Cooper et al.12 In addition, previous studies have shown that the choice of polymerization solvents has an apparent influence on the surface area and pore volume of the resulting CMPs.13 In our previous studies, we found that, in addition to the surface area and pore volume,14 the morphology of CMPs is also affected greatly by the structure of monomers as well as reaction solvents.15 By employment of suitable monomers and polymerization solvents, CMPs with film or nanotube-like morphology can be obtained along this line, in which we suggested that the 2D planar structures formed strongly depended on the polymerization orientation of the phenyl alkyne monomers, and tended to roll up or closely connected to form a 1D cylindrical geometry.15,16 In fact, such nanotube-like CMPs have been previously reported by Müllen et al.17 These findings, however, open a new possibility for synthesis of nanotube-like CMPs via a simple one-step solution polymerization. Compared with those amorphous CMP 3D networks usually formed as unprocessable powders, such a cylindrical geometry of CMPs potentially has a high design flexibility in functionalization, thereby giving an opportunity for the creation of low-dimensional soft materials for specific applications.
So far, only a few examples of CMP nanotubes with low surface areas (<400 m2 g−1) have been reported,15–17 mainly because of the difficulty in seeking suitable monomers and reaction solvents to address much more rigorous requisites for the polymerization orientation of the monomer for construction of such a hollow cylindrical geometry. Therefore, further exploitation of new CMP nanotubes with excellent porous character would be of special interest. In this regard, we report here the synthesis of porous conjugated microporous polymer nanotube (CMPN) materials using Pd(0)/Cu(I)-catalyzed homocoupling polymerization. CMPNs with surface areas of up to 1368 m2 g−1 were obtained. Using the CMPN samples as porous medium, the relationship between the porous properties of the CMPN samples and their uptake capacities for CO2 and I2 capture was investigated. We suggested that the findings from this study may provide guidance for rational design and development of functional CMPs with cylindrical geometry in the future, which would be advantageous for further expanding their application aspects.
In this work, three kinds of CMPNs were synthesized by the palladium-catalyzed Sonogashira–Hagihara crosscoupling reaction according to the literature18 (ESI†) and their structures are shown in Scheme 1. CMPN-1 was synthesized from 1,3,5-tris(4-bromophenyl) benzene with 1,4-diethynylbenzene, which shows a similar net structure to CMPN-2 obtained from 9,10-dibromoanthracene with 1,3,5-triethynylbenzene. It should be noted that CMP-3 (ref. 12) and network 3 (A3 + B2)13 reported by Cooper et al. were synthesized with similar structure nodes to CMPN-1 and CMPN-2, respectively. However, the tubular-like morphology of the resulting CMPN-1 and CMPN-2 is totally different from the amorphous powder appearance of CMP-3 (ref. 12) and network 3 (ref. 13), which were synthesized using different monomers as well as polymerization solvents. The results indicate that the structure of monomers as well as reaction solvents plays an important role in the morphology of the resulting CMP products, which are in good agreement with previous studies.15,16 CMPN-3 was produced using 9,10-dibromoanthracene and 1,4-diethylenzene by employing the same synthesis conditions, showing a linear structure composed of 9,10-substituted anthracene connected by a benzene moiety, which was confirmed by FTIR (Fig. S1†) and solid-state 13C CP/MAS NMR spectra (Fig. S2†).
All three CMPN samples show stable chemical properties and are insoluble in common organic solvents. Thermogravimetric analysis (TGA) shows that three samples have a decomposition temperature over 350 °C, whereas CMPN-3 has the most thermal stability with a temperature over 400 °C (Fig. S3†). The scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images of three CMPN samples are shown in Fig. 1. It is clear that all samples show a nanotube-like morphology. The tips of CMPN-1 are open and it exhibits a smooth and neat tubular surface with ca. 150 nm in diameter and ca. 20 μm in length (Fig. 1a and b). In contrast, both CMPN-2 and CMPN-3 show hollow and hierarchical porous surface morphologies with honeycomb-like pores in tube walls (Fig. 1c–f). To the best of our knowledge, such a cylindrical geometry with hierarchical porous feature of the CMPN-2 and CMPN-3 samples has never been reported.
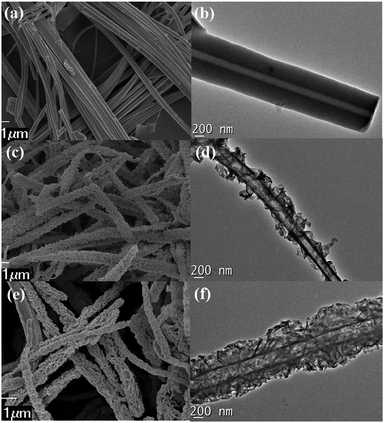 | ||
| Fig. 1 SEM images of (a) CMPN-1, (c) CMPN-2, and (e) CMPN-3. Scale bar: 1 μm. TEM images of (b) CMPN-1, (d) CMPN-2, and (f) CMPN-3. Scale bar: 200 nm. | ||
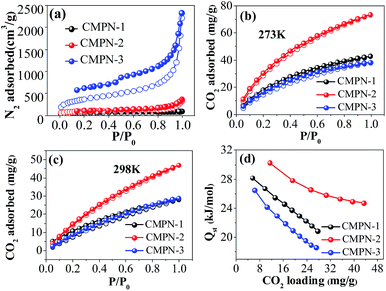
The porosity of the polymers was evaluated by nitrogen adsorption and desorption experiments at 77.3 K and the nitrogen adsorption and desorption isotherms are shown in (Fig. 2a). The details of the porosity parameters of CMPN samples are also listed in Table S1.† The BET surface areas and total pore volumes were calculated to be 230 m2 g−1 and 0.14 cm3 g−1 for CMPN-1 and 339 m2 g−1 and 0.39 cm3 g−1 for CMPN-2 (Fig. 2a and S4†), respectively. For CMPN-3, a large BET surface area of 1368 m2 g−1 was obtained, which is higher than those amorphous conjugated microporous polymers (CMPs, 500–800 m2 g−1),19 polymers of intrinsic microporosity (PIMs, 500–1065 m2 g−1)20 and poly(aryleneethynylene) (PAEs, 512–1018 m2 g−1).12 Also, CMPN-3 possesses a high total pore volume of 2.36 cm3 g−1, which is one of the highest values reported for CMPs to date. Compared with the nitrogen adsorption and desorption isotherms of CMPN-1 and CMPN-2, a large adsorption–desorption hysteresis of CMPN-3 was observed, indicating that CMPN-3 possesses a larger average pore size than that of CMPN-1 and CMPN-2 which was confirmed by the porous property analyses where the adsorption average pore width was calculated to be 2.4 nm, 4.6 nm and 6.9 nm for CMPN-1, CMPN-2 and CMPN-3, respectively. Three CMPN samples have relatively narrow pore size distribution, with the pore widths centering around 2 nm (Fig. S4†). In addition, though CMPN-3 has the largest surface areas and total pore volume, the micropore volume for CMPN-2 (0.07 cm3 g−1) is greater than that of CMPN-1 (0.05 cm3 g−1) and CMPN-3 (0.03 cm3 g−1).
Taking advantage of excellent porous characters and remarkable stability, the as-synthesized CMPN samples should be ideal absorbents which can be expected to be used under harsh conditions. Moreover, results obtained from our previous studies using various CMPs for adsorption of H2,3,14 CO2 (ref. 8a) and organic solvents11,15,16 show that the increase of micropore volume would result in an increase in gas uptake while a larger mesopore volume leads to higher adsorption capacity for oils or organic solvents. In this case, such a difference in pore sizes and micropore volumes of the CMPN samples in comparison with their surface areas makes them multifunctional absorbents for different purposes. Recently, the development of porous absorbents with larger surface areas for efficient CO2 (ref. 21–25) and I2 (ref. 26–30) capture has attracted considerable attention because of severe global climate change and environmental issues in the nuclear energy. In this regard, CO2 and I2 were used as two different guest molecules to evaluate the adsorption performance of the CMPN samples.
The CO2-uptake capacities of the CMPN samples at 273 K and 298 K were investigated under low pressure by volumetric methods. As shown in Fig. 2b and c, all the samples show reversible CO2 uptake with nearly no hysteresis between the absorption and desorption isotherms, implying that CO2 is reversibly physisorbed. At 273 K and 1 bar, the CO2 uptake reaches 73.4 mg g−1 for CMPN-2, which is higher than that of CMPN-1 (42.9 mg g−1) and CMPN-3 (38.2 mg g−1). A similar trend of CO2 uptake was also observed at 298 K and 1 bar (Fig. 2c), though there is an obvious decrease in CO2 uptake for all three CMPN samples (28.2 mg g−1 for CMPN-1, 47.0 mg g−1 for CMPN-2 and 28.8 mg g−1 for CMPN-3).
To better understand the adsorption performance of the CMPN samples, we determined the isosteric heat of adsorption (Qst) for CO2 from dual-site Langmuir fits of the CO2 isotherms at 273 K and 298 K (Fig. 2b and c).24b,c It is clear that, with increasing CO2 uptake, the Qst for CMPN-2 decreased smoothly from 30.2 kJ mol−1 to 24.7 kJ mol−1 while the Qst dropped dramatically from 28.2 kJ mol−1 to 20.9 kJ mol−1 for CMPN-1 and from 26.5 kJ mol−1 to 18.6 kJ mol−1 for CMPN-3, respectively (Fig. 2d). The trend of the CMPN samples in terms of Qst is in the order of CMPN-2 > CMPN-1 > CMPN-3 for the entire CO2 loading range. That is, the better CO2 uptake for CMPN-2 among three samples is dominated by its highest Qst volume as well as the largest micropore volume (Table S1†), instead of their surface areas, which is consistent with previous studies.22–24 Taking into consideration the fact that only a few examples of CMP nanotubes15–17 have been developed so far and no study involving their adsorption performance for CO2 capture has been reported, these findings may provide direct insight into the relationship of porous property–CO2 uptake of the CMPN samples, which would be of importance for further design novel CMP nanotubes towards effective CO2 capture.
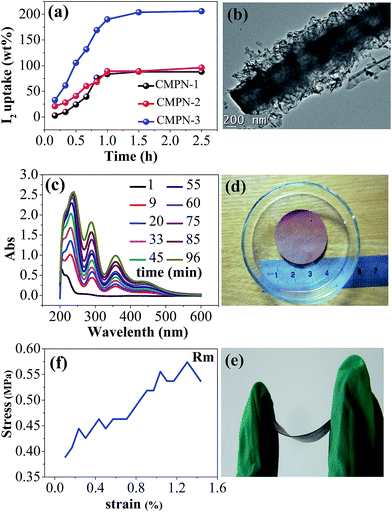
To further investigate their adsorption performance, I2, which is a dangerous radioisotope in nuclear waste,26–30 was also employed as host molecules for the uptake measurement. CMPN samples were exposed to nonradioactive iodine vapor and the I2 capture was conducted at 343.3 K and ambient pressure (ESI†). The I2 sublimed into the porous samples over time at ambient temperature and the equilibrium uptake for the three samples reached quickly (1 h). An apparent color change in the CMPN samples from brown to almost black was observed (Fig. S5†). The equilibrium I2 uptake was measured to be 97 wt%, 110 wt% and 208 wt% for CMPN-1, CMPN-2 and CMPN-3 (Fig. 3a). To the best of our knowledge, the I2 uptake for CMPN-3 is one of the highest values for all porous adsorbents reported to date.26–30 In contrast to that of CO2 uptake, the I2 uptake for three samples is directly proportional to the BET surface areas and total pore volumes of samples. Also, the TEM image of I2 loaded CMPN-3 shows that the I2 crystals were trapped both on the CMPN-3 surface and inside the tube of CMPN-3 (Fig. 3b), which is responsible for high uptake by combination with the largest pore volume and high affinity of the I2 to π-conjugated CMPN network.30 On the other hand, I2 release could occur if the I2 loaded sample, in this case CMPN-3 (Fig. S6†), was placed in organic solvents, for example ethanol. In contrast to conventional adsorbents, the delivery of I2 in ethanol increases linearly with time (Fig. 3c and S7†), indicating that the I2 release is better governed by the host–guest interaction which facilitates the regeneration of the absorbent for reuse. These findings make the CMPN samples valuable and recyclable absorbents for reversible I2 capture.
Interestingly, unlike the amorphous CMP 3D network usually formed as unprocessable powders,31 the tubular-like shape of the as-synthesized CMPN samples makes possible the facile fabrication of film materials for specific applications. To this end, the dried CMPN sample, in this case CMPN-3, was placed in a mould and kept under a pressure of 10 kPa for 10 min to afford a flexible, free-standing film with a size of 3 cm in diameter and 300 μm in thickness (Fig. 3d and e). It is worth noting that both the size and thickness of the film can be tuned by varying the size of mould or quantity of the sample. Dynamic mechanical analysis shows that the as-prepared film has a modulus of 0.57 MPa (Fig. 3f). The formation of such film materials takes great advantage over the porous absorbent powders in practical operation for I2 capture (Fig. S8†), which would be of technological significance for realizing them into real applications. Furthermore, based on its inherent hydrophobic/oleophilic chemistry (Fig. S9†) and high chemical and thermal stability, such a CMPN-based film can be anticipated to be used under harsh conditions by further improving its mechanical strength, which may have great potential for addressing environmental issues in various applications such as separation, purification, water treatment, oil spill cleanup and so on.
Conclusions
In summary, CMP nanotubes with a large surface area of up to 1368 m2 g−1 were synthesized by a simple one-step crosscoupling Sonagashira–Hagihara polymerization and were used for reversible CO2 and I2 capture. The resulting products show adsorption capacity for I2 capture with a maximum uptake of 208 wt%, which is one of the highest values of all porous absorbents reported to date. Owing to their unique cylindrical geometry, robust and stable physicochemical properties, excellent porosity and better processability, these materials might be extended to use as porous medium or film materials for a wide range of applications such as energy storage, catalysis, separation, water treatment and so on.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant no. 51263012, 51262019, 51462021 and 51403092), Fundamental Research Funds for the Universities of Gansu Province, Gansu Provincial Science Fund for Distinguished Young Scholars (Grant no. 1308RJDA012) and the “100-Talent Program” of the Chinese Academy of Sciences.References
- (a) C. D. Wood, B. Tan, A. Trewin, H. J. Niu, D. Bradshaw, M. J. Rosseinsky, Y. Z. Khimyak, N. L. Campbell, R. Kirk, E. Stockel and A. I. Cooper, Chem. Mater., 2007, 19, 2034 CrossRef CAS; (b) J.-X. Jiang, F. Su, H. Niu, C. D. Wood, N. L. Campbell, Y. Z. Khimyak and A. I. Cooper, Chem. Commun., 2008, 486 RSC; (c) C. D. Wood, B. Tan, A. Trewin, F. Su, M. J. Rosseinsky, D. Bradshaw, Y. Sun, L. Zhou and A. I. Cooper, Adv. Mater., 2008, 20, 1916 CrossRef CAS; (d) R. Dawson, D. J. Adams and A. I. Cooper, Chem. Sci., 2011, 2, 1173 RSC.
- (a) P. M. Budd, A. Butler, J. Selbie, K. Mahmood, N. B. McKeown, B. Ghanem, K. Msayib, D. Book and A. Walton, Phys. Chem. Chem. Phys., 2007, 9, 1802 RSC; (b) N. B. McKeown, P. M. Budd and D. Book, Macromol. Rapid Commun., 2007, 28, 995 CrossRef CAS.
- A. Li, R.-F. Lu, Y. Wang, X. Wang, K.-L. Han and W.-Q. Deng, Angew. Chem., Int. Ed., 2010, 49, 3330 CrossRef CAS PubMed.
- (a) X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76 CrossRef CAS PubMed; (b) X. Wang, K. Maeda, X. Chen, K. Takanabe, K. Domen, Y. Hou, X. Fu and M. Antonietti, J. Am. Chem. Soc., 2009, 131, 1680 CrossRef CAS PubMed; (c) M. G. Schwab, M. Hamburger, X. Feng, J. Shu, H. W. Spiess, X. Wang, M. Antonietti and K. Mullen, Chem. Commun., 2010, 46, 8932 RSC.
- (a) F. Goettmann, A. Fischer, M. Antonietti and A. Thomas, Angew. Chem., Int. Ed., 2006, 45, 4467 CrossRef CAS PubMed; (b) P. Kuhn, M. Antonietti and A. Thomas, Angew. Chem., Int. Ed., 2008, 47, 3499 CrossRef; (c) X. Wang, K. Maeda, X. Chen, K. Takanabe, K. Domen, Y. Hou, X. Fu and M. Antonietti, J. Am. Chem. Soc., 2009, 131, 1680 CrossRef CAS PubMed.
- J.-X. Jiang, C. Wang, A. Laybourn, T. Hasell, R. Clowes, Y. Z. Khimyak, J. Xiao, S. J. Higgins, D. J. Adams and A. I. Cooper, Angew. Chem., Int. Ed., 2011, 50, 1072 CrossRef CAS PubMed.
- L. Chen, Y. Yang and D. Jiang, J. Am. Chem. Soc., 2010, 132, 9138 CrossRef CAS PubMed.
- (a) Y. Xie, T. T. Wang, X. H. Liu, K. Zou, X. H. Liu, K. Zou and W. Q. Deng, Nat. Commun., 2013, 4, 1960 Search PubMed; (b) Y. Xie, T. T. Wang, R. X. Yang, N. Y. Huang, K. Zou and W. Q. Deng, ChemSusChem, 2014, 7, 2110 CrossRef CAS PubMed.
- Y. Kou, Y. Xu, Z. Guo and D. Jiang, Angew. Chem., Int. Ed., 2011, 50, 8753 CrossRef CAS PubMed.
- L. Chen, Y. Honsho, S. Seki and D. Jiang, J. Am. Chem. Soc., 2010, 132, 6742 CrossRef CAS PubMed.
- A. Li, H. X. Sun, D. Z. Tan, W. J. Fan, S. H. Wen, X. J. Qing, G. X. Li, S. Y. Li and W. Q. Deng, Energy Environ. Sci., 2011, 4, 2062 CAS.
- J. X. Jiang, F. Su, A. Trewin, C. D. Wood, H. Niu, J. T. A. Jones, Y. Z. Khimyak and A. I. Cooper, J. Am. Chem. Soc., 2008, 130, 7710 CrossRef CAS PubMed.
- R. Dawson, A. Laybourn, Y. Z. Khimyak, D. J. Adams and A. I. Cooper, Macromolecules, 2010, 43, 8524 CrossRef CAS.
- D. Z. Tan, W. J. Fan, W. N. Xiong, H. X. Sun, A. Li, W. Q. Deng and C. G. Meng, Eur. Polym. J., 2012, 48, 705 CrossRef CAS PubMed.
- D. Z. Tan, W. J. Fan, W. N. Xiong, H. X. Sun, Y. Q. Cheng, X. Y. Liu, C. G. Meng, A. Li and W. Q. Deng, Macromol. Chem. Phys., 2012, 213, 1435 CrossRef CAS.
- (a) D. Z. Tan, W. N. Xiong, H. X. Sun, Z. Zhang, W. Ma, C. G. Meng, W. j. Fan and A. Li, Microporous Mesoporous Mater., 2013, 176, 25 CrossRef CAS PubMed; (b) W. J. Fan, X. F. Liu, Z. Zhang, Q. J. Zhang, M. W. Wei, D. Z. Tan and A. Li, Microporous Mesoporous Mater., 2014, 196, 335 CrossRef CAS PubMed.
- X. L. Feng, Y. Y. Liang, L. J. Zhi, A. Thomas, D. Q. Wu, I. Lieberwirth, U. Kolb and K. Müllen, Adv. Funct. Mater., 2009, 19, 2125 CrossRef CAS.
- K. Sonogashira, Y. Tohda and N. Hagihara, Tetrahedron Lett., 1975, 4467 CrossRef CAS.
- J. X. Jiang, F. Su, A. Trewin, C. D. Wood, N. L. Campbell, H. Niu, C. Dickinson, A. Y. Ganin, M. J. Rosseinsky, Y. Z. Khimyak and A. I. Cooper, Angew. Chem., Int. Ed., 2007, 46, 8574 CrossRef CAS PubMed.
- (a) P. M. Budd, B. S. Ghanem, S. Makhseed, N. B. McKeown, K. J. Msayib and C. E. Tattershall, Chem. Commun., 2004, 230 RSC; (b) N. B. McKeown, B. M. Gahnem, K. Msayib, P. M. Budd, C. E. Tattershall, K. Mahmood, S. Tan, D. Book, H. W. Langmi and A. Walton, Angew. Chem., Int. Ed., 2006, 45, 1804 CrossRef CAS PubMed.
- (a) R. Banerjee, A. Phan, B. Wang, C. Knobler, H. Furukawa, M. O'Keeffe and O. M. Yaghi, Science, 2008, 319, 939 CrossRef CAS PubMed; (b) Z. Zhang, Z. Z. Yao, S. Xiang and B. Chen, Energy Environ. Sci., 2014, 7, 2868 RSC.
- (a) R. Dawson, E. Stöckel, J. R. Holst, D. J. Adams and A. I. Cooper, Energy Environ. Sci., 2011, 4, 4239 RSC; (b) J. T. A. Jones, T. Hasell, X. F. Wu, J. Bacsa, K. E. Jelfs, M. Schmidtmann, S. Y. Chong, D. J. Adams, A. Trewin, F. Schiffman, F. Cora, B. Slater, A. Steiner, G. M. Day and A. I. Cooper, Nature, 2011, 474, 367 CrossRef CAS PubMed.
- (a) T. Ben, H. Ren, S. Q. Ma, D. P. Cao, J. H. Lan, X. F. Jing, W. C. Wang, J. Xu, F. Deng, J. M. Simmons, S. L. Qiu and G. S. Zhu, Angew. Chem., Int. Ed., 2009, 48, 9457 CrossRef CAS PubMed; (b) T. Ben, C. Pei, D. Zhang, J. Xu, F. Deng, X. Jing and S. Qiu, Energy Environ. Sci., 2011, 4, 3991 RSC.
- (a) D. Yuan, W. Lu, D. Zhao and H.-C. Zhou, Adv. Mater., 2011, 23, 3723 CrossRef CAS PubMed; (b) W. Lu, J. P. Sculley, D. Yuan, R. Krishna, Z. Wei and H.-C. Zhou, Angew. Chem., Int. Ed., 2012, 51, 7480 CrossRef CAS PubMed; (c) J. A. Mason, K. Sumida, Z. R. Herm, R. Krishna and J. R. Long, Energy Environ. Sci., 2011, 4, 3030 RSC.
- G. Qi, Y. Wang, L. Estevez, X. Duan, N. Anako, A. A. Park, W. Li, C. W. Jonesc and E. P. Giannelis, Energy Environ. Sci., 2011, 4, 444 CAS.
- (a) K. W. Chapman, P. J. Chupas and T. M. Nenoff, J. Am. Chem. Soc., 2010, 132, 8897 CrossRef CAS PubMed; (b) H. Zhao, T. M. Nenoff, G. Jennings, P. J. Chupas and K. W. Chapman, J. Phys. Chem. Lett., 2011, 2, 2742 CrossRef CAS; (c) K. W. Chapman, D. F. Sava, G. J. Halder, P. J. Chupas and T. M. Nenoff, J. Am. Chem. Soc., 2011, 133, 18583 CrossRef CAS PubMed; (d) D. F. Sava, K. W. Chapman, M. A. Rodriguez, J. A. Greathouse, P. S. Crozier, H. Zhao, P. J. Chupas and T. M. Nenoff, Chem. Mater., 2013, 25, 2591 CrossRef CAS.
- (a) M. H. Zeng, Q. X. Wang, Y. X. Tan, S. Hu, H. X. Zhao, L. S. Long and M. Kurmoo, J. Am. Chem. Soc., 2010, 132, 2561 CrossRef CAS PubMed; (b) Z. Yin, Q. X. Wang and M. H. Zeng, J. Am. Chem. Soc., 2012, 134, 4857 CrossRef CAS PubMed.
- (a) Q. K. Liu, J. P. Ma and Y. B. Dong, Chem. Commun., 2011, 47, 7185 RSC; (b) T. Hasell, M. Schmidtmann and A. I. Cooper, J. Am. Chem. Soc., 2011, 133, 14920 CrossRef CAS PubMed.
- Y. C. He, J. Yang, G. C. Yang, W. Q. Kan and J. F. Ma, Chem. Commun., 2012, 48, 7859 RSC.
- A. Sigen, Y. W. Zhang, Z. P. Li, H. Xia, M. Xue, X. M. Liu and Y. Mu, Chem. Commun., 2014, 50, 8495–8498 RSC.
- C. Gu, N. Huang, J. Gao, F. Xu, Y. H. Xu and D. L. Jiang, Angew. Chem., Int. Ed., 2014, 53, 4850 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ta04235b |
| This journal is © The Royal Society of Chemistry 2015 |