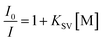Highly sensitive and selective detection of 2,4,6-trinitrophenol using covalent-organic polymer luminescent probes†
Nannan
Sang
a,
Chuanxing
Zhan
b and
Dapeng
Cao
*a
aState Key Lab of Organic–Inorganic Composites, Beijing University of Chemical Technology, Beijing 100029, P. R. China. E-mail: caodp@mail.buct.edu.cn
bDepartment of Materials Science and Engineering, Nanchang University, Nanchang 330031, P. R. China
First published on 4th November 2014
Abstract
Rapid and sensitive detection of nitroaromatic explosives has attracted considerable attention due to their serious harm to our world. In this work, two porous luminescent covalent-organic polymers (COP-401 and COP-301) have been synthesized through copolymerization of double ligands. The results indicate that the two COPs with high thermal stability show significant luminescence quenching effects for nitroaromatic explosives. In particular, the two COPs exhibit not only a high sensitivity (about 1 ppm) for nitoraromatic explosives, but also an extremely high selectivity for 2,4,6-trinitrophenol (PA), which suggests that they are promising luminescent probes for highly sensitive and selective detection of nitroaromatic explosives, especially for PA.
Rapid and selective detection of nitroaromatic explosives has become one of the most pressing issues, because nitroaromatic explosives are serious pollution sources of water and important chemical species in mine fields, munitionary remediation sites, military and security applications.1–5 Currently, nitroaromatic derivatives are being used world-wide to prepare different explosives. Among them, the detection of 2,4,6-trinitrophenol (PA) has become very popular as it is widely used in dyes, fireworks, glass and leather industries. During commercial production and use, PA is released into the environment, leading to the contamination of soil and aquatic biosystems.6–10 Thus, there is an urgent need to develop an efficient and reliable sensor for detection of nitroaromatic explosives, especially for PA, owing to their serious pollution and potential threats to security.
Until now, a variety of methods of sensing nitroaromatic explosives have been reported.11 Metal detectors, widely used as a portable instrument for field explosive detection, cannot locate the plastic casing of modern land mines. Trained dogs are expensive and difficult to maintain and are also easily fatigued.12 Physical detection methods for explosives include gas chromatography coupled with a mass spectrometer, surface-enhanced Raman spectroscopy, nuclear quadrupole resonance, energy-dispersive X-ray diffraction, neutron activation analysis, electron capture detection and cyclic voltammetry.13–19 These techniques are highly selective, but some are expensive and are not easily fielded in a small low-power package. Most detection methods for explosives are only applicable to vapor samples,20–22 because of the interference problems encountered in complex aqueous media.
As a complementary method, however, chemical sensors provide new means for the rapid detection of ultra-trace analytes from explosives, and can be easily incorporated into inexpensive and portable microelectronic devices.23–30 Meanwhile, fluorescence-based sensor schemes are very special promising. Moreover, the fluorescence method has individual advantages in many circumstances. Therefore, the fluorescence-based detection is gaining increasing attention owing to its high sensitivity, simplicity, short response time and its ability to be employed both in solution and solid phases.31–35 Numerous electron-rich fluorescent conjugated polymers have been synthesized and used in the detection of trace amounts of nitroaromatic explosives.36–38 Fluorescence-quenching-based chemical detection represents one of the most sensitive and convenient methods that have been widely employed in explosive identification.39–43 By means of the specific recognition of TNT antibodies, Goldman et al. fabricated some fluorescence immunoassays for TNT.44 Li et al. synthesized two new luminescent metal–organic frameworks as probe materials to sense RDX.45 Wang et al. reported a convenient and rapid paper sensor for PA-selective detection.46 Shyamal et al. reported a graphene-based composite, which could be potentially useful for selective detection of PA.9 Although many chemical sensors with fluorescence toward nitroaromatic explosives have been proposed, developing quick and highly selective sensors for nitroaromatic explosives is still an imperative issue.
Covalent-organic polymers (COPs) are a new class of porous network materials, which are connected by stable covalent C–C bonds. Recent research indicates that COPs have shown great potential for a wide range of applications such as gas storage and separation, luminescence sensing and templates for new energy materials.47–50 In particular, COPs are a kind of moisture-resistant porous materials, possessing high hydrothermal stability and a large specific surface of 1000–3000 m2 g−1,43 which may be promising reversible luminescent sensing materials. In this work, we report two porous luminescent COP probes that are capable of fast and sensitive detection for nitroaromatic explosives.
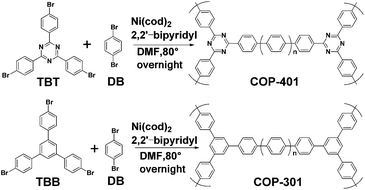
The synthesis route of the two COPs is depicted in Scheme 1. The porous COPs were successfully synthesized using 1,4-Dibromobenzene (DB) with 2,4,6-Tris-(4-bromo-phenyl)-[1,3,5]-triazine (TBT) and 1,3,5-Tris(4-bromophenyl)benzene (TBB), respectively, by copolymerization. The success of the phenyl–phenyl coupling in the two porous COPs can be certified by the Fourier transform infrared (FTIR) measurement, as shown in Fig. S1 and S2 in the ESI,† in which the absence of the C–Br stretching peak around 500 cm−1 in the FTIR spectra indicates that the Br functional groups in the reactant have been consumed completely by phenyl–phenyl coupling.
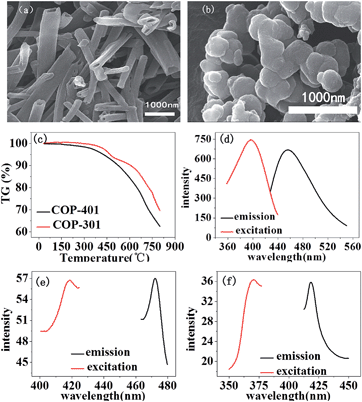
The SEM image of COP-401 shows hollow pipe morphology (Fig. 1(a)), while the SEM image of COP-301 exhibits a spherical shape (Fig. 1(b)). Fig. 1(c) shows the TGA of two COPs under an Ar atmosphere. There is nearly no weight loss before 300 °C for the two COPs, and less than 5% weight loss at T = 400 °C. Beyond 550 °C, COP-401 has a large weight loss, while COP-301 has a large weight loss after 650 °C, i.e. the framework decomposition thresholds for COP-401 and COP-301 are about 550 and 650 °C, respectively, which indicates that both COPs are highly thermally stable.
Fig. 1(d–f) show the solid-state photoluminescence (PL) spectra of COP-401 and the respective monomers (TBT and DB) at room temperature. Both organic linkers show the emission peak upon excitation. The organic linker DB shows an emission at 418 nm upon excitation at 371 nm. TBT shows an emission at 472 nm upon excitation at 416 nm. The maximum emission peak of COP-401 is located at 456 nm upon excitation at 396 nm. Compared to TBT and DB, the luminescence behavior of COP-401 is probably caused by the ligand to ligand charge transfer, because the interactions between linkers can shorten the distance between the luminophores, enabling electron transfer between the luminophores.38 Actually, the luminescence principles of COP-301 are similar to COP-401. The maximum emission peak of COP-301 is located at 432 nm, upon excitation at 372 nm (Fig. S3a†). The solid-state PL spectra of TBB monomer are shown in Fig. S3b.†
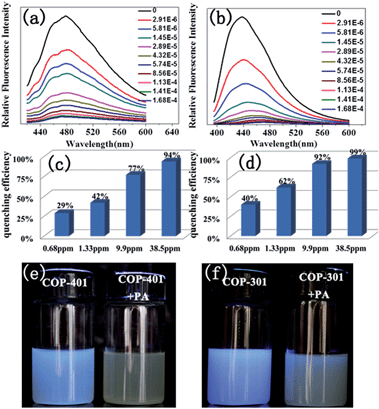
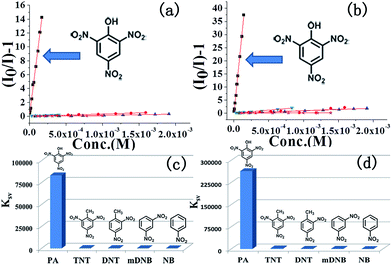
The ability of COPs to detect nitroaromatic explosives by luminescence quenching was investigated by adding successive aliquots of PA, TNT, DNT, mDNB, and NB into methanol solutions of COP-401 and COP-301. The quenching behaviors of PL spectra of COP-401 and COP-301 upon addition of various concentrations of PA, TNT, DNT, mDNB, and NB are shown in Fig. 2(a) and (b), S4 and S5 in the ESI,† respectively. All nitroaromatic explosives lead to luminescence quenching, compared to COP matrices. The luminescence intensity of nitroaromatic explosives-incorporated COPs is largely dependent on the concentration of the nitroaromatic explosives. With the increase of the nitroaromatic explosive concentration, the luminescence intensity decreases at different levels. However, the quenching degree of the same COP for different nitroaromatic explosives is clearly different. Among the nitroaromatic explosives, the quenching effects of the two COPs for PA are very apparent, especially the sensing of COP-301 for PA.
In addition, both COP-401 and COP-301 can detect PA at a very low concentration (<1 ppm), i.e. they show high sensitivity for PA. As shown in Fig. 2(c) and (d), when the concentration of PA is only 0.68 ppm, the quenching efficiency of COP-401 is 29%, and the quenching efficiency of COP-301 is 40%. With the increase of the concentration of PA, the luminescence intensity decreases drastically. When the concentration of PA is 38.5 ppm, the quenching efficiency of COP-401 reaches 94%, while it is almost quenched completely for COP-301. The visible bright blue emission of COP-401 and COP-301 in UV light vanishes upon the addition of the PA solution, which are quenched almost completely (Fig. 2(e) and (f)). Therefore, both COP-401 and COP-301 can be used to detect nitroaromatic explosives, especially for PA, with high sensitivity.
According to previous literature,23 the quenching effect can be calculated quantitatively by the Stern–Volmer equation:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KSV[TNT] = 201.1) greater than those for TNT and other nitroaromatic explosives, and the quenching constant of COP-301 for PA is at least is 116 times greater than those for other nitroaromatic explosives. The observation above indicates that COP-401 and COP-301 might be promising candidates to selectively detect PA for security or environmental applications.
KSV[TNT] = 201.1) greater than those for TNT and other nitroaromatic explosives, and the quenching constant of COP-301 for PA is at least is 116 times greater than those for other nitroaromatic explosives. The observation above indicates that COP-401 and COP-301 might be promising candidates to selectively detect PA for security or environmental applications.
In both COP-401 and COP-301 cases, the quenching efficiencies are in the order of PA > TNT > DNT > mDNB > NB. To better understand this observation we measured the absorption spectrum of PA and the spectrum is shown in Fig. S6 in the ESI.† The absorption of PA and the emission of COPs have almost no spectral overlap, suggesting that it is not the energy transfer mechanism.51,56,57 Therefore, we calculated the molecular orbits (MO) (Fig. S7 in ESI†). This quenching order can be explained by the donor–acceptor electron transfer mechanism with COPs as the donor and PA as the acceptor. Upon excitation, the electrons in the conduction band of COPs are transferred to the lowest unoccupied molecular orbital (LUMO) of the analytes, which results in the quenching effect. With the increase of such electron withdrawing groups (–NO2) in analytes, the quenching efficiencies are therefore enhanced,38 which can be explained by the order of LUMO levels of explosives, i.e. NB > mDNB > DNT > TNT > PA. As a result, the transferred electron efficiency follows the order of NB < mDNB < DNT < TNT < PA, thereby yielding the above quenching order. In addition, the KSV[PA] of COP-301 is three times higher than the KSV[PA] of COP-401, mainly because the electronegativity of N is larger than C and the LUMO level of COP-301 is higher than that of COP-401 (see Fig. S7†). Accordingly, the electron transformation from the conduction band of COP-301 to the LUMO of the analyte becomes faster than that of COP-401 upon excitation, which results in higher quenching efficiencies of COP-301 for the analyte, compared to COP-401.
In summary, we have synthesized two porous luminescent COPs by copolymerization. The two COPs possess high thermal stability and good luminescence intensities. Moreover, the two COPs show fast response and high sensitivity for detection of the nitroaromatic explosives, especially for PA. In particular, KSV[PA] of COP-301 and COP-401 for PA reaches 2.6 × 105 and 8.3 × 104, which is higher than that of other chemical sensors.48–51 Furthermore, both COP-401 and COP-301 exhibit not only high sensitivity for PA at a low concentration about 1 ppm, but also extremely high selectivity for PA, which suggests that the COPs are promising luminescent probes for detection of nitroaromatic explosives, especially for PA.
Acknowledgements
This work is supported by the National 973 Program (2011CB706900), NSF of China (no. 91334203, 21274011, 21121064), National 863 Program (2013AA031901), Central Scientific Research Funding (ZZ1304), and Outstanding Talents Plan from BUCT.Notes and references
- H. Sohn, M. J. Sailor, D. Magde and W. C. Trogler, J. Am. Chem. Soc., 2003, 125, 3821–3830 CrossRef CAS PubMed.
- Y. Long, H. Chen, H. Wang, Z. Peng, Y. Yang, G. Zhang, N. Li, F. Liu and J. Pei, Anal. Chim. Acta, 2012, 744, 82–91 CrossRef CAS PubMed.
- M. E. Germain and M. J. Knapp, Chem. Soc. Rev., 2009, 38, 2543–2555 RSC.
- N. Hannink, S. J. Rosser, C. E. French, A. Basran, J. A. Murray, S. Nicklin and N. C. Bruce, Nat. Biotechnol., 2001, 19, 1168–1172 CrossRef CAS PubMed.
- E. S. Forzani, D. Lu, M. J. Leright, A. D. Aguilar, F. Tsow, R. A. Iglesias, Q. Zhang, J. Lu, J. Li and N. Tao, J. Am. Chem. Soc., 2009, 131, 1390–1391 CrossRef CAS PubMed.
- S. S. Nagarkar, B. Joarder, A. K. Chaudhari, S. Mukherjee and S. K. Ghosh, Angew. Chem., 2013, 125, 2953–2957 CrossRef.
- G. He, H. Peng, T. Liu, M. Yang, Y. Zhang and Y. Fang, J. Mater. Chem., 2009, 19, 7347–7353 RSC.
- B. Roy, A. K. Bar, B. Gole and P. S. Mukherjee, J. Org. Chem., 2013, 78, 1306–1310 CrossRef CAS PubMed.
- D. Dinda, A. Gupta, B. K. Shaw, S. Sadhu and S. K. Saha, ACS Appl. Mater. Interfaces, 2014, 6, 10722–10728 CAS.
- S. Madhu, A. Bandela and M. Ravikanth, RSC Adv., 2014, 4, 7120–7123 RSC.
- S. J. Toal and W. C. Trogler, J. Mater. Chem., 2006, 16, 2871–2883 RSC.
- A. W. Czarnik, Nature, 1998, 394, 417–418 CrossRef CAS PubMed.
- J. M. Sylvia, J. A. Janni, J. Klein and K. M. Spencer, Anal. Chem., 2000, 72, 5834–5840 CrossRef CAS.
- R. Luggar, M. Farquharson, J. Horrocks and R. Lacey, X-Ray Spectrom., 1998, 27, 87–94 CrossRef CAS.
- A. Hilmi and J. H. Luong, Anal. Chem., 2000, 72, 4677–4682 CrossRef CAS.
- M. Krausa and K. Schorb, J. Electroanal. Chem., 1999, 461, 10–13 CrossRef CAS.
- M. Kandpal, A. K. Bandela, V. K. Hinge, V. R. Rao and C. P. Rao, ACS Appl. Mater. Interfaces, 2013, 5, 13448–13456 CAS.
- B. Zu, Y. Guo and X. Dou, Nanoscale, 2013, 5, 10693–10701 RSC.
- S. R. Wallenborg and C. G. Bailey, Anal. Chem., 2000, 72, 1872–1878 CrossRef CAS.
- S. Tao, G. Li and H. Zhu, J. Mater. Chem., 2006, 16, 4521–4528 RSC.
- L. Pinnaduwage, A. Gehl, D. Hedden, G. Muralidharan, T. Thundat, R. Lareau, T. Sulchek, L. Manning, B. Rogers and M. Jones, Nature, 2003, 425, 474 CrossRef CAS PubMed.
- L. Guo, B. Zu, Z. Yang, H. Cao, X. Zheng and X. Dou, Nanoscale, 2014, 6, 1467–1473 RSC.
- S. W. Thomas, G. D. Joly and T. M. Swager, Chem. Rev., 2007, 107, 1339–1386 CrossRef CAS PubMed.
- L. Sun, H. Xing, J. Xu, Z. Liang, J. Yu and R. Xu, Dalton Trans., 2013, 42, 5508–5513 RSC.
- H. Xu, F. Liu, Y. Cui, B. Chen and G. Qian, Chem. Commun., 2011, 47, 3153–3155 RSC.
- A. Rose, Z. Zhu, C. F. Madigan, T. M. Swager and V. Bulović, Nature, 2005, 434, 876–879 CrossRef CAS PubMed.
- S. R. Zhang, D. Y. Du, J. S. Qin, S. J. Bao, S. L. Li, W. W. He, Y. Q. Lan, P. Shen and Z. M. Su, Chem.–Eur. J., 2014, 20, 3589–3594 CrossRef CAS PubMed.
- S. Singh, J. Hazard. Mater., 2007, 144, 15–28 CrossRef CAS PubMed.
- D. Wang, Y. Shiraishi and T. Hirai, Chem. Commun., 2011, 47, 2673–2675 RSC.
- Y. Peng, A.-J. Zhang, M. Dong and Y.-W. Wang, Chem. Commun., 2011, 47, 4505–4507 RSC.
- S. Liu, Z. Xiang and D. Cao, J. Mater. Chem., 2011, 21, 6649–6653 RSC.
- S. Shaligram, P. P. Wadgaonkar and U. K. Kharul, J. Mater. Chem., 2014, 2, 13983–13989 RSC.
- X. Zhou, H. Li, H. Xiao, L. Li, Q. Zhao, T. Yang, J. Zuo and W. Huang, Dalton Trans., 2013, 42, 5718–5723 RSC.
- T. Naddo, Y. Che, W. Zhang, K. Balakrishnan, X. Yang, M. Yen, J. Zhao, J. S. Moore and L. Zang, J. Am. Chem. Soc., 2007, 129, 6978–6979 CrossRef CAS PubMed.
- Z. Xiang, C. Fang, S. Leng and D. Cao, J. Mater. Chem. A, 2014, 2, 7662–7665 CAS.
- Y.-N. Gong, L. Jiang and T.-B. Lu, Chem. Commun., 2013, 49, 11113–11115 RSC.
- P. Beyazkilic, A. Yildirim and M. Bayindir, ACS Appl. Mater. Interfaces, 2014, 6, 4997–5004 CAS.
- S. Pramanik, C. Zheng, X. Zhang, T. J. Emge and J. Li, J. Am. Chem. Soc., 2011, 133, 4153–4155 CrossRef CAS PubMed.
- D. Banerjee, Z. Hu and J. Li, Dalton Trans., 2014, 43, 10668–10685 RSC.
- Y. Ma, H. Li, S. Peng and L. Wang, Anal. Chem., 2012, 84, 8415–8421 CrossRef CAS PubMed.
- B. Gole, A. K. Bar and P. S. Mukherjee, Chem. Commun., 2011, 47, 12137–12139 RSC.
- W.-S. Zou, F.-H. Zou, Q. Shao, J. Zhang, Y.-Q. Wang, F.-Z. Xie and Y. Ding, J. Photochem. Photobiol., A, 2014, 278, 82–88 CrossRef CAS PubMed.
- Z. Xiang and D. Cao, Macromol. Rapid Commun., 2012, 33, 1184–1190 CrossRef CAS PubMed.
- E. R. Goldman, I. L. Medintz, J. L. Whitley, A. Hayhurst, A. R. Clapp, H. T. Uyeda, J. R. Deschamps, M. E. Lassman and H. Mattoussi, J. Am. Chem. Soc., 2005, 127, 6744–6751 CrossRef CAS PubMed.
- Z. Hu, K. Tan, W. P. Lustig, H. Wang, Y. Zhao, C. Zheng, D. Banerjee, T. J. Emge, Y. J. Chabal and J. Li, Chem. Sci., 2014, 5, 4873–4877 RSC.
- Y. Ma, H. Li, S. Peng and L. Wang, Anal. Chem., 2012, 84, 8415–8421 CrossRef CAS PubMed.
- Z. Xiang, X. Zhou, C. Zhou, S. Zhong, X. He, C. Qin and D. Cao, J. Mater. Chem., 2012, 22, 22663–22669 RSC.
- Z. Xiang, D. Cao, W. Wang, W. Yang, B. Han and J. Lu, J. Phys. Chem. C, 2012, 116, 5974–5980 CAS.
- Y. Zhou, Z. Xiang, D. Cao and C.-J. Liu, Chem. Commun., 2013, 49, 5633–5635 RSC.
- Z. Xiang and D. Cao, J. Mater. Chem. A, 2013, 1, 2691–2718 CAS.
- S. M. Aly, M. R. Parida, E. Alarousu and O. F. Mohammed, Chem. Commun., 2014, 50, 10452–10455 RSC.
- N. Venkatramaiah, S. Kumar and S. Patil, Chem. Commun., 2012, 48, 5007–5009 RSC.
- Y. Wang and Y. Ni, Anal. Chem., 2014, 86, 7463–7470 CrossRef CAS PubMed.
- N. Dey, S. K. Samanta and S. Bhattacharya, ACS Appl. Mater. Interfaces, 2013, 5, 8394–8400 CAS.
- S. K. Ghosh, S. S. Nagarkar and A. V. Desai, Chem. Commun., 2014, 50, 8915–8918 RSC.
- A. a. O. El-Ballouli, E. Alarousu, M. Bernardi, S. M. Aly, A. P. Lagrow, O. M. Bakr and O. F. Mohammed, J. Am. Chem. Soc., 2014, 136, 6952–6959 CrossRef CAS PubMed.
- S. M. Aly, S. Goswami, Q. A. Alsulami, K. S. Schanze and O. F. Mohammed, J. Phys. Chem. Lett., 2014, 5, 3386–3390 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ta04903a |
| This journal is © The Royal Society of Chemistry 2015 |