New versatile Pt supports composed of graphene sheets decorated by Fe2O3 nanorods and N-dopants with high activity based on improved metal/support interactions†
Yunqian
Dai
*a,
Yunling
Chai
a,
Yibai
Sun
a,
Wanlin
Fu
a,
Xiaotian
Wang
a,
Qing
Gu
a,
Tingying Helen
Zeng
b and
Yueming
Sun
*a
aSchool of Chemistry and Chemical Engineering, Southeast University, Nanjing, Jiangsu 211189, P. R. China. E-mail: daiy@seu.edu.cn; sun@seu.edu.cn
bCenter for Excitonics, Research Laboratory for Electronics, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA
First published on 11th November 2014
Abstract
Supported metal catalysts are critical to many important chemical reactions, but the weak metal/support interaction is an obstacle to the success of remarkable catalytic performance. This paper reports rational-designed novel Pt supports, consisting of reduced graphene oxide sheets decorated with both Fe2O3 nanorods and N-dopants (denoted as Fe2O3/N-RGO), for Pt photodeposition driven by visible light in a controllable fashion. The 2–3 nm Pt nanocrystals primarily nucleated on rough Fe2O3 nanorods, and interacted strongly with special sites on the Fe2O3 surface using unsaturated vacant orbitals. At the same time, the accelerated photodegradation of undesirable PVP allowed the Pt nanocrystals with clean active sites. The supported Pt showed impressive activity and had a 7-times higher reaction rate constant (11.4 s−1 mg−1) towards 4-nitrophenol reduction, compared with that of free Pt, due to the synergetic effect within the whole Pt/Fe2O3/N-RGO catalysts and the doping of N atoms which acted as new metal-free catalytic centers in N-RGO sheets. We further demonstrated that the ternary catalyst could be easily removed through magnetic separation from the system. This new strategy is extendible to other heterogeneous catalysts with different components.
Improved catalytic and photocatalytic performance is usually achieved in many important chemical reactions, when the catalysts are supported on specific substrates, mainly due to the change of electronic states in noble metals.1 The remarkable activities of supported catalysts are greatly dependent on their chemical states, metal/support interactions, and their sizes. Typically, the catalysts, such as Pt nanocrystals, are dispersed finely on the porous carbon or metal oxide support,2 which has abundant active sites to interact with metals. As a new emergence in the carbon family, graphene conjugated with sp2-hybridized carbon domains has been widely explored as a new two-dimensional (2D) scaffold in catalytic science.3 Doping heteroatoms, such as electron-rich N atoms, in graphene and/or its derivatives is a promising way to manipulate their intrinsic electronic features and thus better interactions with catalysts to achieve enhanced performance.4 From this perspective, several successful applications of this strategy have been demonstrated but are still limited in reactions related to electrocatalytic or photocatalytic scopes,4b–d like oxygen reduction reaction (ORR), water splitting and organic pollutant degradation. But the metal/carbon interaction is still weak at the interface, and thus is an obstacle to the success of impressive catalytic performance.
The metal oxide (e.g. Fe2O3, TiO2, CeO2 and ZrO2) is another widely explored ideal substrate that has excellent physical and chemical stability, especially the unique thermal stability required in many important industrial reactions, in which the carbon-based supports may not participate. An important breakthrough has been recently achieved in utilizing FeOx nanocrystallites as metal supports for anchoring active single Pt atoms in CO oxidation, due to a facile charge transfer from Pt to FeOx at metal/metal oxide interfaces.5 Recent efforts on TiO2 supported Pt nanocrystals have demonstrated their excellent sinter-resistant ability,2b,6 but these pre-synthesized PVP-stabilized Pt nanocrystals only exhibited weaker activities,6 as the presence of PVP at the interface hindered a direct contact between the metal and metal oxide. Several studies have shown that calcination is a simple way to efficiently remove the surfactants, but the metal nanocrystals dramatically aggregate and thus lose active sites in high temperatures.7 Therefore, building a closed metal/support interface with a strong interaction at ambient temperature through a green chemistry approach is highly demanded but remains a significant challenge.
Herein, we report that the Pt nanocrystals can be selectively in situ photodeposited on a well-designed support, consisting of reduced graphene oxide sheets decorated with both Fe2O3 nanorods and N-dopants (denoted as Fe2O3/N-RGO). The versatile Fe2O3/N-RGO sheets here offer three functions: (i) new photocatalyts for the deposition of Pt atoms and the simultaneous photodecomposition of the PVP surfactant driven by visible-light, thus allowing Pt nanocrystals to contact with supports directly, (ii) new substrates for the growth of Pt nanocrystals with well-established Pt/support interactions at a closed interface, and (iii) magnetic materials for the rapid separation of the catalyst from the reaction system. By using this strategy, we were able to achieve a 7-times higher reaction rate than that of free-Pt nanocrystals.
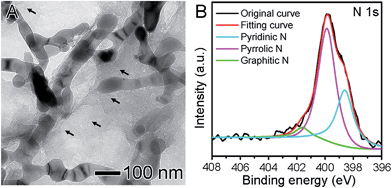
Fig. 1A shows a typical TEM image of the Fe2O3/N-RGO hierarchical sheets. The graphene oxide (GO) sheets were simultaneously functionalized with Fe2O3 nanorods and N-dopants, via a one-pot hydrothermal reaction in ammonia aqueous solution by mixing GO sheets and electrospun α-Fe2O3 nanofibers (Fig. S1†). After the reaction, the pristine Fe2O3 nanofibers in fact converted to nanorods, but these morphology changes had no impact on their application described here, as the Fe2O3 nanorods maintained their highly irregular and rough surfaces, which can serve as primary nucleation sites for catalyst growth.8 Interestingly, we also observed nanopores (highlighted by black arrows in Fig. 1A) with a size of ∼3 nm evenly dispersed in entire sheets, and the N-RGO sheets became holey. These nanosized holes are able to transport ions and molecules, and allow the graphene to be permeable to water desalination, gas separation, and DNA sequencing.9 Herein, they will also benefit the molecular migration during the catalytic reaction. The N 1s XPS spectrum in Fig. 1B could be deconvoluted into three peaks for pyridinic N (398.6 eV), predominant pyrrolic N (399.9 eV), and graphitic N (401.6 eV).10 Their atomic ratios to the total N 1s atoms are calculated to be 0.33, 0.60, and 0.065, respectively. No peak in the N 1s spectrum could be assigned to the Fe–N bonding, suggesting that the N-dopants most likely bonded with C atoms selectively and thus had a very high N/C ratio up to 14.9 at% in N-RGO sheets, compared with elsewhere observed for N-RGOs recently.11 These N-dopants in graphene could enhance visible activities of Fe2O3 nanocrystallines greatly by improving the charge separation.4a Therefore, the Fe2O3/N-RGO sheets are expected to have good performance in visible-light driven photocatalytic reactions and photovoltaic devices.
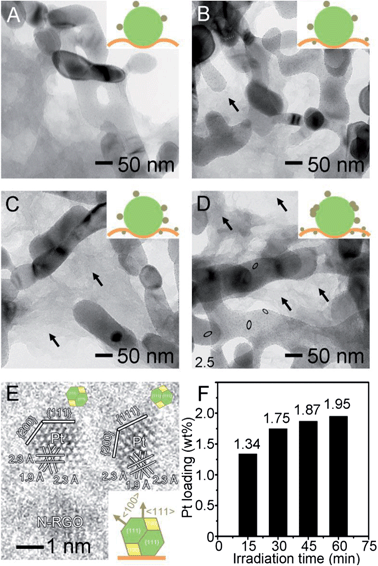
The Fe2O3/N-RGO sheets were demonstrated as new substrates for a facile photodeposition of Pt nanocrystals driven by visible-light (>420 nm) in a controllable fashion. The evolution of Pt nanocrystals was investigated as a function of irradiation time, as shown in Fig. 2. After 15 min, a number of Pt nanocrystals with a size of 2.1 nm discretely and evenly dispersed on each Fe2O3 nanorods (Fig. 2A). The Fe2O3 nanorods acted as both a photocatalyst and a robust substrate for the growth of Pt nanocrystals. Interestingly, the Pt nanocrystals were rarely observed on the surface of N-RGO sheets, showing their favor of the rough Fe2O3 surface, as depicted in the inset of Fig. 2A. The irregularities on the Fe2O3 surface, such as indentions, step edges, protrusions, or defective sites, significantly lowered the energy barrier for heterogeneous nucleation,8 and thus functioned as primary nucleation sites for the photodeposition of Pt atoms. And the selective deposition of Pt on Fe2O3 nanorods also confirmed that the Fe2O3 interacted strongly with a late transition metal. When the irradiation time was prolonged to 30 min, several tiny Pt nanocrystals appeared on the N-RGO sheets with a size of sub-2 nm, as indicated by a black arrow in Fig. 2B, while the Pt on Fe2O3 nanorods had a bigger size of 2.4 nm and a higher density. Further prolonging the irradiation time to 45 min, the densities and sizes of Pt nanocrystals further increased on both Fe2O3 nanorods and the N-RGO sheets as shown in Fig. 2C. These results clearly show that our photodeposition approach provides a superb control over the formation and distribution of Pt nanocrystals on the Fe2O3/N-RGO surface. These supports provide a large number of active sites for Pt nucleation which were evenly distributed over the entire surface, and thus ensured the generation of Pt nanocrystals without an observed overlap between them. However, further prolonging the irradiation time to 60 min, a few of quasi-linear Pt agglomerations (highlighted by black ellipses in Fig. 2D) appeared on the Fe2O3 surface only, which were in fact composed of smaller nanocrystals loosely bonded together.12 These linear-like Pt agglomerations had random orientation, which did not exhibit any preference in the direction. In contrast, the Pt nanocrystal on N-RGO sheets still kept their individuality well and had smaller sizes and lower coverages. Therefore the growth rates of Pt at different supports were different, and more than 90% of Pt nanocrystals were selectively deposited on the Fe2O3 nanorods. As shown in the HRTEM image (Fig. 2E), two truncated octahedra supported on the N-RGO sheets, and the lattice distance of 2.3 Å and 1.9 Å were the {111} and {200} facets of Pt nanocrystals.6 The inset in Fig. 2E illustrates a truncated octahedron supported on the N-RGO sheets. The nanopores on N-RGO sheets are less observable, which most likely have served as the favorable nucleation sites for Pt deposition. The Pt loadings were proportional to the irradiation time (Fig. 2F), and the success of Pt growth at a low loading fashion (<2 wt%) is beneficial for decreasing noble metal consumption in a cost-saving mode.
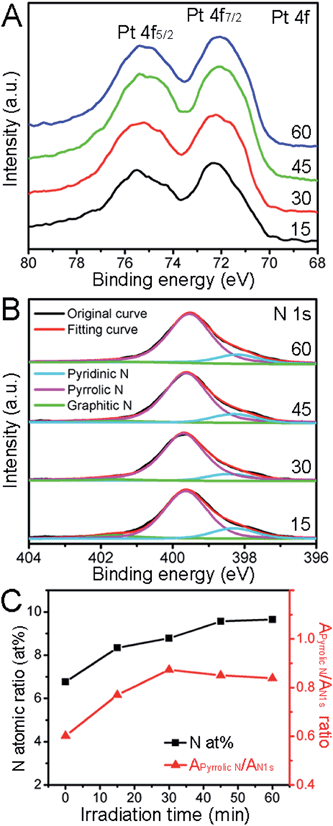
Fig. 3A shows the Pt 4f XPS spectra of ternary Pt/Fe2O3/N-RGO catalysts, two predominant peaks centered at 75.6 eV and 72.2 eV belong to Pt 4f5/2 and Pt 4f7/2, respectively. The Pt 4f7/2 had a ∼1.0 eV up-shift, compared with the characteristics of metallic Pt0 (∼71.2 eV).12 This shift was assigned to the electron-deficient Ptδ+ state (0 < δ < 2),8b which indicated that anchored Pt nanocrystals had unsaturated vacant orbitals possibly resulting from the transferring of their 5d electrons to the Fe2O3 nanorods,1c,5 and thus interacted strongly with special sites on supports. These low-coordination atoms on the metal surface often act as excellent active sites, and are highly desirable in catalytic reactions. It can thus be concluded that the metallic Pt nanocrystals have been efficiently reduced by using Fe2O3/N-RGO sheets as photocatalysts, and the metal/support interactions also have been well-established that may play a vital role in anchoring catalysts and enhance the remarkable catalytic activity. Bonding analysis of N 1s atoms (Fig. 3B and C) shows that the N atoms had a predominant pyrrolic configuration and the N atomic ratio was directly proportional to the irradiation time. The increase in N at% implied the PVP, which contained pyrrolic N, gradually adsorbed on the Pt and/or the Fe2O3/N-RGO surface. But the PVP, particularly those on the Pt surface, encapsulate the Pt surface via the carbonyl bonds like a nanocage and thus are not favorable for the catalytic reactions.12 We calculated the peak area ratio of pyrrolic N dopants to all N 1s atoms (referred as Apyrrolic N/AN 1s) for a simple comparison, on the basis of a hypothesis that the N-dopants in N-RGO sheets preserved their pristine bonding structures under irradiation. Thus the increase of the Apyrrolic N/AN 1s ratio could be a facile indicator of the adsorption of PVP also. Surprisingly, the Apyrrolic N/AN 1s ratio decreased when more PVP were adsorbed as implied by a higher N at%, and was anti-proportional to the irradiation time after 45 min. This tendency uncovered the pyrrolic N structures that were disrupted in PVP, and their degradation rate became faster than their adsorption rate. It has been reported the Fe2O3 have a superior ability to photodegrade many organic molecules especially when decorated with metal nanocrystals even at a low level, and the metal surfaces are often the active centers for photocatalytic reactions.4a,8b,13 Therefore it is rational for us to deduce that the Pt/Fe2O3/N-RGO hybrids have plenty of active sites for the photodegradation of PVP, particularly at the Pt surfaces, and the degradation rate of PVP on Pt is possibly faster than that on Fe2O3/N-RGO. Moreover, the removal of PVP is also confirmed by the appearance of several loosely packed Pt structures in TEM analysis (Fig. 2D). Herein, the PVP had been selectively and partially removed, thus the Pt nanoparticles exposed more desired clean active sites for the catalytic reaction.
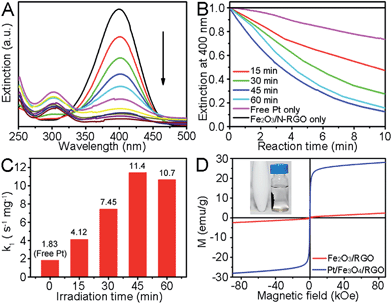
We chose the reduction of 4-nitrophenol to 4-aminophenol by NaBH4 as a model reaction to quantitatively evaluate their catalytic performances. This model reaction has a pseudo-first-order kinetics with respect to 4-nitrophenol (or 4-nitrophenolate).14 After adding the Pt/Fe2O3/N-RGO catalyst, a new peak at 315 nm for 4-aminophenol appeared, and the intensity of the adsorption peak for 4-nitrophenol at 400 nm gradually dropped in a deceleration fashion, as the catalytic reaction processed (Fig. 4A). The kinetic process of the catalytic reduction could be conventionally monitored by measuring the normalized extinction at 400 nm as a function of time (Fig. 4B). We then use previously defined rate constant k1,15 a normalized apparent rate constant (kapp) by mass (defined as k1 = kapp/m, where m is the weight of the catalyst), to exclude the influence of the catalyst mass on the reaction rate. Therefore, the k1 reflects the intrinsic catalytic activity. As shown in Fig. 4C, the free PVP-stabilized Pt nanoparticles (2.9 nm, Fig. S2†) had a lowest k1 value of 1.83 s−1 mg−1 similar to other reports.6 In contrast, the supported Pt had a high k1, up to 7-times higher (11.4 s−1 mg−1) than that of free Pt. And this impressive activity is advancing among other building blocks for Pt-based catalysts anchored on specific supports with different components consisting of metal oxides and/or graphene sheets recently (see Table S1†).6,14b–d The unexpected, surprisingly better catalytic performance of our supported Pt could be mainly ascribed to the partially vacant 5d orbital of Pt atoms, less residual PVP on the Pt surface, and strong Pt/support interaction by which the Pt/Fe2O3 interface may serve as the primary active site for the activation of 4-nitrophenol.1c Surprisingly, we observed that the Fe2O3/N-RGO sheets were in fact catalytically active towards the reduction of 4-nitrophenol to 4-aminophenol despite a weak activity without any Pt (Fig. S3A†). To have a deeper insight into this unusual Pt-free catalytic activity, we evaluated the activity of N-RGO sheets and the Fe2O3 nanorods respectively, and found that only the N-RGO sheets exhibited detectable activity for this reaction (Fig. S3B and C†). Without the N-RGO sheets, the Pt/Fe2O3 nanorods, fabricated by a similar approach to the one for making the Pt/Fe2O3/N-RGO shown in Fig. 2C, only had a smaller k1 of 7.98 s−1 mg−1 (Fig. S4†). Such weaker activity indicated that the versatile N-RGO sheets in fact acted as a new metal-free catalyst besides an ideal metal support, and had played a vital role in achieving the excellent catalytic performance of the ternary Pt/Fe2O3/N-RGO catalysts. The N-dopants in N-RGO sheets were believed to facilitate the adsorption and activation of 4-nitrophenol, and the carbon atoms bonded to them also became the active sites for the reaction,15 and were responsible to our observed catalyst activity. Moreover, the synergistic effect between the N-dopants and the Pt on N-RGO sheets is also beneficial to a superior performance in this well-designed catalyst system. The turnover frequency (TOF) is another important indicator in the evaluation of catalytic efficiency. The approximate TOF expressed as ([4-nitrophenol] × conversion)/([Pt] × t) when the conversion reaches 90% is calculated to be as high as ∼646 h−1, indicating its good catalytic performance.3c,14c It is worth noting that the Pt/Fe2O3/N-RGO sheets maintained a high stability over the course of another five cycles of reactions (Fig. S5†), exhibiting a good resistance against losing of activity due to Pt aggregation. Generally, the positively charged reaction product (4-aminophenol) could easily adsorb on the PVP-stabilized Pt surface that has a negative charge, and thus causes poisoning of catalysts.14b,16 Therefore, our observed good recyclability also uncovered the purified Pt, which had excellent stability against poisoning, thanks to the efficient degradation of PVP.
The performance of supported Pt nanocrystals was strongly dependent on their irradiation time in photodeposition, during which their sizes gradually increased from 2.1 nm to 3.1 nm. k1 increased with longer irradiation time and reached the maxima of 11.4 s−1 mg−1 at 45 min. This tendency is consistent with the gradual degradation of PVP on the Pt surface under irradiation, and demonstrates that the clean surfaces have more active sites. These cleaner surfaces in an increased proportion could promote the total activity towards the reaction in spite of bigger sizes. The k1 value only slightly decreased to 10.7 s−1 mg−1 at 60 min, due to the formation of quasi-linear Pt agglomerations. Thus, the chemical state of exposed Pt species is of great importance in determining the activity of Pt/Fe2O3/N-RGO ternary catalysts.
As an important precursor, α-Fe2O3 can also be conventionally converted into magnetic materials, such as magnetite (Fe3O4) by simple reduction. In an effort to easily separate catalysts from the reaction system, we simply reduced the Fe2O3 nanorods after finishing the catalytic reaction, by adding NaNH4 into the solution system.17 The ternary catalysts exhibited a rapid response to an external magnetic field (inset in Fig. 4D) that allowed facile and rapid separation. These observations allow us to deduce the desired conversion of Fe2O3 to Fe3O4. And this hypothesis was then confirmed by the dramatically increased saturation magnetization (Ms) of 28.1 emu g−1, from 2.5 emu g−1 of pristine Fe2O3/N-RGO sheets at room temperature.
In summary, a new rational-designed Pt-based ternary catalyst system with significantly improved activity was achieved by a green chemistry approach. In such a system, the Fe2O3/N-RGO sheets showed versatile functions as visible active photocatalysts, ideal metal supports and magnetic materials. The supported 2–3 nm Pt nanoparticles exhibited an unexpected 7-times enhanced reaction rate up to 11.4 s−1 mg−1, owing to the advantages brought by the well-established metal/support interaction during in situ photodeposition, a big portion of an exposed clean surface, metal-free catalytic abilities arose from N-dopants, and the synergetic effect within the whole Pt/Fe2O3/N-RGO catalysts. The success of the strategy demonstrated may provide some inspirations that the utilization of strongly interacted catalysts could open up a brand new approach to advanced heterogeneous catalysts with enhanced performances.
Acknowledgements
This work was financially supported by the National Basic Research Program (973 program, 2013CB932902), the National Natural Science Foundation of China (21201034, 21173042, 21310102005 and 21345008), the Science and Technology Support Program (Industry) Project of Jiangsu Province (BE 2013118) and the Fundamental Research Funds for the Central Universities (3207044403). T. Z. acknowledges funding from MIT Energy Initiative (MITEI) through MITEI-Weatherford International Corporation (Grant no. 6925033) and the MITEI seed funding (Grant no. 6925587) and Center for Excitonics at the Massachusetts Institute of Technology, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, and Office of Basic Energy Sciences under Award Number DE-SC0001088.References
-
(a) C. T. Campbell, Nat. Chem., 2012, 4, 597 CrossRef CAS PubMed
; (b) Y. Li and G. A. Somorjai, Nano Lett., 2010, 1, 2289 CrossRef PubMed
; (c) N. An, S. Li, P. N. Duchesne, P. Wu, W. Zhang, J. Lee, S. Cheng, P. Zhang, M. Jia and W. Zhang, J. Phys. Chem. C, 2013, 117, 21254 CrossRef CAS
; (d) S. Bai, J. Ge, L. Wang, M. Gong, M. Deng, Q. Kong, L. Song, J. Jiang, Q. Zhang, Y. Luo, Y. Xie and Y. Xiong, Adv. Mater., 2014, 26, 5689 CrossRef CAS PubMed
.
-
(a) P. Trogadas, T. F. Fuller and P. Strasser, Carbon, 2014, 75, 5 CrossRef CAS PubMed
; (b) Y. Dai, B. Lim, Y. Yang, C. M. Cobley, W. Li, E. C. Cho, B. Grayson, P. T. Fanson, C. T. Campbell, Y. Sun and Y. Xia, Angew. Chem., Int. Ed., 2010, 49, 8165 CrossRef CAS PubMed
.
-
(a) C. Du, Y. Liao, X. Hua, W. Luo, S. Chen and G. Chenga, Chem. Commun., 2014, 50, 12843 RSC
; (b) C. A. Tsang, K. N. Hui, K. S. Hui and L. Ren, J. Mater. Chem. A, 2014, 2, 17986 RSC
; (c) X. Lu, L. Yang, X. Bian, D. Chao and C. Wang, Part. Part. Syst. Charact., 2014, 31, 245 CrossRef CAS
; (d) S. Li, A. Wang, Y. Hu, K. Fang, J. Chen and J. Feng, J. Mater. Chem. A, 2014, 2, 18177 RSC
; (e) Q. Luo, X. Yu, B. Lei, H. Chen, D. Kuang and C. Su, J. Phys. Chem. C, 2012, 116, 8111–8117 CrossRef CAS
.
-
(a) L. Zhao, R. He, K. T. Rim, T. Schiros, K. S. Kim, H. Zhou, C. Gutiérrez, S. P. Chockalingam, C. J. Arguello, L. Pálová, D. Nordlund, M. S. Hybertsen, D. R. Reichman, T. F. Heinz, P. Kim, A. Pinczuk, G. W. Flynn and A. N. Pasupathy, Science, 2011, 333, 999 CrossRef CAS PubMed
; (b) L. He, L. Jing, Y. Luan, L. Wang and H. Fu, ACS Catal., 2014, 4, 990 CrossRef CAS
; (c) J. Zhu, M. Xiao, X. Zhao, K. Li, C. Liu and W. Xing, Chem. Commun., 2014, 50, 12201–12203 RSC
; (d) Y. Shao, S. Zhang, M. H. Engelhard, G. Li, G. Shao, Y. Wang, J. Liu, I. A. Aksay and Y. Lin, J. Mater. Chem., 2010, 20, 7491 RSC
.
- B. Qiao, A. Wang, X. Yang, L. F. Allard, Z. Jiang, Y. Cui, J. Liu, J. Li and T. Zhang, Nat. Chem., 2011, 3, 634 CrossRef CAS PubMed
.
-
(a) L. Ping, C. T. Campbell and Y. Xia, Nano Lett., 2013, 13, 4957 CrossRef PubMed
; (b) L. Ping and Y. Xia, ACS Appl. Mater. Interfaces, 2013, 5, 6391 CrossRef PubMed
.
-
(a) L. Liu, S. Ouyang and J. Ye, Angew. Chem., Int. Ed., 2013, 52, 6689 CrossRef CAS PubMed
; (b) Z. Wang, H. Fu, D. Han and F. Gu, J. Mater. Chem. A, 2014, 2, 20374 RSC
.
-
(a) E. Formo, P. Camargo, B. Lim, M. Jiang and Y. Xia, Chem. Phys. Lett., 2009, 476, 56 CrossRef CAS PubMed
; (b) L. Chen, F. Li, B. Ni, J. Xu, Z. Fu and Y. Lu, RSC Adv., 2012, 2, 10057 RSC
.
-
(a) D. Cohen-Tanugi and J. C. Grossman, Nano Lett., 2012, 12, 3602 CrossRef CAS PubMed
; (b) D. Jiang, V. R. Cooper and S. Dai, Nano Lett., 2009, 9, 4019 CrossRef CAS PubMed
.
- Y. Dai, H. Long, X. Tian, Y. Wang, Q. Gu, W. Jiang, Y. Wang, C. Li, T. H. Zeng, Y. Sun and J. Zeng, Part. Part. Syst. Charact., 2014, 31, 597 CrossRef CAS
.
- H. Wang, T. Maiyalagan and X. Wang, ACS Catal., 2012, 2, 781 CrossRef CAS
.
- Y. Borodko, P. Ercius, D. Zherebetskyy, Y. Wang, Y. Sun and G. Somorjai, J. Phys. Chem. C, 2013, 117, 26667 CAS
.
- B. Xu, B. Huang, H. Cheng, Z. Wang, X. Qin, X. Zhang and Y. Dai, Chem. Commun., 2012, 48, 6529 RSC
.
-
(a) R. He, Y. Wang, X. Wang, Z. Wang, G. Liu, W. Zhou, L. Wen, Q. Li, X. Wang, X. Chen, J. Zeng and J. G. Hou, Nat. Commun., 2014, 5, 4327 Search PubMed
; (b) T. Yu, J. Zeng, B. Lim and Y. Xia, Adv. Mater., 2010, 22, 5188 CrossRef CAS PubMed
; (c) X. Wang, D. Liu, S. Song and H. Zhang, J. Am. Chem. Soc., 2013, 135, 15864 CrossRef CAS PubMed
; (d) Z. Ji, X. Shen, Y. Xu, G. Zhu and K. Chen, J. Colloid Interface Sci., 2014, 432, 57 CrossRef CAS PubMed
.
-
(a) X. Kong, Z. Sun, M. Chen, C. Chen and Q. Chen, Energy Environ. Sci., 2013, 6, 3260 RSC
; (b) X. Kong, C. Chen and Q. Chen, Chem. Soc. Rev., 2014, 43, 2841 RSC
.
- H. Li, S. Gan, D. Han, W. Ma, B. Cai, W. Zhang, Q. Zhang and L. Niu, J. Mater. Chem. A, 2014, 2, 3461 CAS
.
- F. Jiao, J. Jumas, M. Womes, A. V. Chadwick, A. Harrison and P. G. Bruce, J. Am. Chem. Soc., 2006, 128, 12905 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ta05869k |
| This journal is © The Royal Society of Chemistry 2015 |