Atomic layer deposition of ruthenium at 100 °C using the RuO4-precursor and H2
Matthias M.
Minjauw
a,
Jolien
Dendooven
a,
Boris
Capon
a,
Marc
Schaekers
b and
Christophe
Detavernier
*a
aDepartment of Solid State Sciences, CoCooN, Ghent University, Krijgslaan 281-S1, B-9000 Ghent, Belgium. E-mail: Christophe.Detavernier@ugent.be; Tel: +32 92644996
bIMEC, Kapeldreef 75, B-3001 Leuven, Belgium
First published on 24th October 2014
Abstract
In this paper we report a low temperature (100 °C) ALD process for Ru using the RuO4-precursor (ToRuS™) and H2 as the reactant. The thermal decomposition behaviour of the precursor in the range of 50 °C–250 °C was investigated and it was found that thermal decomposition of RuO4 to RuO2 starts at a sample temperature of 125 °C. The RuO4/H2 process (0.0045 mbar/4 mbar) was attempted at temperatures below this decomposition limit and it was found that ALD growth of pure Ru is possible in a narrow temperature window near 100 °C. The growth rate during steady state growth was found to be 0.1 nm per cycle. The Ru film nucleated easily on a wide range of substrates (H-terminated Si, TiN, Pt and Al2O3). Although the films are grown at a low temperature, they are considerably pure and are of good quality as evidenced by a resistivity of 18 μΩ cm for an 18 nm film and a relative atomic concentration of impurities <5% as determined by XPS. It is hypothesized that the reaction of the RuO4 molecule with the Ru-surface leading to a monolayer of RuO2 is the mechanism that ensures a self-saturated behaviour of the first half reaction, which is a critical requirement to achieve a well-behaved ALD process.
Introduction
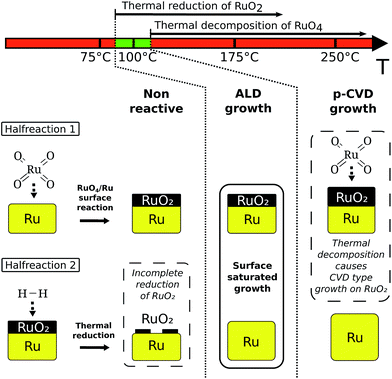
Due to its high work function (>4.7 eV), low bulk resistivity (7 μΩ cm), high chemical and thermal stability and conductive oxide, Ru is a candidate material for various applications in microelectronics. For example a thin film of Ru may replace the currently used TiN capacitor electrode in DRAM and because of its adhesive properties towards Cu, Ru thin films can be used as a seed layer for Cu electroplating in combination with a TaN barrier.1,2 Ru has a broad range of possible oxidation states, rendering it also of interest for applications in catalysis.3As in microelectronics the substrate consists of high aspect ratio structures and in catalysis Ru must be deposited onto complex surfaces to ensure a high effective area, for both applications it is important to be able to deposit Ru films in a conformal way. Atomic layer deposition (ALD) is a thin film deposition technique which offers unrivalled conformality and thickness control. Over the past decade, ALD of Ru has been actively pursued.4–7 Most of this research focussed on metal–organic (MO) precursors, such as the RuCp2- and Ru(EtCp)2-precursors.8,9 Gatineau et al. proposed RuO4 as an inorganic precursor (ToRuS™: solution of RuO4 in an inert organic solvent, Air Liquide), aiming to reduce the amount of C-impurities in the deposited films.10 The precursor is used in a H2-reduction chemistry5 at temperatures higher than 180 °C, where in the first half-reaction RuO2 is deposited by pulsing the RuO4-precursor, and this oxide is then reduced to Ru by H2 in the second half-reaction.10–14 Although ALD type growth of Ru was first reported10 for temperatures above 180 °C, it was later found11 that at these temperatures the precursor decomposes thermally such that the process is in fact pulsed CVD (p-CVD) rather than ALD.
In this paper a genuine Ru ALD process with the RuO4-precursor is reported at a temperature of 100 °C. This is the lowest temperature ever reported for thermal ALD of Ru, as for most MO-precursors the temperature window lies above 200 °C.7 The low deposition temperature enables deposition on temperature-sensitive substrates. A common problem with MO-precursors is a rather long incubation time from several tens to hundreds of cycles on different substrates.6,7 This is important as the incubation period increases the deposition time, leads to a waste of expensive precursors and generally results in films with a high roughness. The growth rate after incubation is also typically low with MO-precursors, lying generally below 0.1 nm per cycle, which again increases the cost for growing a Ru film of a certain thickness.7,15 Here we report rather low incubation times (0–40 cycles) on different substrates and a high growth rate of 0.1 nm per cycle for ALD with the RuO4-precursor.
Experimental procedures
The Ru thin films were grown in an experimental cold wall ALD reactor16 equipped with a turbomolecular pump connected through a gate valve, such that a base pressure of 10−6 mbar is achieved. The ALD-reactor is also equipped with a rotary vane pump connected through a separate gate valve. The sample is heated inside the chamber with a resistive heater. Because of the high vapor pressure of the precursor (13.3 mbar at 25 °C),10 no carrier gas was used and the precursor was put at a stabilized, constant temperature of 30 °C. The walls of the chamber were put at temperatures below that of the sample, but sufficiently high to avoid condensation of the precursor (30 °C–90 °C).During the precursor-pulse (RuO4), the gate valve to the turbomolecular pump remained open, and the flow of the precursor gas through a needle valve caused the chamber pressure to increase to ∼4.5 × 10−3 mbar. Because higher pressures of ∼4 mbar are needed during the reactant pulse (20/80 H2/Ar-mixture), the valve to the turbo pump was closed during this pulse and the chamber was pumped down to a pressure of about 10−1 mbar with the rotary vane pump before opening the valve to the turbo pump again. The pulsing was of a static nature, in which the pressure was built up for 8 s, followed by a period at constant pressure which is defined as the pulse time of the reactant pulse. Unless stated otherwise, both the RuO4-pulse and the H2-pulse lasted 10 s.
The ALD-reactor is equipped with a Woollam M-2000 spectroscopic ellipsometer, enabling in situ metrology (ISE). The crystallographic structure and thickness of the thin films were determined by X-ray Diffraction (XRD) and X-ray Reflection (XRR), respectively, using a Bruker D8 diffractometer with a CuKα source. Resistivity measurements were performed by the four-point probe method. Atomic Force Microscopy (AFM) and Scanning Electron Microscopy (SEM) were used to determine the surface roughness and morphology of the films, using a Bruker Dimension Edge system and a FEI Quanta 200F instrument, respectively. The purity and chemical composition of the thin films were investigated by X-ray Photoelectron Spectroscopy (XPS) using a Theta Probe (Thermo Scientific) instrument.
Many experiments were performed on sputtered Ru to avoid nucleation issues, as the presence of a metallic Ru surface can be expected to result in steady state ALD of Ru on Ru right from the start. XPS indicated that the sputtered Ru substrates had a thin RuO2 layer on top, resulting from surface oxidation in air. In what follows, the term “Grown film thickness” will indicate the thickness of the Ru thin film subtracted by that of the PVD-Ru film. The H-terminated Si substrates were obtained by dipping them in a 2% HF solution for approximately 60 s and they were directly transferred to the reactor afterwards. The TiN and Al2O3 substrates were grown in situ before the Ru deposition, with the TDMAT/NH3 plasma enhanced16 and the TMA/H2O thermal ALD process,4 respectively, both at 100 °C. The Si micro-pillar substrates used to verify the conformality of the Ru ALD process were fabricated using the Bosch Deep Reactive Ion Etching process.17
The optical model used for ISE was a combination of a Drude model with two Lorentzian oscillators. Unless stated otherwise, the optical model was optimized uniquely for every deposition. This was performed by fixing the thickness in the model to the value found by ex situ XRR, and optimizing all other parameters to give a good fit to the final ISE spectrum of the deposition. This model could then be used to fit the thickness parameter for each ISE-spectrum acquired during that deposition. The substrate optical model was always kept fixed during this procedure.
Experimental results
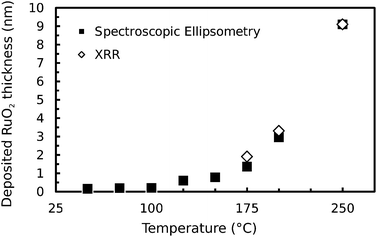
To obtain an ALD process one must work at temperatures where thermal decomposition of the precursor does not occur. As the thermal decomposition of RuO4:11,18| RuO4 → RuO2 + O2 | (1) |
To check the validity of this experimental approach, initial tests were performed at temperatures higher than 150 °C, where the precursor is known to thermally decompose. ISE clearly indicated the growth of a thin film at these temperatures. Deposition was verified by performing ex situ XRR, and excellent fits were found for films of RuO2 with a certain thickness, shown in Fig. 1. An Arrhenius plot was constructed using the XRR data points, and an activation energy of 0.423 eV could be derived from a linear fit with a χ2-value of 0.9997 indicating a thermally activated process. Finally, performing XPS on the material deposited at 250 °C further confirmed that stoichiometric RuO2 had been deposited. We can hence conclude that thermal decomposition occurred according to (1), and that we can detect the occurrence of growth of RuO2 using ISE.
Following this, experiments were conducted in the temperature range at and below 150 °C. From the ISE data no clear evidence of growth was found for deposition temperatures at and below 100 °C, in contrast to higher temperatures. This is illustrated in Fig. 1 with a thickness-fit of the grown RuO2-film at the end of every experiment. From this we can conclude that the thermal decomposition must be negligible at temperatures below 125 °C. Secondly, we can conclude that no other reactions between the RuO2 surface and RuO4 or catalytic decomposition of RuO4 on the RuO2 surface takes place which could lead to deposition of the material.
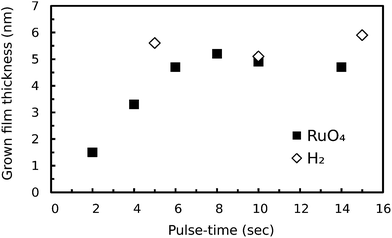
Consequently, growth of Ru with the RuO4/H2 process was attempted at 100 °C. The process was performed over 50 cycles on a surface oxidized, sputtered Ru-substrate, and ISE was used to monitor deposition. Growth occurred, and was linear with a growth rate of 0.1 nm per cycle (Fig. 2). Also shown in Fig. 2 is the full dataset of the earlier thermal decomposition experiment at 100 °C, of which only the last data point is shown in Fig. 1. Comparing both curves in Fig. 2 reveals that the growth we are obtaining at 100 °C is entirely driven by surface reactions which are occurring when the precursor and reactant are being alternated, which is typical for ALD growth.
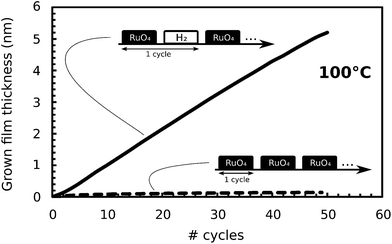 | ||
| Fig. 2 Grown film thickness as a function of the number of cycles from in situ SE. For each curve, the corresponding process is denoted by a diagram. The optical model for the (RuO2) film deposited by the process with only RuO4-pulses was the same as the one used for the SE data in Fig. 1. | ||
The ALD behaviour of the process at 100 °C was confirmed by saturation experiments. This was done by determining the thickness of the grown Ru layers on surface oxidized, sputtered Ru substrates by ex situ XRR for different lengths of the RuO4- and H2-pulses (Fig. 3). Both half-reactions show a self-terminating behaviour.
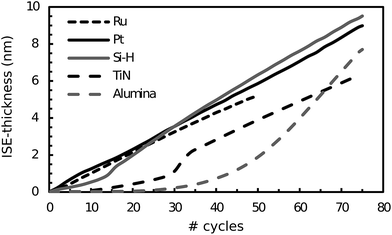
The RuO4/H2 process at 100 °C was also attempted on other substrates, and the nucleation behaviour was investigated by monitoring the deposition with ISE. The used substrates were PVD Pt, ALD TiN, ALD Al2O3 and H-terminated Si (Si–H). The results of these experiments are shown in Fig. 4, together with the data for PVD Ru from Fig. 2, and it is clear that although growth occurs from the start on Pt, Ru and Si–H, it is not linear until a transition regime has ended. The latter is probably linked to island growth.19 The fact that growth occurs from the start means that we have swift nucleation on the Pt, Ru and Si–H substrates. For the TiN and Al2O3-substrates, growth does not occur from the start but an incubation period seems to be present. The nucleation is faster on TiN compared to Al2O3. Apart from Al2O3, a fit to the linear regime led to a growth per cycle (GPC) ranging from 0.1 to 0.13 nm per cycle.
Growth with the RuO4/H2 process was also attempted at temperatures below 100 °C. The process was performed over 50 cycles on surface oxidized, sputtered Ru-substrates at temperatures of 50 °C and 75 °C, and ISE was used to monitor deposition. ISE showed that growth occurred from the start at 75 °C, while no appreciable growth occurred at 50 °C (Fig. 5). XRR revealed that the film grown at 75 °C had a lower density than the underlying sputtered Ru substrate, which was not observed for the films grown at 100 °C. The origin of this lower density will become clear in what follows.
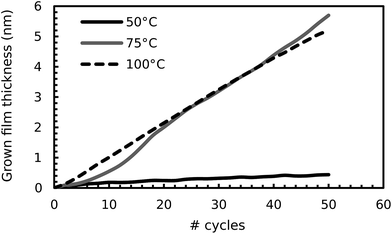 | ||
| Fig. 5 Thickness of the films, as determined by ISE, during their growth on sputtered Ru at 50 °C, 75 °C and 100 °C. The data at 100 °C are the same as has already been shown in Fig. 2 and 4. The optical model found for the film grown at 75 °C was used to fit the data at 50 °C. | ||
A set of 4 reference films of comparable thickness were prepared on Si–H substrates at temperatures of 75 °C, 100 °C, 175 °C and 250 °C using the same RuO4/H2 process (Table 1), to evaluate physical properties.
| T | # Cycles | Δ (nm) | Θ (%) | O (at%) | ρ (μΩ cm) |
|---|---|---|---|---|---|
| 75 °C | 175 | 20.0 | 53 | 44 | 48 |
| 100 °C | 125 | 17.8 | 100 | 2 | 18 |
| 175 °C | 105 | 14.0 | 81 | 4 | 27 |
| 250 °C | 115 | 20.4 | 74 | 9 | 46 |
XPS revealed oxygen as the only impurity in the bulk of all reference films, while all other impurities were below the detection limit. The film grown at 100 °C showed the lowest oxygen content (Table 1), indicating that the ALD-regime results in the purest films. The lower density of the films grown at 75 °C and 250 °C as found by XRR can be attributed to the O-impurities incorporated in these films. As a final remark, a layer of SiO2 was found to be present in all samples at the Si/Ru interface, which can be explained by the oxidation of the Si–H substrate by RuO4.
The resistivity measurements showed that the reference film with the lowest resistivity is the one grown at 100 °C (Table 1). This can easily be understood by the lower amount of oxygen impurities and associated higher density found by XPS and XRR, respectively. The value is higher than the bulk one (7 μΩ cm), but comparable to the values reported for Ru films grown with other ALD-processes.8,9 It is worth mentioning that even for a very thin film of 3.8 nm grown by the ALD-process at 100 °C a value of 20 μΩ cm was found.
The crystallinity of the thin films was examined by performing XRD in the range 2θ = [20° and 60°], and the patterns are shown in Fig. 6. It is clear that the films at 100 °C and 250 °C are polycrystalline hcp Ru as-deposited, with the (100)- (002)- and (101)-peaks appearing at 2θ = 38.5°; 42.3° and 44.1°, respectively, while the film grown at 75 °C seems to be amorphous.
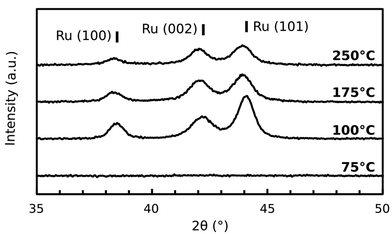 | ||
| Fig. 6 Diffractograms of the four reference films. The expected peak positions for hcp Ru are indicated by the vertical bars. | ||
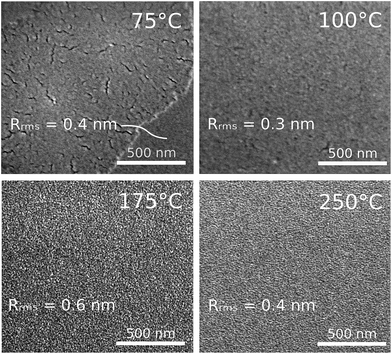
The surface morphology of the four reference films was studied by AFM and SEM. The RMS roughness of the film grown at 100 °C was found to have a value of 0.3 nm, while for the other films slightly higher values were found (Fig. 7). Though the RMS roughness of the films as determined by AFM does not seem to differ considerably, SEM revealed a clear difference in surface morphology (Fig. 7). For the film grown in the ALD regime weaker contrasts are visible compared to the films grown in the p-CVD regime, while the contrasts in the p-CVD regime are present on a smaller scale. For the film grown at 75 °C, the surface morphology is of a more exotic nature, as bright patches surrounded by a darker matrix are found in the SEM-micrograph. Energy-dispersive X-ray spectroscopy (EDX) revealed that the oxygen content in the dark matrix was significantly higher than that in the bright patches, while the carbon content was found to be higher in the bright patches.
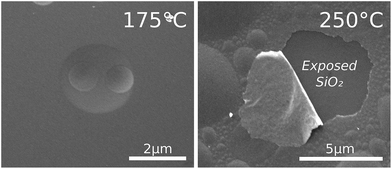
As a side remark, we report that for deposition on sputtered Ru at temperatures >125 °C (i.e. in the p-CVD regime), the films started to reflect diffusely, in contrast to the specular reflection which was observed for the films grown at 100 °C. This effect further increased with increasing temperatures. SEM revealed that this derived from bubbles which had formed in the films (Fig. 8, left). Note that bubbles have formed on top of each other, which can be explained by formation of bubbles in both the sputtered Ru and the grown p-CVD Ru film. For growth at elevated temperatures (>200 °C) this bubble formation led to the delamination of the film, and it was found by EDX that the sputtered film delaminated together with the grown film (Fig. 8, right). XPS revealed that the films contained O-impurities (∼10 at%). These results can be explained by the previously hypothesized mechanism20 of diffusion of O-atoms to both the native SiO2/PVD Ru- and the PVD Ru/p-CVD Ru-interfaces with the successive formation of O2 gas which then inflates both films.
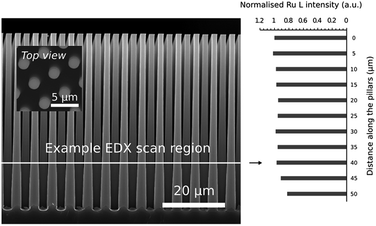
As a final experiment, the conformality of the RuO4/H2 process at 100 °C was verified by deposition on 50 μm high Si micro-pillars. The substrate was HF cleaned, resulting in H-terminated Si, before subjecting it to 125 ALD cycles. The optimized cycle times for steady state Ru growth on planar substrates were found to be insufficient for conformal coating of the micro-pillar sample, and therefore largely increased precursor and reactant exposures were used to ensure conformal growth in a second feasibility test. The first half cycle comprised of 8 static RuO4-pulses of 10 s at 1 mbar, and the second half cycle comprised of 1 static H2-pulse of 40 s at 5 mbar. In between subsequent RuO4-pulses, and in between the first and second half cycle, the chamber was evacuated to ∼10−5 mbar using both the rotation and the turbo pump. The depth profile of the Ru coating was determined by EDX, and the results are shown in Fig. 9. It is clear that a conformal film was obtained for nearly the entire structure.
Discussion
In the p-CVD regime, the proposed reaction-mechanism for the first half cycle during steady state growth is the parallel occurrence of thermal decomposition (1) and the reaction of RuO4 with the Ru surface (2).12| Ru + RuO4 → 2RuO2 | (2) |
Our experiments showed that an ALD regime for the RuO4/H2 process exists at temperatures where reaction (1) does not occur, such that (2) becomes entirely responsible for the first half reaction. Backman et al. showed that a RuO2 surface does not assist the decomposition of RuO4.21 Our thermal decomposition experiments also demonstrate that no chemical reactions between the RuO2 surface and RuO4 take place which could lead to deposition of the material. This explains the self-saturated behaviour of the first half reaction as (2) stops once the unreacted metal is fully covered by RuO2.
To reach steady state growth, the second half-reaction (3) must of course also be occurring.
| RuO2 + 2H2 → Ru + 2H2O | (3) |
The slow kinetics of this reaction at temperatures below 100 °C explains the absence of growth at 50 °C.22 The presence of considerable amounts of oxygen in the films grown at 75 °C can be explained by an incomplete removal of oxygen from the surface as (3) is only partially completed. Growth can proceed in this case because for an incomplete reduction of RuO2, metallic Ru islands23 are present within the RuO2, and they can react with RuO4. Most likely, the bright patches found by SEM analysis on the reference film grown at 75 °C correspond to such metal islands. To explain the larger amount of oxygen impurities for the p-CVD grown film relative to the ALD grown film, both half reactions have to be considered. First, both (1) and (2) are occurring during the first half reaction in the p-CVD regime, leading to more oxygen being deposited in every cycle. Secondly, the reduction rate of a (110) RuO2 film on (002) Ru is known to decrease with increasing temperature in the range of 200 °C–300 °C, such that the extra oxygen is removed less efficiently at higher substrate temperatures.22
For the reaction-mechanism at the early stages of growth on the other hand, we have found that the reactivity of the substrate with the precursor facilitates the initiation of growth, just as was the case for the p-CVD regime.13 The swift nucleation on Si–H and enhanced nucleation on TiN relative to Al2O3 for example can be explained by the oxygen consuming nature of Si–H and TiN. The swift nucleation on Pt can in turn be explained by the catalytic activity of the surface for dissociation of RuO4.13
Conclusions
We have shown that an ALD-process for Ru using the RuO4-precursor and H2 is possible at a temperature of 100 °C. At slightly higher temperatures, the precursor starts decomposing thermally, while at lower temperatures the process is limited by the thermal H2-reduction being inefficient below 100 °C (Fig. 10). Thanks to the high reactivity of the precursor, a high growth per cycle of 0.1 nm per cycle and rather low incubation periods of the order of tens of cycles were found on various substrates. The films were polycrystalline hcp Ru as-deposited and though they were grown at a low temperature, they had a low impurity content according to XPS-measurements and a low resistivity of the order of 20 μΩ cm, even for films of only 3.8 nm thickness.Acknowledgements
This research was supported by the European Research Council (Starting Grant No. 239865), by the Flemish Research Foundation FWO (Project G.0209.11), GOA no. 01G01513. Matthias M. Minjauw and Jolien Dendooven acknowledge funding by FWO Vlaanderen. The authors acknowledge P. M. Vereecken (IMEC) for providing Si micro-pillar samples.Notes and references
- International Technology Roadmap for Semiconductors, 2011.
- D.-S. Kil, H.-S. Song, K.-J. Lee, K. Hong, J.-H. Kim, K.-S. Park, S.-J. Yeom, J.-S. Roh, N.-J. Kwak, H.-C. Sohn, J.-W. Kim and S.-W. Park, Digest of Technical Papers, in 2006 Symposium on VLSI Technology, IEEE, 2006, pp. 38–39 Search PubMed.
- A. S. Seddon and K. R. Seddon, The chemistry of Ruthenium, Elsevier, Amsterdam, 1984 Search PubMed.
- R. L. Puurunen, J. Appl. Phys., 2005, 97, 121301 CrossRef PubMed.
- S. M. George, Chem. Rev., 2010, 110, 111 CrossRef CAS PubMed.
- V. Miikkulainen, M. Leskelä, M. Ritala and R. L. Puurunen, J. Appl. Phys., 2013, 113, 021301 CrossRef PubMed.
- J. Hämäläinen, M. Ritala and M. Leskelä, Chem. Mater., 2014, 26, 786 CrossRef.
- T. Aaltonen, P. Alén, M. Ritala and M. Leskelä, Chem. Vap. Deposition, 2003, 9, 45 CrossRef CAS.
- O.-K. Kwon, J.-H. Kim, H.-S. Park and S.-W. Kang, J. Electrochem. Soc., 2004, 151, G109 CrossRef CAS PubMed.
- J. Gatineau, K. Yanagita and C. Dussarrat, Microelectron. Eng., 2006, 83, 2248 CrossRef CAS PubMed.
- J. H. Han, S. W. Lee, G.-J. Choi, S. Y. Lee, C. S. Hwang, C. Dussarrat and J. Gatineau, Chem. Mater., 2009, 21, 207 CrossRef CAS.
- J. H. Han, S. W. Lee, S. K. Kim, S. Han, C. S. Hwang, C. Dussarrat and J. Gatineau, Chem. Mater., 2010, 22, 5700 CrossRef CAS.
- J. H. Han, S. W. Lee, S. K. Kim, S. Han, W. Lee, C. S. Hwang, C. Dussarat and J. Gatineau, Chem. Mater., 2012, 24, 1407 CrossRef CAS.
- J. H. Han, W. Lee, W. Jeon, S. W. Lee, C. S. Hwang, C. Ko and J. Gatineau, Chem. Mater., 2012, 24, 4686 CrossRef CAS.
- K. Gregorczyk, L. Henn-Lecordier, J. Gatineau, C. Dussarrat and G. Rubloff, Chem. Mater., 2011, 23, 2650 CrossRef CAS.
- J. Musschoot, Q. Xie, D. Deduytsche, S. Van den Berghe, R. L. Van Meirhaeghe and C. Detavernier, Microelectron. Eng., 2009, 86, 72 CrossRef CAS PubMed.
- B. Majeed, N. Tutunjyan, B. Jones, P. Fiorini and D. S. Tezcan, in 2012 IEEE 14th Electronics Packaging Technology Conference (EPTC), IEEE, 2012, pp. 52–56 Search PubMed.
- Z. Yuan and R. J. Puddephatt, Chem. Mater., 1993, 5, 908 CrossRef CAS.
- R. Puurunen and W. Vandervorst, J. Appl. Phys., 2004, 96, 7686 CrossRef CAS PubMed.
- H. Wang, R. G. Gordon, R. Alvis and R. M. Ulfig, Chem. Vap. Deposition, 2009, 15, 312 CrossRef CAS.
- U. Backman, M. Lipponen, A. Auvinen, U. Tapper, R. Zilliacus and J. K. Jokiniemi, Radiochim. Acta, 2005, 93, 297 CrossRef CAS.
- D. Ugur, A. J. Storm, R. Verberk, J. C. Brouwer and W. G. Sloof, J. Phys. Chem. C, 2012, 116, 26822 CAS.
- H. Over, Y. B. He, A. Farkas, G. Mellau, C. Korte, M. Knapp, M. Chandhok and M. Fang, J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct.--Process., Meas., Phenom., 2007, 25, 1123 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |