A new insight into the interaction of cisplatin with DNA: ROA spectroscopic studies on the therapeutic effect of the drug†
M.
Gąsior-Głogowska
a,
K.
Malek
ab,
G.
Zajac
ab and
M.
Baranska
*ab
aJagiellonian Centre for Experimental Therapeutics (JCET), Jagiellonian University, Krakow, 30-348, Poland
bFaculty of Chemistry, Jagiellonian University, Krakow, 30-060, Poland. E-mail: baranska@chemia.uj.edu.pl; Fax: +48 12 634 0515; Tel: +48 12 663 2253
First published on 11th November 2015
Abstract
Raman optical activity (ROA) spectroscopy has been applied for the first time to study the interaction of cisplatin with DNA. The knowledge about the structure of DNA–metal ion cross-links and hence the mechanism of the drug action is fundamental for the development of new antitumor drugs. At the same time, there is an urgent need to search for new methods for monitoring of this effect at the therapeutic dose of a drug. We have demonstrated that ROA spectroscopy is a sensitive technique with the capability to follow the structural alteration of the whole DNA molecule upon drug binding via a direct observation of transformation undergoing within chiral sugar moieties. A ROA profile delivers clear evidence of a partial transition from the B-DNA to the A-form due to the formation of cisplatin–DNA cross-links.
Introduction
Nowadays, many transition metal complexes have been commonly used as antitumor drugs and diagnostic agents.1–5 Despite their high efficiency, newer, safer and more effective pharmaceuticals have been looked for. There are increasing demands for maximizing clinical benefits and minimising the side effects, bypassing drug resistance and individualization of drug therapy to the patient. The understanding of the molecular mechanism of interactions between deoxyribonucleic acid (DNA) and metal ions is an essential part of the anticancer drug development.We focus our attention on cisplatin (cis-DDP, Pt(NH3)2Cl2), the first clinically applied platinum antitumor drug,6 widely used for treatment of many types of cancers e.g. testicular cancer, ovarian cancer, bladder cancer, squamous cell lung cancers and small-cell carcinoma.7,8 Cisplatin interacts with DNA within the cell nucleus, inducing cell death via apoptosis. It reacts with the DNA bases, primarily with guanine (G) and adenine (A). The most preferred sites of platinum binding are the guanine-N(7) and adenine-N(3), as the most nucleophilic atoms in DNA.5,6,9cis-DDP forms mainly intrastrand 1,2-GG (∼65%) and 1,2-AG adducts (∼25%). However, adducts involving cisplatin intrastrand crosslinks with 1,3-GXG as well as different interstrand crosslinks are also possible platinum complexes which cause significant distortion of the DNA double helical structure.5,6,10,11 Crystallographic studies have shown that the Pt adducts induce a global bending toward the major groove in DNA, local unwinding of the helix, and widening and flattening of the minor groove.11 Conformational changes occurring upon cisplatin binding have been observed also by AFM,10 NMR12 and VCD spectroscopy.13,14 It has been found that the transition of the native B-form into other DNA forms occurs as a result of platination. The formation of a Z-form stabilizing the effect of cisplatin has been postulated,15,16 but the formation of this left-handed double helix has not yet been confirmed.13 Raman spectroscopic studies have revealed only a partial transition from the B- to the A-form,17 whereas the X-ray crystallographic results have shown that DNA intercalated with cisplatin adopts predominantly the A-form.18 Although DNA–cisplatin interactions have been widely investigated by numerous techniques, a full explanation of this process has remained unclear. It should also be stressed here that all these studies have been conducted for the drug interacting with DNA at higher concentrations than the therapeutic dose.
The purpose of this work is to demonstrate the ability of Raman Optical Activity (ROA) spectroscopy to assess the changes in the DNA structure caused by the interaction with cisplatin. To the best of our knowledge, this is the first time when the ROA spectrum of DNA affected by a platinum(II)-containing drug is presented. Moreover, the first ROA spectra of DNA and RNA were recorded in 1998 by Bell and co-workers,19 and since that time, no further work has been undertaken on this topic.
ROA spectroscopy has been already proved to be a very sensitive tool for structure exploration of biomolecules that provides complementary information to other analytical techniques. A major advantage of ROA is its effectiveness to examine the chirality of biomolecules in solution and to determine their absolute configuration.20–23 Chiroptical methods follow even slight conformational and stereochemical changes in the secondary and tertiary structures of macrobiomolecules. The ROA spectra of nucleic acids provide information on the sugar ring conformation, the base-stacking arrangement of base rings and mutual orientation of the sugar and base rings.19,21,23–25 Based on this, the authors are convinced that ROA spectroscopy is able to expand knowledge about the interactions between DNA molecules and antitumor drugs.
We investigated herring sperm DNA type XIV (Sigma D6898). This popular spectroscopic standard of DNA posseses a similar average base content like calf thymus DNA (G–C 42%, A–T 58%) but exhibits higher solubility in water and physiological buffers than the latter. From this reason, we collected the ROA spectrum of DNA without ultrasonication of the solution. Omitting this step in the sample preparation is crucial for preserving the native structure of the nucleic acid, because ultrasonication leads to degradation of DNA by breaking the hydrogen bonds and rupturing single- and double-strands of the helix.26 Consequently, we recorded the Raman and ROA spectra of relatively long DNA molecules (av. ∼700 bp vs. <50 bp19) in their native, double-stranded B-DNA form. Moreover, we investigated DNA-platination at two concentrations of cisplatin, i.e. 0.01 and 0.02 molar ratios of cis-Pt/nucleotide, used commonly as therapeutic doses. We found that ROA spectroscopy is a very sensitive technique to study the metal–DNA binding process at a very low concentration of the intercalator, under the nearly-physiological conditions. For the first time, we show a detailed comparative analysis of the ROA spectra of DNA before and after the cis-DDP treatment and we propose ROA spectroscopic markers for the drug–DNA interactions.
Experimental
DNA samples were prepared by dissolving 50 mg of lyophilised herring sperm DNA (Sigma-Aldrich) in 1.0 mL MOPS (3-(N-morpholino)propane sulfonic acid) buffer (pH = 7.2, c = 50 mM). The ionic strength of the DNA solution was approx. 0.1. Prior to the spectral analysis, the samples were filtered by using a syringe filter with a 0.45 μm nylon membrane. The concentration of DNA was determined spectrophotometrically by measuring the absorbance value at 260 nm, according to the procedure of Gallagher et al.27Cisplatin (cis-diammineplatinum(II) dichloride) was added to the DNA solution from an original solution (1.0 mg mL−1) manufactured by Accord Healthcare Limited, UK. Cisplatin–DNA solutions were prepared in two molar ratios of Pt/nucleotide, 0.01 and 0.02. All samples were prepared in ultrapure water (18.2 MΩ, Milli-Q system).
Raman and ROA spectra were recorded simultaneously on a Chiral RAMAN-2X spectrometer (BioTools Inc.) with an excitation wavelength at 532 nm and an output laser power of 600 mW. All spectra were recorded in the range of 2000–50 cm−1 with a spectral resolution of 7 cm−1. The data collection time was 24 hours for all spectra. No melting of any sample was observed. Three spectra of triplicates were collected.
Then, the spectra were processed using OriginPro 9.1 software (OriginLab Corp.). After background subtraction, the spectra were smoothed using a second-order Savitzky-Golay filter with a 15-point window. The corrected spectra were next normalised to a band at 1097 cm−1 (the symmetric stretching phosphate mode), which is often used as an intensity standard.17,28–30
Results and discussion
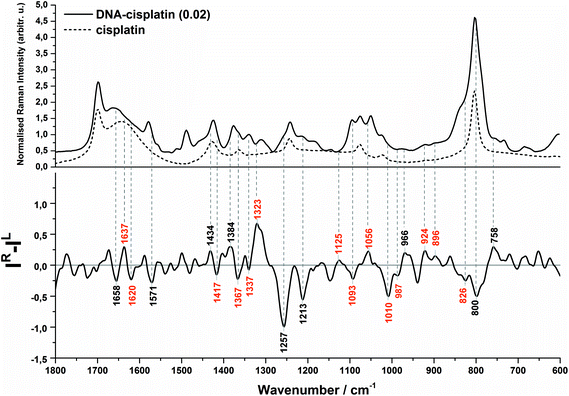
The Raman and ROA spectra in the 1800–600 cm−1 region of DNA and its complex with cisplatin at the molar ratio of cis-Pt/nucleotide = 0.01 are presented in Fig. 1. The spectra in the entire range from 2000 to 50 cm−1 are presented in Fig. S1 (ESI†), however the low-wavenumber region is overlapped by the bands of the fused silica of quartz cuvettes used for spectra collection.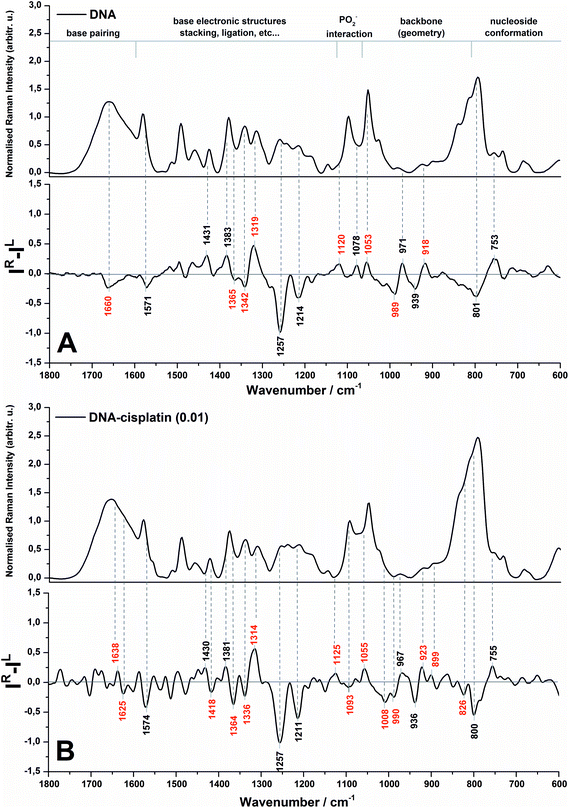 | ||
| Fig. 1 Backscattered SCP Raman and Raman optical activity (ROA) spectra of DNA (A) and the DNA complex with cisplatin at 0.01 molar ratio of Pt/nucleotide (B). | ||
The spectral regions sensitive to various conformational features of DNA are shown in Fig. 1 according to Duguid et al.31 while the most dominant spectral changes are labelled in red. A detailed assignment of the observed Raman and ROA bands of herring sperm DNA and its Pt complex is given in the ESI (Table S1†).
An analysis of the Raman spectra of DNA and DNA treated with cis-DDP leads to the conclusion that cisplatin binds to DNA and guanine is the most preferred site of platination. Upon Pt-binding the predominant spectral changes are observed specifically for the stretching modes of the guanine ring (1579, 1490, 1341 and 1311 cm−1).31,32 Moreover, the bands originating from hydrogen bonding between G–C17,31–35 may be also correlated with cisplatin biadducts appealing. The lack of alterations in the Raman features assigned to dA (1513 cm−1),32,34–36 dT and dC (1185 cm−1)37 confirm no or weak interactions between Pt and adenine and pyrimidines. This observation is rather expected since we used a relatively low cis-Pt/nucleotide ratio (0.01), and consequently N7-guanine monoadducts are created at the first step of the cross-linking process. The Raman spectra of DNA and its Pt-complex also show the formation of crosslinks by cis-DDP causing marked conformational transformation of DNA. A decrease in the intensity of bands characteristic for the C2′-endo conformation of sugar moieties (843 and 684 cm−1) and an increase of features assigned to C3′-endo (815 and 605 cm−1)17,19,29,31,35 endorse the conclusion of the B- to A-DNA transition, reported previously by Vrána et al.17. However, the suggested Z-form37,38 is not noticed in our work.
It is worth stressing here that the ROA spectrum of herring sperm DNA (cf.Fig. 1A bottom) shows some differences in comparison to the ROA spectrum of calf thymus DNA – the only one spectrum reported so far by Bell and co-workers.19 Likely, the observed differences result from several issues associated with sample preparation and collection of ROA spectra such as compensation of Raman bands, background correction, differences in hydration and aggregation of both types of DNA. First of all, we do not observe in the ROA spectrum recorded here a strong positive band at ca. 1480 cm−1 (see Fig. 1A bottom), which has been found in the ROA spectra of ultrasonicated calf thymus DNA, tRNA19 and other RNA types.30 The Raman counterpart is assigned to guanine and it is found to be sensitive to sugar-base torsion angles and base–base stacking interactions.31,32,34–36 In addition, this band exhibits a lower intensity in the ROA spectrum of Mg2+-free tRNAPhe, which adopts an open clover leaf form upon binding of magnesium ions. A stabilization role of Mg2+ species for the quaternary structure of tRNA is a commonly known fact.19 Hobro et al.30 have also observed a weak signal at ca. 1480 cm−1 in the ROA spectrum of the RNA 37-nucleotide based on EMC IRES domain I (internal ribosomal entry site (IRES) from encephalomyocarditis virus), which exhibits a complex cloverleaf type structure. In turn, Blanch et al.39 have associated the absence of this band in the ROA spectrum of viral RNA with a single-strand helix present in these nucleic acids, proposing the origin of the signal from the vibrations of bases within the base-paired regions of tRNAPhe. Therefore, the fact that this band is not observed in our work can be primarily explained by the presence of a long chain of herring sperm DNA as well as by preservation of the native, double-stranded form of the helix.
Evidence of a partial transition from the B-DNA to the A-form upon the interaction with cis-DDP is clearly visible from the ROA profile. A spectral marker of an A-type double helical form is a negative–positive–negative triplet at 1093, 1055, and 1008 cm−1 (Fig. 1B, bottom). The negative components of this triplet are more intense in the spectrum of DNA treated with a higher concentration of cisplatin (Fig. 2, bottom). A similar signature has been found in the ROA spectra of A-type polynucleotides,24,25 and Mg2+-free tRNA (1089, 1047, 992 cm−1), which adopt mainly the A-form by losing the C2′-endo conformation of the sugar moieties.19,30,40,41 Next, the ROA spectrum of untreated DNA shows the presence of a broad negative peak with a maximum at 989 cm−1 (Fig. 1A) which disappears after addition of cisplatin and instead of that a doublet at 1008 and 990 cm−1 occurs (Fig. 1B). The intensity of the 1008 cm−1 band increases along with an increase of the drug dose (Fig. 2). A similar effect is noticed for a negative band at 1093 cm−1. We propose that these features are ROA markers for the transformation of the C2′-endo conformation into C3′-endo. In turn, a positive band at ca. 918 cm−1 is considered to be an indicator of the C2′-endo pucker in a molecule of nucleic acids.19,24,25,40,41 This band is assigned to the stretching vibrations of the deoxyribose ring in the Raman spectrum of herring sperm DNA32 and is still observed after platination, see Fig. 1B and 2. Its presence indicates the incomplete transition of DNA from the B- to A-form. Upon the interaction with cisplatin, a broadening and shift to the higher wavenumbers of this band are found followed by the occurrence of a weak band at 899 cm−1. In addition, the spectral profile in the region of 1550–1200 cm−1 represents changes in the sugar pucker conformation from C2′-endo–C3′-endo. This is manifested by the decreasing intensity of a positive band at 1319 cm−1 and the appearance of a negative band at 1418 cm−1.24
Next, an increase in the intensity of a ROA negative doublet at 1364 and 1336 cm−1 in cisplatin-bound DNA and changes in their intensity ratio confirm the spatial rearrangement of mutual orientation of the sugar and base rings around the glycosidic link.24,41 The Raman counterparts in the DNA spectrum are mainly assigned to vibrations of guanine and adenine.32,34,35 This observation supports the formation of 1,2-GG and 1,2-AG cisplatin adducts. At the current stage of our studies, we are not able to identify the type of crosslink. However, only a little decrease in the intensity of a band at 1120 cm−1, which is specific for adenine,32,34,35 suggests that the guanine residues are rather involved in the crosslink formation.
Another spectral feature of the drug action on the chiral structure of DNA is a weak negative–positive doublet at ca. 1638 and 1625 cm−1, respectively, characteristic for both doses of cisplatin, see Fig. 1B and 2. This doublet has been proposed as a ROA marker of the right-handed helix of RNA.30 In turn, the ∼1750–1550 cm−1 region exhibits the presence of ROA bands attributed to bases involved in stacking arrangements.41 For example, a weak positive band at 1495 cm−1per analogue to the Raman band is assigned to guanine, while a band at 1462 cm−1 to adenine and thymine (see Table S1†). The changes observed in this region identify distortions of base stacking interactions and disruptions of hydrogen bonding due to cross-linking of cisplatin to a DNA molecule, cf.Fig. 1.
We summarise the most prominent markers specific for the DNA–cisplatin interaction in Table 1.
| DNA–cisplatin (r = 0.01) | DNA–cisplatin (r = 0.02) | Assignment | |
|---|---|---|---|
| DNA | Wavenumber/cm−1 | ||
| r – molar ratio of the Pt/nucleotide. ROA band: −negative, +positive. Band intensity: vs – very strong, s – strong, m – medium, w – weak, vw – very weak. Components: dA – deoxyadenosine, dC – deoxycytidine, dG – deoxyguanosine, dT – thymidine, d – deoxyribose. | |||
| +1638 w | +1637 m | B-DNA → A-form | |
| −1625 w | −1620 m | ||
| −1418 w | −1417 w | C2′-endo → C3′-endo | |
| −1365 w | +1364 m | +1367 m | dT, dA, dG |
| −1342 m | −1336 m/w | −1337 w/vw | dG, dA |
| +1120 w | +1125 w | +1125 vw | dA |
| −1093 vw | −1093 w | C2′-endo → C3′-endo | |
| +1053 w | +1055 w | +1056 w | |
| −1008 m | −1010 s | ||
| −989 m | −990 w | −987 w | |
| +899 w | +896 w | ||
| +918 w | +923 w | +924 w | |
Conclusions
We demonstrate that ROA spectroscopy is a powerful tool to monitor the DNA conformational changes occurring upon drug binding. Our results clearly show the sensitivity of this technique in following the structural alteration of the whole DNA molecule upon cisplatin action. A direct observation of DNA transformation is identified by conformational rearrangement within the chiral sugar moieties. A wide application of ROA spectroscopy for such a challenging task as the determination of the drug action on chiral molecules is to some extent limited by the requirements of the sample preparation and problems with spectra collection. This technique requires a high concentration and high purity of a sample. In addition, commercially available instrumentation employs only an excitation wavelength at 532 nm that can induce a high fluorescence background obscuring the weak ROA signal. Despite this, our work evidently shows that the description of the chiral nature of a biomolecule like nucleic acid or a receptor provides a complementary insight into the drug action.Acknowledgements
This study was supported by the European Union from the resources of the European Regional Development Fund under the Innovative Economy Programme [grant coordinated by JCET-UJ, No. POIG.01.01.02-00-069/09] and by the National Centre of Science [DEC-2013/08/A/ST4/00308].Notes and references
- J. K. Barton, in Bioinorganic chemistry, ed. I. Bertini, H. B. Gray, S. J. Lippard and J. S. Valentine, University Science Books, Mill Valley, CA, USA, 1994, pp. 455–504 Search PubMed.
- M. Egli, Chem. Biol., 2002, 9, 277–286 CrossRef CAS PubMed.
- S. Rafique, M. Idrees, A. Nasim, H. Akbar and A. Athar, Biotechnol. Mol. Biol. Rev., 2010, 5, 38–45 CAS.
- M. H. Shamsi and H. B. Kraatz, J. Inorg. Organomet. Polym. Mater., 2013, 23, 4–23 CrossRef CAS.
- I. Turel and J. Kljun, Curr. Top. Med. Chem., 2011, 11, 2661–2687 CrossRef CAS PubMed.
- C. F. J. Barnard, Platinum Met. Rev., 1989, 33, 162–167 CAS.
- R. A. Alderden, M. D. Hall and T. W. Hambley, J. Chem. Educ., 2006, 83, 728–734 CrossRef CAS.
- P. J. Loehrer and L. H. Einhorn, Ann. Intern. Med., 1984, 100, 704–713 CrossRef CAS PubMed.
- M.-H. Baik, R. A. Friesner and S. J. Lippard, J. Am. Chem. Soc., 2003, 125, 14082–14092 CrossRef CAS PubMed.
- Y.-R. Liu, C. Ji, H.-Y. Zhang, S.-X. Dou, P. Xie, W.-C. Wang and P.-Y. Wang, Arch. Biochem. Biophys., 2013, 536, 12–24 CrossRef CAS PubMed.
- P. M. Takahara, A. C. Rosenzweig, C. A. Frederick and S. J. Lippard, Nature, 1995, 377, 649–652 CrossRef CAS PubMed.
- C. J. van Garderen and L. P. van Houte, Eur. J. Biochem., 1994, 225, 1169–1179 CrossRef CAS PubMed.
- D. Tsankov, B. Kalisch, H. Van de Sande and H. Wieser, J. Phys. Chem. B, 2003, 107, 6479–6485 CrossRef CAS.
- V. Andrushchenko, H. Wieser and P. Bouř, J. Phys. Chem. A, 2007, 111, 9714–9723 CrossRef CAS PubMed.
- V. M. González, M. A. Fuertes, A. Jiménez-Ruíz, C. Alonso and J. M. Pérez, Mol. Pharmacol., 1999, 55, 770–778 Search PubMed.
- J. M. Pérez-Martín, J. M. Requena, D. Craciunescu, M. C. López and C. Alonso, J. Biol. Chem., 1993, 268, 24774–24778 Search PubMed.
- O. Vrána, V. Mašek, V. Dražan and V. Brabec, J. Struct. Biol., 2007, 159, 1–8 CrossRef PubMed.
- R. C. Todd and S. J. Lippard, J. Inorg. Biochem., 2010, 104, 902–908 CrossRef CAS PubMed.
- A. F. Bell, L. Hecht and L. D. Barron, J. Am. Chem. Soc., 1998, 120, 5820–5821 CrossRef CAS.
- N. Berova, P. L. Polavarapu, K. Nakanishi and R. W. Woody, Comprehensive chiroptical spectroscopy, John Wiley & Sons, New Jersey, 2012 Search PubMed.
- L. D. Barron and L. Hecht, in Comprehensive Chiroptical Spectroscopy, ed. N. Berova, P. L. Polavarapu, K. Nakanishi and R. W. Woody, John Wiley & Sons, Hoboken, 2012, vol. 2, pp. 759–793 Search PubMed.
- L. A. Nafie, Vibrational optical activity: principles and applications, John Wiley & Sons, UK, 2011 Search PubMed.
- V. Parchaňský, J. Kapitán and P. Bouř, RSC Adv., 2014, 4, 57125–57136 RSC.
- A. F. Bell, L. Hecht and L. D. Barron, J. Chem. Soc., Faraday Trans., 1997, 93, 553–562 RSC.
- A. F. Bell, L. Hecht and L. D. Barron, J. Am. Chem. Soc., 1997, 119, 6006–6013 CrossRef CAS.
- H. I. Elsner and E. B. Lindblad, DNA, 1989, 8, 697–701 CrossRef CAS PubMed.
- S. R. Gallagher, Curr. Protoc. Neurosci., 2011, 56, A.1 K.1–A.1 K.14 Search PubMed.
- J. M. Benevides, A. H.-J. Wang, G. A. Van Der Marel, J. H. Van Boom and G. J. Thomas Jr., Biochemistry, 1988, 27, 931–938 CrossRef CAS PubMed.
- W. Ke, D. Yu and J. Wu, Spectrochim. Acta, Part A, 1999, 55, 1081–1090 CrossRef.
- A. J. Hobro, M. Rouhi, E. W. Blanch and G. L. Conn, Nucleic Acids Res., 2007, 35, 1169–1177 CrossRef CAS PubMed.
- J. Duguid, V. A. Bloomfield, J. Benevides and G. J. Thomas Jr., Biophys. J., 1993, 65, 1916–1928 CrossRef CAS PubMed.
- S. Olsztyńska-Janus, M. Gasior-Głogowska, K. Szymborska-Małek, M. Komorowska, W. Witkiewicz, C. Pezowicz, S. Szotek and M. Kobielarz, Acta Bioeng. Biomech., 2012, 14, 121–133 Search PubMed.
- E. W. Blanch, L. Hecht and L. D. Barron, Methods, 2003, 29, 196–209 CrossRef CAS PubMed.
- S. Ponkumar, P. Duraisamy and N. Iyandurai, Am. J. Biochem. Biotechnol., 2011, 7, 135–140 CrossRef CAS.
- V. Vaverkova, O. Vrana, V. Adam, T. Pekarek, J. Jampilek and P. Babula, Biomed Res. Int., 2014 Search PubMed , 461393.
- A. J. Ruiz-Chica, M. A. Medina, F. Sánchez-Jiménez and F. J. Ramírez, Nucleic Acids Res., 2004, 32, 579–589 CrossRef CAS.
- L. Movileanu, J. M. Benevides and G. J. Thomas Jr., Biopolymers, 2002, 63, 181–194 CrossRef CAS PubMed.
- J. M. Benevides and G. J. Thomas, Nucleic Acids Res., 1983, 11, 5747–5761 CrossRef CAS PubMed.
- E. W. Blanch, L. Hecht, C. D. Syme, V. Volpetti, G. P. Lomonossoff, K. Nielsen and L. D. Barron, J. Gen. Virol., 2002, 83, 2593–2600 CrossRef CAS PubMed.
- L. D. Barron, L. Hecht, E. W. Blanch and A. F. Bell, Prog. Biophys. Mol. Biol., 2000, 73, 1–49 CrossRef CAS PubMed.
- L. D. Barron, E. W. Blanch, I. H. McColl, C. D. Syme, L. Hecht and K. Nielsen, Spectroscopy, 2003, 17, 101–126 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5an02140e |
| This journal is © The Royal Society of Chemistry 2016 |