Voltammetric behavior of HeLa cells on a carbon nanotube/ionic liquid modified electrode for cytotoxicity evaluation of chlorophenols
Xiaolin
Zhu†
a,
Hongwei
Qin†
b,
Guanlan
Wu
a,
Jinlian
Li
c,
Xing
Yuan
*a and
Dongmei
Wu
*c
aState Environmental Protection Key Laboratory of Wetland Ecology and Vegetation Restoration, School of Environment, Northeast Normal University, Changchun 130117, P.R. China. E-mail: yuanx@nenu.edu.cn; Fax: +86 431 89165606; Tel: +86 431 89165600
bCollege of Chemistry and Chemical Engineering Bohai University, Jinzhou 121013, P.R. China
cCollege of Pharmacy, Jiamusi University, Jiamusi 154007, P.R. China. E-mail: dmwu@jmsu.edu.cn; Fax: +86 454 8618460; Tel: +86 454 8618461
First published on 16th November 2015
Abstract
In the present study, the electrochemical behavior of human cervical carcinoma (HeLa) cells on a purified-multiwall carbon nanotube (p-MWCNT) and ionic liquid (IL) modified glass carbon electrode was studied. Two voltammetric signals of HeLa cells were detected, which were attributed to intracellular xanthine, guanine, hypoxanthine and adenine. The existence of these purines was further verified by the high-performance liquid chromatography (HPLC) assay. Based on the variation of the intensity of the two voltammetric signals, the cytotoxicity of 2,4-dichlorophenol (2,4-DCP), 2,4,6-trichlorophenol (2,4,6-TCP) and pentachlorophenol (PCP) was evaluated, which was also compared with the traditional methyl tetrazolium (MTT) assay. This work is expected to provide a label-free method providing more exact and perfect information to study the purine metabolism, as well as to evaluate the influence of chemicals from different perspectives.
1. Introduction
Chlorophenols (CPs), which are listed as priority pollutants, are widely used in pesticides, germicides, dyestuffs, phenolic resins, etc. They are recalcitrant to biodegradation and pose a serious threat to the environment and humans.1–3 Therefore, sensitive and reliable toxicity evaluation of CPs in the environment is greatly important. In recent years, the in vitro toxicity testing technique has been approved as an alternative to the whole-animal test.4–6 Some assays including neutral red assay, methyl tetrazolium (MTT) assay, lactate dehydrogenase leakage assay and deoxyribonucleic acid fragmentation assay have been used in the cytotoxicity evaluation of CPs.7–9 However, these marking methods have several disadvantages, such as the complexity of operation, high cost, and time-consumption. The emerging in vitro electrochemical method provides a new insight for scientists owing to its simple instrumentation, rapidness, high sensitivity, and non-toxicity.10,11 Nevertheless, the mechanism of the electrochemical behavior of cells is poorly studied due to the complexity of intracellular biological characters in the field of cell electrochemistry.In our previous study, we found that the cell viability was strongly related to intracellular nucleotide metabolism. Thus, the effects of different substances on cell activity could be attributed to the influence on purine bases, which are the intermediate products of nucleotide metabolism. The electrochemical behaviours of human prostate cancer (PC-3) and breast cancer (MCF-7) were studied and the voltammetric signal at approximately +0.7 V derived from guanine (G) and xanthine (XA) was verified.12–14 The sensitivities of several drugs were tested using the electrochemical method.12 Compared with the above cells, cervical carcinoma (HeLa) cells are good model organisms.15,16 Like normal cells, they can produce proteins, express genes, communicate with each other, and are susceptible to infections. They can also proliferate abnormally rapidly, when compared to other cancer cells.16–19 However, due to the restrictions of current graphene or carbon nanotube modified electrodes in terms of sensitivity and stability, the electrochemical detection could only be carried out in the potential range from 0.0 to +0.8 V.13–15,20 Herein, to the best our knowledge, there is no report on the electrochemical response related to the purine metabolism of HeLa cells. If more purine bases can be detected, the toxic effects of different chemicals on nucleotide metabolism will be evaluated more comprehensively by the electrochemical method.
Herein, a more sensitive and stable purified-multiwall carbon nanotube (p-MWCNT) and ionic liquid (IL) modified glass carbon electrode (p-MWCNT/IL/GCE) was prepared. Then the electrochemical behavior of HeLa cells based on the p-MWCNT/IL/GCE was studied. A new signal at +1.034 V was found for the first time and confirmed from the oxidation of adenine (A) and hypoxanthine (HX). The cytotoxicity of three CPs including 2,4-dichlorophenol (2,4-DCP), 2,4,6-trichlorophenol (2,4,6-TCP) and pentachlorophenol (PCP) is evaluated based on the two electrochemical signals, and then the results are compared with those of the MTT assay. This work will be beneficial in providing more information of effects on purine metabolism caused by hazardous pollutants.
2. Experimental
2.1 Chemicals and reagents
G, A, XA, HX, and trypsinase were purchased from Sigma. Phosphate buffer saline (PBS, pH 7.4, 0.2 M) containing KCl, Na2HPO4 and KH2PO4 was used. IL ([BMIM]PF6) was obtained from J&K Scientific Ltd. (China). Three CPs of analytical grade were purchased from Sigma. For the experiments, several serial dilutions of CP stocks were prepared in the culture medium to concentrations of 199.6, 319.4, 511.0, 817.6, and 1308.2 μM for 2,4-DCP, 49.0, 85.7, 150.0, 262.5, and 459.4 μM for 2,4,6-TCP and 29.3, 46.9, 75.0, 120.0, and 192.0 μM for PCP. All other chemicals were of analytical grade and used without further purification.2.2 Cell culture and treatment
HeLa cells were cultured in Dulbecco's Modified Eagle Medium supplemented with 10% fetal calf serum, 100 μg mL−1 penicillin and streptomycin in an incubator (37 °C, 5% CO2). The DRM-700 counting plate (China) was used for the determination of cell concentrations. Prior to the CP exposure, the medium was replaced with the media with various concentrations of CPs. After a certain incubation time, the culture medium was removed, and the adherent cells were washed with PBS for three times. Next, a certain amount of pH 7.4 PBS was added, and finally they were heated in a 50 °C water bath for 0.5 h to obtain the HeLa cell suspension.2.3 Preparation of the p-MWCNT/IL/GCE
Purification procedures were applied to MWCNTs (diameter: 10–20 nm, Shenzhen Nanotech Port Co. Ltd, China) by refluxing in 30% HNO3 at 100 °C for 1 d. They were then separated, washed with double-distilled water, filtered through a 0.45 μm filter membrane and dried at 120 °C to obtain the p-MWCNTs. The GCE was polished with 1.0, 0.3 and 0.05 μm alumina slurry, followed by rinsing thoroughly with double-distilled water and ethanol in an ultrasonic bath. Then the GCE was allowed to dry at room temperature. 1 mg p-MWCNTs and 20 μL IL were ground together. A portion of p-MWCNTs/IL was coated on the GCE to fabricate the p-MWNTs/IL/GCE. For comparison, a MWCNT/GCE was prepared according to our group's previous work.12 Prior to use, all the electrodes were scanned in pH 7.4 PBS for twenty cycles to obtain a stable background.2.4 Electrochemical experiments
In this work, the p-MWCNT/IL/GCE (working electrode), the Pt wire (counter electrode), and the Ag/AgCl (reference electrode) were tested on a LK2005 electrochemical workstation (Lanlike Instruments, China) for electrochemical measurements. After each measurement, the p-MWCNT/IL/GCE was renewed by scanning five times in pH 7.4 PBS from 0.0 to +1.1 V. 480 s was selected as the best accumulation time for the electrochemical experiments. The cytotoxicity of CPs was calculated from the formula:| Cytotoxicity = [(iblank − iCP)]/iblank]100% | (1) |
2.5 MTT assay
1.2 × 104 cells per mL HeLa cells in 200 μL of either the medium alone or the medium containing different CPs were added to the 96-well microtitre plates. The plates were incubated at 37 °C for 30 h, and then 20 μL of 5 mg mL−1 MTT (Sigma) was added to the wells. Four hours later, the medium containing MTT was removed and 150 μL sodium dodecyl sulfate was added. The absorbance was recorded at 490 nm using a microplate spectrophotometer system. The cytotoxicity of CPs was calculated from the formula:| Cytotoxicity = [(Ablank − ACP)/Ablank]100% | (2) |
2.6 High-performance liquid chromatography (HPLC) measurements
Prior to HPLC, the proteins in the HeLa cell suspension were removed by subsiding the denatured protein at 50 °C for 0.5 h. The cell suspension was filtered through a 0.22 μM filter after centrifugation at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for about 50 min. An HPLC system (Agilent 1100 series) equipped with an Ascentis RP-Amide column (4.0 × 250 mm), a quaternary pump, an inline degasser and a diode array detector (DAD) was used for the analysis of samples. The mobile phase with 0.02 M KH2PO4 was run at 1.0 mL min−1. The injection volume was 20 μL for all samples.
000 × g for about 50 min. An HPLC system (Agilent 1100 series) equipped with an Ascentis RP-Amide column (4.0 × 250 mm), a quaternary pump, an inline degasser and a diode array detector (DAD) was used for the analysis of samples. The mobile phase with 0.02 M KH2PO4 was run at 1.0 mL min−1. The injection volume was 20 μL for all samples.
2.7 Statistical analysis
Each experiment was carried out at least three times. The results were expressed as mean ± standard deviation (SD). Data from all experiments were analyzed with Statistical Product and Service Solutions (SPSS) 19.0 and Origin 8.0. Statistical differences were evaluated by using student's t-test. A p-value of less than 0.05 was considered statistically significant.3. Results and discussion
3.1 Voltammetric behavior of HeLa cell suspension
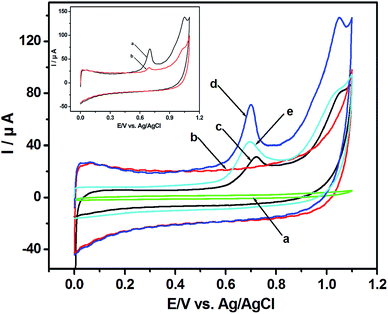
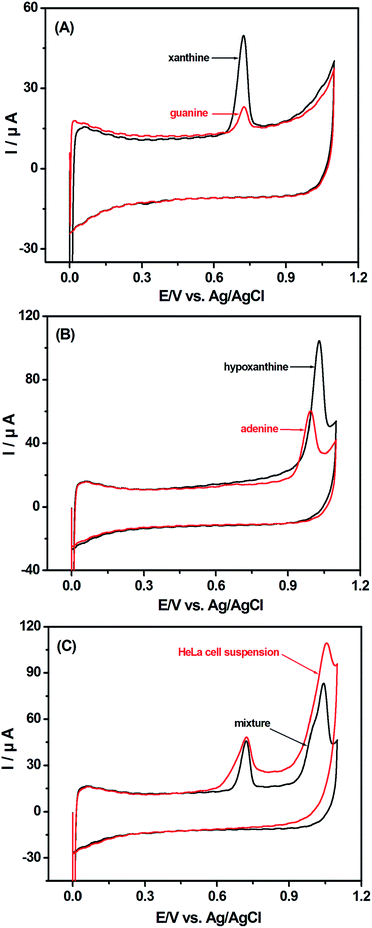
Fig. 1 shows the cyclic voltammograms (CVs) of HeLa cell suspension. No peak is detected with the GCE in HeLa cell suspension (Fig. 1a) and with the p-MWCNT/IL/GCE in PBS (Fig. 1b). However, the background current of the p-MWCNT/IL/GCE is higher than that of the GCE, which reflects its more effective surface area. With the p-MWCNT/IL/GCE in HeLa cell suspension, two anodic peaks at +0.720 V and +1.034 V were observed obviously (Fig. 1d). Additionally, the oxidation potentials shift to less positive ones and the currents are greater than those of the MWCNT/GCE (Fig. 1c) used commonly in our previous study.12 Moreover, compared with the unpurified-MWCNT/IL/GCE (Fig. 1e), the p-MWCNT/IL/GCE exhibits higher electrocatalytic activities owing to its hydrophilic functional groups such as COOH and OH obtained from the purification. These results imply that the p-MWCNTs/IL film possesses excellent electrocatalytic activities towards electroactive species in the HeLa cell suspension, and promotes the electron-transfer rate on the surface of the electrode. No reduction peak is found in the reverse scan, which indicates that the electrooxidation reaction is an irreversible process. Compared with the first scan (curve a in the inset of Fig. 1), the peak current reduces greatly in the second scan (curve b in the inset of Fig. 1) possibly due to the products of the oxidation reaction that has blocked the p-MWCNT/IL/GCE surface.15 Hence the first scan was chosen for the voltammetric analysis.The possible biochemistry mechanism of the two voltammetric signals is studied. As shown in Fig. 2A and B, the peak potentials of XA (+0.718 V) and G (+0.722 V) are very close, as well as those of A (+1.029 V) and HX (+1.035 V). The irreversible anodic peaks of the mixture of XA, G, A and HX are at +0.719 V and +1.031 V, which are almost the same as those of the HeLa cell suspension (+0.720 V and +1.034 V) (Fig. 2C). The negligible discrepancies in peak potentials between the cell suspension and the mixture of G/XA and A/HX are probably ascribed to the weak pH increase caused by the bases secreted into the cell suspension.22 The above results show that the peaks could be caused by the oxidation of G/XA and A/HX in HeLa cell suspension.
3.2 HPLC confirmation of purine bases in HeLa cell suspension
The HPLC method was employed to further verify the existence of the four purine bases in the HeLa cell suspension. As shown in Fig. 3, the chromatogram indicates that HX, G, XA and A have a retention time of 9.823, 10.80, 11.77 and 14.90 min, respectively (Fig. 3A). They are in good agreement with the four chromatographic peaks of the HeLa cell suspension at 9.864, 10.75 11.79 and 14.89 min, respectively (Fig. 3B), which illustrates the existence of the above four purine bases secreted into the HeLa cell suspension.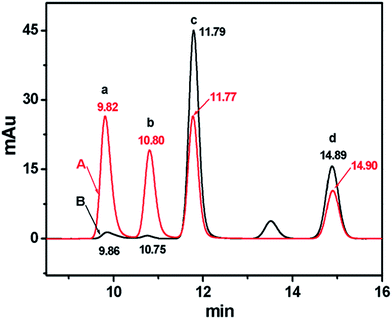 | ||
| Fig. 3 Chromatograms of (A) the mixture of 50.0 μg mL−1 HX (a), G (b), XA (c) and A (d), and (B) the HeLa cell cytoplasm eluent. Cell concentration: 7.2 × 106 cells per mL. | ||
3.3 Description of HeLa cell growth
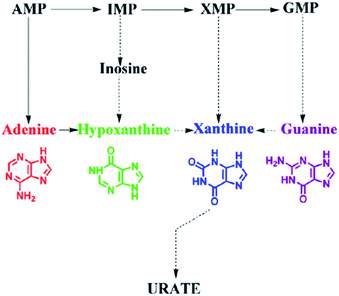
G, XA, A and HX are metabolic intermediates of intracellular guanine nucleotide (GMP), xanthine nucleotide (XMP), adenine nucleotide (AMP) and inosinic acid (IMP) as interpreted in Fig. 4. The high cell viability could accelerate the intracellular metabolic process and then more G, XA, A and HX were metabolized. Thence, the electrochemical measurement of purines which act as intracellular signaling molecules could be selected to reflect the cell viability.HeLa cells with the same concentration were cultured under the same conditions. They were harvested and heated for detection every 6 h. Fig. 5A shows the cell growth curves depicted by the two electrochemical signals of HeLa cell suspension. The peak currents increase gradually in 30 h because of the proliferation of HeLa cells, and then decrease sharply, owing to the apoptosis of cells attributed to the nutrient deficiency. The results are similar to the findings of the cell counting method (Fig. 5B). Therefore, both signals I and II could be used to describe the growth of HeLa cells.
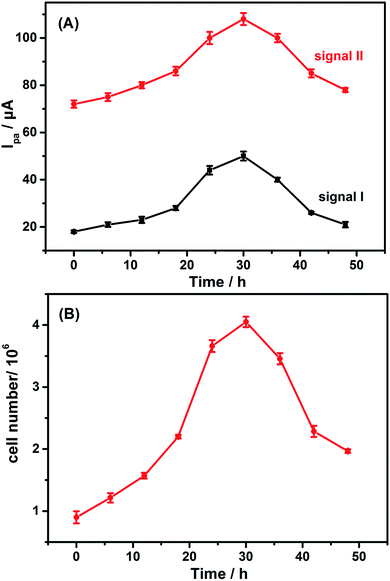 | ||
| Fig. 5 Growth curves of the HeLa cells described by (A) the electrochemical method-signal I (a) and signal II (b), and (B) the cell counting method. | ||
3.4 Cytotoxicity evaluation of 2,4-DCP, 2,4,6-TCP and PCP
The voltammetric behavior of the HeLa cell suspension after the cells were treated with different CPs was studied. All the peak currents of treated CPs are less than those of the control, which indicates that the three CPs have inhibitory effects on HeLa cells. The cytotoxicity reached the maximum after CPs were added to the cell culture medium for 30 h. Therefore, 30 h was chosen as the optimal CP-treatment time in the experiment.Fig. 6 shows the cyclic voltammograms of HeLa cells treated by using CPs with various concentrations for 30 h. The peak currents of CP-treated groups are less than those of the control groups. All the treatment groups had significant differences compared with the control group. The oxidation peak currents depend on the concentration of CPs. As the concentration of 2,4-DCP, 2,4,6-TCP and PCP increases, the peak currents reduce. The cytotoxicity of CPs can act in several ways including inducing chromosome breakage and aneuploidy, damaging the cell membrane and cytoplasm and inhibiting ATP synthesis.23–26 The above results could induce the reduction of cell viability, and reduce the peak currents attributed to the four purine bases, which indicates the change of the physiological characteristics of HeLa cells.
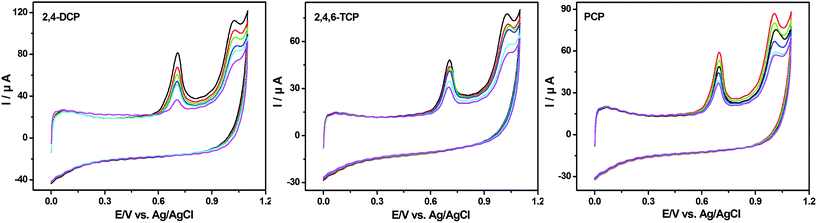 | ||
| Fig. 6 CVs of HeLa cell suspension treated by using three CPs with various concentrations. Cell inoculation concentration: 6.0 × 105 cells per mL; treatment time: 30 h; scan rate: 50 mV s−1. | ||
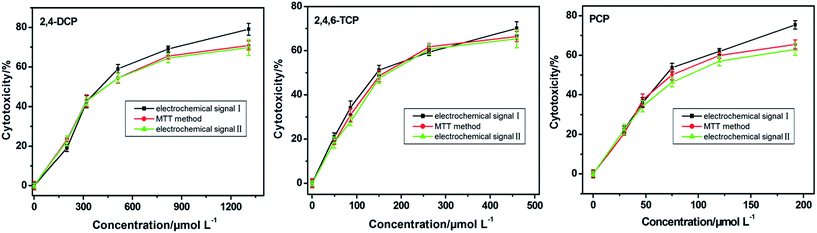
Fig. 7 displays the cytotoxicity curves of HeLa cells exposed to 2,4-DCP, 2,4,6-TCP and PCP for 30 h. The cytotoxicity is linear with the negative logarithm concentration of CP. The IC50 values obtained by the electrochemical signal I and signal II are 451.8 and 495.8 μM for 2,4-DCP, 172.5 and 198.5 μM for 2,4,6-TCP, and 75.91 and 96.40 μM for PCP, respectively. The obtained cytotoxic tendency is PCP > 2,4,6-TCP > 2,4-DCP, which is consistent with the conclusions in other literature and also confirms a correlation between the cytotoxicity of CPs and the number of substituted chlorine atoms.22,27 However, the IC50 obtained by signal I is lower than that of signal II, which may reflect that 2,4-DCP, 2,4,6-TCP and PCP have greater impacts on xanthine/guanine than on hypoxanthine/adenine. The results indicate the different effects of CPs on diverse levels of intracellular purine metabolism. Compared with the previous one-signal electrochemical method, the two electrochemical signals can provide more information about effects of CPs with different parameters, which also has significance for research of the physiological process about purine metabolism. Moreover, the MTT assay was compared with the results obtained by the electrochemical method based on HeLa cells. The IC50 values obtained by the MTT assay are 484.2, 187.9 and 86.81 μM for 2,4-DCP, 2,4,6-TCP and PCP, respectively. The toxicity orders are consistent with those obtained by the electrochemical test. The results suggest that the electrochemical method based on the intracellular purine metabolism of HeLa cells was reliable to assess the cytotoxicity of CPs. Moreover, compared with the traditional MTT assay, the label-free electrochemical method is much easier and faster. The electrochemical detection process only needs 0.5 h, while the MTT assay takes about 5 h.
Finally, the repeatability and reproducibility of the p-MWCNT/IL/GCE were investigated by twenty successive voltammetric measurements. The relative standard deviation (RSD) of 1.0 × 106 cells per mL HeLa cell suspension is 4.31%. Besides, six p-MWCNT/IL/GCEs were independently fabricated to be applied to 1.0 × 106 cells per mL HeLa cell suspension. Statistical differences among the results were tested by student's t-test. The p-value is 0.155, which indicates that there is no significant change among the six p-MWCNT/IL/GCEs (p > 0.05). The results demonstrate that the p-MWCNT/IL/GCE possesses good repeatability and reproducibility. Additionally, the linear detection ranges of the p-MWCNT/IL/GCE are 0.5 to 220.0 μM for G, 0.25 to 200.0 μM for XA, 0.5 to 250.0 μM for A, 0.5 to 250.0 μM for HX, and 5.0 × 103 to 4.5 × 106 cells per mL for HeLa cells, respectively. The lower detection limits are 0.1 μM, 0.03 μM, 0.04 μM, 0.04 μM and 1.0 × 103 cells per mL.
4. Conclusion
In summary, the biochemical mechanism of the voltammetric response for the HeLa cell suspension on a p-MWCNT/IL/GCE was explained. The cytotoxicity of 2,4-DCP, 2,4,6-TCP and PCP was evaluated by the two voltammetric signals. Further, the results were compared with those of the conventional MTT assay. The electrochemical assay developed here is faster and simpler and can be usefully employed to assess more thoroughly the cytotoxicity of CPs. This study provides a basis for future research aiming to evaluate the effects of different substances based on purine metabolism.Acknowledgements
We gratefully acknowledge the National Natural Science Foundation (NNSF) of China (51178092 and 81273132), the Fundamental Research Funds for the Central Universities (14ZZ1517), the Nature Science Foundation of Heilongjiang Province (D201005 and D201016 a), the Scientific Research Fund of Heilongjiang Provincial Education Department (12511542) and the PhD Start-up Fund of Bohai University (bsqd201429).References
- S. S. Gupta, M. Stadler, C. A. Noser, A. Ghosh, B. Steinhoff, D. Lenoir, C. P. Horwitz, K. W. Schramm and T. J. Collins, Science, 2002, 296, 326–328 CrossRef PubMed.
- C. S. Evans and B. Dellinger, Environ. Sci. Technol., 2005, 39, 122–127 CrossRef CAS PubMed.
- A. Karci, Chemosphere, 2014, 99, 1–18 CrossRef CAS PubMed.
- M. Davoren and A. M. Fogarty, Toxicol. In Vitro, 2006, 20, 1190–1201 CrossRef CAS PubMed.
- X. Liu, J. Chen, H. Yu, J. Zhao, J. P. Giesy and X. Wang, Chemosphere, 2006, 64, 1619–1626 CrossRef CAS PubMed.
- G. O. Rankin, A. Sweeney, C. Racine, T. Ferguson, D. Preston and D. K. Anestis, Chem.–Biol. Interact., 2014, 222, 126–132 CrossRef CAS PubMed.
- A. Castaño and M. J. Gómez-Lechó, Toxicol. In Vitro, 2005, 19, 695–705 CrossRef PubMed.
- J. Chen, J. Jiang, F. Zhang, H. Yu and J. Zhang, Cell Biol. Toxicol., 2004, 20, 183–196 CrossRef CAS PubMed.
- H. Saito, J. Koyasu, T. Shigeoka and I. Tomita, Toxicol. In Vitro, 1994, 8, 1107–1112 CrossRef CAS PubMed.
- L. Ding, D. Du, J. Wu and H. Ju, Electrochem. Commun., 2007, 9, 953–958 CrossRef CAS.
- D. L. Robinson, T. J. Volz, J. O. Schenk and R. M. Wightman, Alcohol.: Clin. Exp. Res., 2005, 29, 746–755 CrossRef CAS.
- D. M. Wu, G. L. Fu, J. T. Wang, L. Y. Ge, J. H. Zhu, X. Yuan and J. G. Liu, Electrochem. Commun., 2011, 13, 623–626 CrossRef CAS.
- H. Qin, Q. Gao, H. Niu, Z. Wang, X. Zhu, J. Li, X. Yuan and D. Wu, Analyst, 2013, 138, 3372–3375 RSC.
- X. Guo, Q. Wang, J. Li, J. Cui, S. Zhou, S. Hao and D. Wu, Biosens. Bioelectron., 2015, 64, 594–596 CrossRef CAS PubMed.
- X. Zhu, H. Qin, J. Liu, Z. Zhang, Y. Lu, X. Yuan and D. Wu, J. Hazard. Mater., 2014, 271, 210–219 CrossRef CAS PubMed.
- M. Ivanković, A. Ćukušić, I. Gotić, N. Škrobot, M. Matijašić, D. Polančec and I. Rubelj, Biogerontology, 2007, 8, 163–172 CrossRef PubMed.
- B. Ekwall, Toxicity to HeLa cells of 205 drugs as determined by the metabolic inhibition test supplemented by microscopy, Toxicology, 1980, 17, 273–295 CrossRef CAS PubMed.
- R. A. Garduño, F. D. Quinn and P. S. Hoffman, Can. J. Microbiol., 1998, 44, 430–440 CrossRef.
- S. Patel, N. Gheewala, A. Suthar and A. Shah, Int. J. Pharm. Pharm. Sci., 2009, 1, 38–46 Search PubMed.
- K. Chen, J. Chen, M. Guo, Z. Li and S. Yao, Electroanalysis, 2006, 18, 1179–1185 CrossRef CAS.
- K. Thomulka, C. Abbas, D. Young and J. Lange, Bull. Environ. Contam. Toxicol., 1996, 56, 446–452 CrossRef CAS PubMed.
- C. Y. Chen and J. H. Lin, Chemosphere, 2006, 62, 503–509 CrossRef CAS PubMed.
- M. J. Armstrong, S. M. Galloway and J. Ashby, Mutat. Res. Lett., 1993, 303, 101–108 CrossRef CAS.
- P. Fernández Freire, V. Labrador, J. M. Pérez Martín and M. J. Hazen, Toxicology, 2005, 210, 37–44 CrossRef PubMed.
- T. T. Liao, Y. L. Shi, J. W. Jia, R. W. Jia and L. Wang, J. Hazard. Mater., 2010, 179, 1055–1064 CrossRef CAS PubMed.
- Y. J. Wang, Y. S. Ho, J. H. Jeng, H. J. Su and C. C. Lee, Chem.–Biol. Interact., 2000, 128, 173–188 CrossRef CAS PubMed.
- T. Tiensing, N. Strachan and G. I. Paton, J. Environ. Monit., 2002, 4, 482–489 RSC.
Footnote |
| † Xiaolin Zhu and Hongwei Qin have equal contribution to this work. |
| This journal is © The Royal Society of Chemistry 2016 |