Gas-phase chemistry of ruthenium and rhodium carbonyl complexes
Shiwei
Cao
ab,
Yang
Wang
abc,
Zhi
Qin
*a,
Fangli
Fan
a,
Hiromitsu
Haba
d,
Yukiko
Komori
d,
Xiaolei
Wu
a,
Cunmin
Tan
abc and
Xin
Zhang
ab
aNuclear Chemistry, Institute of Modern Physics, Chinese Academy of Sciences, No. 509 Nanchang Rd., 730000, Lanzhou, China. E-mail: qinzhi@impcas.ac.cn
bSchool of Physical Science and Technology, University of Chinese Academy of Sciences, No. 19A Yuquan Rd., 100049, Beijing, China
cSchool of Nuclear Science and Technology, Lanzhou University, No. 222 Tianshuinan Rd., 730000, Lanzhou, China
dNishina Center for Accelerator-Based Science, RIKEN, Wako, Saitama 351-0198, Japan
First published on 5th November 2015
Abstract
Short-lived ruthenium and rhodium isotopes were produced from a 252Cf spontaneous fission (SF) source. Their volatile carbonyl complexes were formed in gas-phase reactions in situ with the carbon-monoxide containing gas. A gas-jet system was employed to transport the volatile carbonyls from the recoil chamber to the chemical separation apparatus. The gas-phase chemical behaviors of these carbonyl complexes were studied using an online low temperature isothermal chromatography (IC) technique. Long IC columns made up of FEP Teflon were used to obtain the chemical information of the high-volatile Ru and Rh carbonyls. By excluding the influence of precursor effects, short-lived isotopes of 109–110Ru and 111–112Rh were used to represent the chemical behaviours of Ru and Rh carbonyls. Relative chemical yields of about 75% and 20% were measured for Ru(CO)5 and Rh(CO)4, respectively, relative to the yields of KCl aerosols transported in Ar gas. The adsorption enthalpies of ruthenium and rhodium carbonyl complexes on a Teflon surface were determined to be around ΔHads = –33+1−2 kJ mol−1 and −36+2−1 kJ mol−1, respectively, by fitting the breakthrough curves of the corresponding carbonyl complexes with a Monte Carlo simulation program. Different from Mo and Tc carbonyls, a small amount of oxygen gas was found to be not effective for the chemical yields of ruthenium and rhodium carbonyl complexes. The general chemical behaviors of short-lived carbonyl complexes of group VI–IX elements were discussed, which can be used in the future study on the gas-phase chemistry of superheavy elements – Bh, Hs, and Mt carbonyls.
Introduction
The synthesis of superheavy elements (SHEs; Z ≥ 104) and their chemistry studies are attractive but challenging in the field of nuclear science. The superheavy elements can only be produced in heavy-ion reactions at the “one-atom-at-a-time” level with very short half-lives. These extreme conditions require the development of unique components and methods to achieve the ultimate goal of chemically isolating one single atom that lives for only a few seconds. Due to very strong relativistic effects on the valence electron shells, the SHEs should behave dissimilarly to their lighter homologs in the chemical groups.1 Significant deviations of the chemical behaviors of SHEs require the studies of their lighter homologs, which can provide corresponding chemical information, and contribute to the further studies of SHEs.Simple inorganic compounds like oxides, halides, hydroxides, and oxyhalides of SHEs and their homologs have been investigated in the past few decades.2–5 In recent years, transition metal – organic complexes have been drawing much attention, and the new classes of carbonyl complexes opened the perspectives to the chemical studies of SHEs.1 As these complexes were known to be volatile under ambient conditions, gas-phase chromatography techniques were used to study their chemical properties.6–11
The EAN rule (effective atomic number rule), known as the 18-electron rule, primarily gives the qualitative predictions for the coordination and structures of many transition metal complexes.12 In previous work, Mo(CO)6 and W(CO)6 were observed in the gas-phase experiments,7 and were confirmed in matrix isolation experiments.13 For groups VII–IX, Tc(CO)5, Ru(CO)5, and Rh(CO)4 were proposed according to the 18-electron rule.14 In other studies, Tc(CO)5 and Re(CO)5 were considered to be the only possible structures, which should thermodynamically exist under specific conditions.10,11 Short-lived Re and Ir carbonyls were studied using a TransActinide Separator and Chemistry Apparatus (TASCA),10 and the coordination numbers of 170Re(CO)x and 178Ir(CO)x were proposed to be x = 5 and 4, respectively.10
The chemical behaviors of Mo and W carbonyl complexes were studied systematically in ref. 8, 10, 11 and 14. Furthermore, the first synthesis of an organometallic compound of a superheavy element, seaborgium hexacarbonyl (Sg(CO)6), was recently successfully achieved, and its adsorption enthalpy on the quartz surface was deduced.9 So far, the gas-phase chemistry of carbonyl complexes of group VI has been studied adequately. The chemical information on group VI–IX carbonyls was reported by Even et al.10 The adsorption enthalpy of Tc carbonyls and the thermal stabilities of Mo, W, Tc, Ru, and Rh carbonyl complexes were also studied in other groups.8,11,14 However, in ref. 10, the authors did not give final conclusions on the adsorption enthalpies or chemical yields of Tc, Ru or Rh carbonyls, since these observed isotopes were influenced by strong precursor effects in the experiment with a 249Cf target irradiated at the nuclear reactor; no chemical yields of Ru and Rh carbonyls were reported. Therefore, the systematic studies on Ru and Rh carbonyl complexes are requisite and urgent for the future chemical investigation of carbonyl complexes of element 108, Hs and element 109, Mt, respectively.
In the present work, short-lived ruthenium and rhodium isotopes were generated from a 252Cf spontaneous fission (SF) source, and their volatile carbonyl complexes were formed in gas-phase reactions. 109–110Ru and 111–112Rh carbonyls, which could exclude the precursor effects, were studied using a low-temperature IC technique. The adsorption enthalpies were obtained from the breakthrough curves of the corresponding carbonyl complexes. The relative chemical yields and the influence of a small amount of oxygen gas were also studied.
Experimental
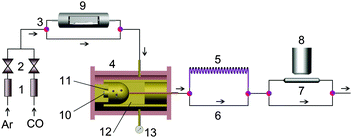
The same experimental setup was used in the previous work,11 see Fig. 1. The system consists of (a) the gas-jet transport system, (b) the 252Cf source chamber, (c) the low-temperature isothermal chromatography apparatus, and (d) the collecting, detection, and data acquisition system. The components were connected via fluorinated ethylene propylene (FEP) Teflon tubes. For more details, please see ref. 11.According to the Monte Carlo simulation, a 5-m-long FEP IC column (i.d. = 4 mm) was used to obtain the complete breakthrough curves of high-volatile ruthenium carbonyls in the limited temperature range. And a 3-m-long (i.d. = 2 mm) FEP IC column was used in the rhodium carbonyl experiment.
All experiments were performed at 19 °C and at 1.05 bar with a total gas flow rate of 1000 mL min−1, except for these with oxygen containing gas with a gas flow rate of 1500 mL min−1. The online collecting time for each experiment was 30 min.
Results and discussion
Formation of Ru and Rh carbonyl complexes
Fig. 2 shows the typical γ-ray spectra of 252Cf fission fragments transported with KCl aerosols in Ar gas. All fission fragments with suitable half-lives and γ-ray energies can be found without chemical selectivity. Fig. 3 shows only the volatile species among the fission products, e.g.137–139Xe, transported in pure Ar gas without any aerosol.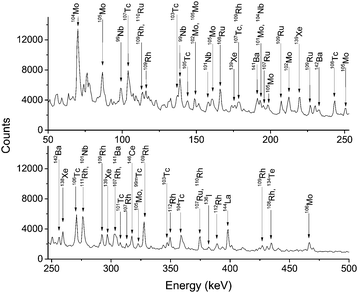 | ||
| Fig. 2 γ-ray spectra of fission products transported in Ar gas with KCl aerosol particles. The total gas flow rate was 1 L min−1. The sample was collected and detected on-line for 30 min. | ||
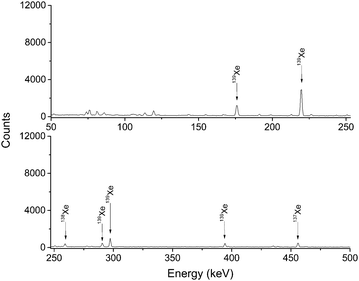 | ||
| Fig. 3 γ-ray spectra of fission products transported in pure Ar gas. The total gas flow rate was 1 L min−1. The sample was collected and detected on-line for 30 min. | ||
When CO gas was added to Ar gas, several Mo, Tc, Ru, and Rh isotopes appeared in the spectra, see Fig. 4. This proved that volatile carbonyl complexes of these 4d elements were synthesized and transported from the 252Cf source chamber to the charcoal trap.
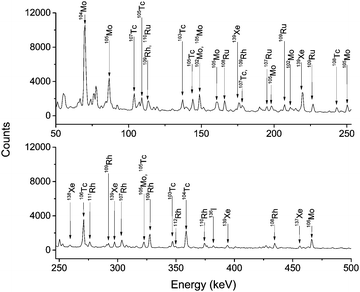 | ||
| Fig. 4 γ-ray spectra of fission products transported in a mixed gas of 50% Ar and 50% CO. The gas flow rate was 1 L min−1. The total sample was collected and detected on-line for 30 min. | ||
Since there is no direct experimental evidence to provide any information on the configuration of Ru or Rh complexes under such conditions, the structures of these complexes can only be inferred theoretically.
As the production rates of metal atoms from the 252Cf fission source are very low (not higher than few thousands per second), the probability of atoms meeting each other in the gas-phase, and forming polynuclear complexes, e.g. Mx(CO)y (x ≥ 2), can be excluded. It is convinced that these short-lived isotopes would form mononuclear complexes rather than polynuclear complexes.10,11
Fig. 5 shows the valence electron (VE) configurations of central atoms in Ru, Rh, Mo, and Tc carbonyls according to the valence bond (VB) theory. A free ruthenium atom has 8 VEs. The bonding arrangement results in four paired electrons in two of the 4d orbitals and four unpaired electrons in the other three 4d orbitals and 5s orbitals. When ruthenium forms a carbonyl complex, one of the 4d orbitals, one 5s and three 5p orbitals are made available to form a set of empty dsp3 hybrid orbitals by means of electron pairing.12 Five empty orbitals can accept at most five pairs of electrons which are donated by CO ligands, and reach the number of electrons in the next noble gas atom. As a result, Ru(CO)5 is the final saturated structure, which is known to be stable in macrochemistry, see Fig. 5a.
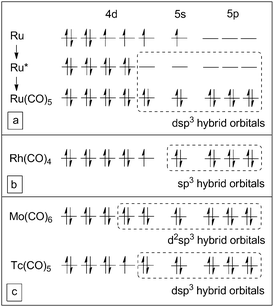 | ||
| Fig. 5 The valence electron configurations of central atoms in Ru(CO)5, Rh(CO)4, Mo(CO)6, and Tc(CO)5. | ||
In the formation of rhodium carbonyls, 9 VEs from the Rh atom require all the five 4d orbitals, and one electron remains unpaired. Only one 5s and three 5p orbitals are used in the hybrids. The resulting sp3 hybrid orbitals can accommodate at most four pairs of electrons, and for CO ligands the maximum coordination number is 4. In this case, Rh(CO)4 gives a total of 17 electrons in the valence shell of Rh, see Fig. 5b. Since the formation of polynuclear carbonyls is excluded, this may be the only possibility which is closest to the stable structure according to the EAN rule.
In the same way, the maximum coordination number of Mo and Tc mononuclear carbonyls, which were mentioned in previous studies,7–11 can also be inferred to be 6 and 5, respectively, see Fig. 5c.
In a laser-ablation matrix-isolation infrared spectroscopy experiment,13 the free Mo/W atoms form carbonyl complexes directly in CO and inert gas mixtures. The low-coordinate complexes were found to be spontaneously transformed into high-coordinate complexes under such conditions, and saturated Mo(CO)6/W(CO)6 was the final stable product. It can be deduced that the low-coordinate carbonyl complexes of Ru/Rh have the same trend to transform into saturated complexes under the same conditions. Therefore, Ru(CO)5 and Rh(CO)4 should be the final products in reactions with free atoms and CO containing inert gas.
Additionally, Bogdan et al. reported that the ruthenium carbonyls like Ru(CO)5 could be observed using transient infrared spectroscopy.15,16 Ozin et al. reported that Rh(CO)1–4 was observed in solid argon in earlier thermal atom experiments.17 Even et al. also proposed that ruthenium and rhodium were most likely to form pentacarbonyl ruthenium and tetracarbonyl rhodium under similar conditions.10 Though Ru(CO)5 and Rh(CO)4 could be reactive and unstable in macrochemistry, they are the only possible binary, mononuclear, neutral and saturated chemical forms in the current study.
Relative chemical yields
In this work, the first step is to decide which isotopes can be used to represent the corresponding elements.In Even's work,10 short-lived isotopes of 106–107Tc, 107–108Ru and 107–110,110mRh produced using the 249Cf source were identified in the spectra. The transport yields of 106–107Tc, 108Ru, and 109Rh as a function of CO concentrations were illustrated.
But these isotopes were all influenced by precursor effects, and cannot represent the behaviors of the corresponding elements. As a result, no quantified chemical information of ruthenium and rhodium could be deduced in that study.
In our recent study,11 for a certain isotope the precursor effects can be excluded only if (1) the fraction (Fr) of the independent yields (Yi) to the cumulative yields (Yc) is large enough, and (2) the precursor's half-life is short enough to decay in the recoil chamber.
The Yi and Yc values of short-lived Tc, Ru, and Rh isotopes observed in the γ-ray spectra are shown in Table 1. For ruthenium, most of the 107Ru (t1/2 = 3.8 min) atoms are formed from the β−-decay of 107Tc (t1/2 = 21.2 s), and only 13% are formed directly. This means that the 107Tc atoms form carbonyl complexes and pass through the IC column, but give the signals of 107Tc and 107Ru in the charcoal trap during the measurement, giving strong precursor effects on the daughter 107Ru. 108Tc and 108Ru are in the same situation. These two isotopes should show almost the same behaviors as 108Tc.
| Isobar | Nuclide | Half-life | Independent yield (Yi) | Cumulative yield (Yc) | Fraction (%) (Fr) |
|---|---|---|---|---|---|
| 107 | 107Tc | 21.2 s | 3.630 | 5.730 | 63 |
| 107Ru | 3.8 min | 0.872 | 6.600 | 13 | |
| 108 | 108Tc | 5.17 s | 3.330 | 4.010 | 83 |
| 108Ru | 4.5 min | 1.980 | 5.990 | 33 | |
| 109 | 109Tc | 0.86 s | 1.890 | 2.040 | 93 |
| 109Ru | 34.5 s | 2.990 | 5.030 | 59 | |
| 109mRh | 50 s | 0.254 | 2.770 | 9 | |
| 109Rh | 1.34 min | 0.652 | 5.930 | 11 | |
| 110 | 110Tc | 0.83 s | 0.855 | 0.878 | 97 |
| 110Ru | 11.6 s | 3.620 | 4.500 | 80 | |
| 110mRh | 29 s | 0.675 | 0.675 | 100 | |
| 110Rh | 3.1 s | 0.672 | 5.170 | 13 | |
| 111 | 111Ru | 2.12 s | 2.260 | 2.440 | 93 |
| 111Rh | 11 s | 2.460 | 4.890 | 50 | |
| 112 | 112Ru | 1.75 s | 0.939 | 0.962 | 98 |
| 112Rh | 6.73 s | 2.390 | 3.350 | 71 |
For 109Ru (t1/2 = 34.5 s; Eγ = 206 keV, Iγ = 22%; Eγ = 226 keV, Iγ = 20%), the Yi value is large enough (59%), and the half-life of its mother 109Tc (t1/2 = 0.86 s) is too short to be transported from the recoil chamber to the charcoal trap. This means that the precursor effect of 109Tc on 109Ru can be completely excluded. Similarly, 110Ru (t1/2 = 11.6 s; Eγ = 112.2 keV, Iγ = 23%) will not be influenced by precursor effects, since its mother 110Tc (t1/2 = 0.83 s) has a very short half-life.
For rhodium, the isotopes of 109–112Rh can be observed. In view of above-mentioned reasons, 109–110Rh is strongly affected by the precursor effects and showed the same behavior as 109–110Ru. Only 111Rh (t1/2 = 11 s; Eγ = 275 keV; Iγ = 100%) and 112Rh (t1/2 = 6.8 s; Eγ = 348.7 keV; Iγ = 89%) can represent the behavior of element Rh.
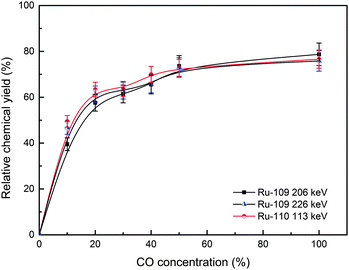
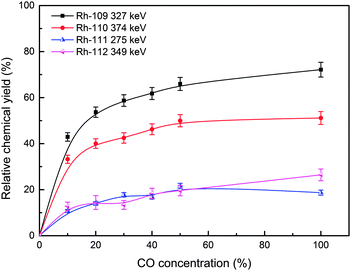
The relative chemical yields of the investigated carbonyl complexes as a function of CO concentration are shown in Fig. 6 and 7. The data were fixed by the constant yields of the volatile fission product 139Xe, to eliminate the influence of the gas flow rate. The yields are given relative to those of KCl aerosol transport. The relative chemical yields of 109–110Ru carbonyl complexes reach the maximum of about 75% (Fig. 6). Meanwhile, the maximum yields of 111–112Rh approach about 20% (Fig. 7). As mentioned above, the maximum yields of 109–110Rh isotopes are far higher than those of 111–112Rh, and similar to their precursors 109–110Ru. For all these carbonyl complexes, the relative chemical yields reach the maximum basically when CO concentration is higher than 40%.
The results show the difference in chemical yields of the carbonyl complexes of group VIII and group IX. According to the EAN rule, the numbers of effective electrons in Ru(CO)5 are 18, and it suggests that Ru(CO)5 has a stable structure, and may have a relatively high chemical yield. However, Rh(CO)4 has an unstable structure, because of its 17 effective electrons. This could make its chemical yields much lower than Ru(CO)5.
Adsorption enthalpies
Fig. 8 shows the breakthrough curves of 109–110Ru with a 5-m-long FEP IC column (i.d. = 4 mm) using 1 L min−1 mixed gas (Ar![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO = 1
CO = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1.05 bar; 19 °C). Since the Ru carbonyls are very volatile,8 a Monte Carlo simulation program18 was used to select the length and the radius of the IC column according to the estimated adsorption enthalpy values and simulated breakthrough curves. At each experimental temperature, the products were collected and measured on-line for 30 minutes.
1; 1.05 bar; 19 °C). Since the Ru carbonyls are very volatile,8 a Monte Carlo simulation program18 was used to select the length and the radius of the IC column according to the estimated adsorption enthalpy values and simulated breakthrough curves. At each experimental temperature, the products were collected and measured on-line for 30 minutes.
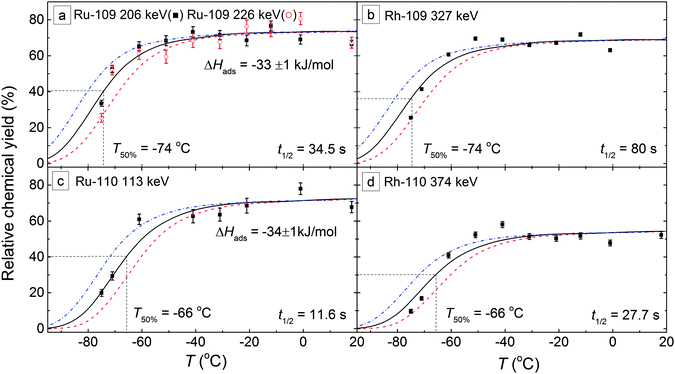 | ||
| Fig. 8 Breakthrough curves of 109–110Ru and 109–110Rh carbonyls with a 5-m-long FEP Teflon IC column (i.d. = 4 mm). The symbols (black boxes and circles) are the experimental data. The solid curves are the results of the Monte Carlo simulations18 with the corresponding adsorption enthalpies. For Rh isotopes, the curves are consistent with the simulation results of their precursors. | ||
In Fig. 8, the simulation results show a near-perfect match with the breakthrough curves. By fitting the experimental data with the Monte Carlo simulation, the adsorption enthalpy of Ru carbonyls was determined to be ΔHads = –33+1−2 kJ mol−1 on the FEP surface. The T50% values, where 50% of the molecules pass the IC column, are −74 °C and −66 °C for 109Ru and 110Ru carbonyls, respectively, due to the difference of their half-lives.
Since most of the 109Rh atoms are formed from the β−-decay of 109Ru in the charcoal trap, the precursor effects strongly affect 109Rh. The breakthrough curve of 109Rh is similar to 109Ru and shows an equal T50% value (Fig. 8a and b). 110Rh is in the same situation and shows the behaviors of 110Ru (Fig. 8c and d).
Fig. 9 shows the breakthrough curves of 111–112Rh with a 3-m-long FEP IC column (i.d. = 2 mm), fitting with the Monte Carlo simulation. The gas flow rate was 1 L min−1 (Ar![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO = 1
CO = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1.05 bar; 19 °C). An adsorption enthalpy of ΔHads = −36+2−1 kJ mol−1 was deduced for Rh carbonyls on the FEP Teflon surface. The T50% appears at −58 °C for both 111Rh and 112Rh. In contrast to Ru carbonyls, Rh carbonyl complexes are less volatile and have lower relative chemical yields.
1; 1.05 bar; 19 °C). An adsorption enthalpy of ΔHads = −36+2−1 kJ mol−1 was deduced for Rh carbonyls on the FEP Teflon surface. The T50% appears at −58 °C for both 111Rh and 112Rh. In contrast to Ru carbonyls, Rh carbonyl complexes are less volatile and have lower relative chemical yields.
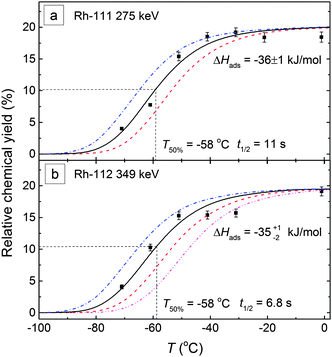 | ||
| Fig. 9 Breakthrough curves of Rh carbonyls on the FEP Teflon surface, fitting with Monte Carlo simulation. The symbols are the experimental data. The solid curves are the results of Monte Carlo simulations18 with the corresponding adsorption enthalpies. | ||
Influence of oxygen on the chemical yields of ruthenium and rhodium carbonyl complexes
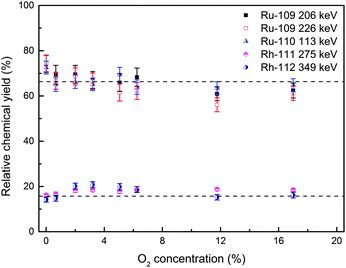
In previous studies, a trace amount of O2 was found to have a strong negative influence on chemical yields of Mo, Tc, and W carbonyl complexes.10,11 Wang et al. reported that 2% of oxygen gas already makes the relative yields drop from 100% to 70% for Mo, and from 25% to 15% for Tc. With 6% of O2 gas, about 70% of Mo and Tc carbonyls were lost.11To verify such an influence on carbonyl complexes of group VIII and group IX elements, experiments under the same conditions were performed. A small amount of O2 was added into the gas mixture (Ar![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO = 1
CO = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with a total flow rate of nearly 1.5 L min−1. The relative chemical yields of Ru and Rh were obtained in different O2 concentrations. The relative chemical yields of corresponding carbonyls were obtained, relative to the transport yields with KCl aerosol in Ar gas.
1) with a total flow rate of nearly 1.5 L min−1. The relative chemical yields of Ru and Rh were obtained in different O2 concentrations. The relative chemical yields of corresponding carbonyls were obtained, relative to the transport yields with KCl aerosol in Ar gas.
In Fig. 10, we can see that with the O2 concentrations varying from 0% to 17%, the relative chemical yields of Ru and Rh carbonyls remain basically constant, which remain around 70% and 20%, respectively. Thus, no significant influence of O2 gas was observed on the chemical yields of Ru and Rh carbonyls under the present experimental conditions.
Gas-phase behaviors of group VI–IX element carbonyl complexes
The chemical information of group VI–IX element carbonyl complexes is shown in Table 2. The VE numbers of the corresponding carbonyls are listed. For group VI and group VIII elements, their carbonyls have 18 VEs, and should be stable according to the EAN rule. Not surprisingly, they were found to have high chemical yields in the experiments. For group VII and group IX elements, their carbonyls have 17 VEs. They would be unstable and low-yield according to the EAN rule. The low chemical yields were confirmed in the present experiments.| Group | VE numbers | Complexes | Relative chemical yield (%) | −ΔHads on the Teflon surface (kJ mol−1) | −ΔHads on the SiO2 surface (kJ mol−1) |
|---|---|---|---|---|---|
| a Data from ref. 11. b Data from ref. 10. c Data from ref. 8. | |||||
| VI | 18 | Mo(CO)6 | 100a | 38 ± 2a | 39 ± 2a |
| W(CO)6 | 46.5 ± 2.5b | ||||
| VII | 17 | Tc(CO)5 | 25a | 43 ± 2a | 43 ± 2a |
| Re(CO)5 | 43 ± 3b | ||||
| VIII | 18 | Ru(CO)5 | 75 | 33+2−1 | 35 ± 1c |
| Os(CO)5 | 43.5+3.5−2.5b | ||||
| IX | 17 | Rh(CO)4 | 20 | 36+1−2 | 37 ± 3b |
| Ir(CO)4 | |||||
For group VI and group VIII elements, the −ΔHads values of W/Os carbonyls on the quartz surface are about 8 kJ mol−1 higher than those of Mo/Ru carbonyls. However, for group VI and group VIII elements, Re/Ir and Tc/Rh carbonyls have basically the same adsorption enthalpies, respectively.
This work gives important information for the future study on the carbonyls of SHEs – 108Hs and 109Mt. If their chemical properties are not strongly affected by the relativistic effects, it may suggest that Hs carbonyl complexes will have higher stability than Mt carbonyl complexes.
Additionally, the Hs carbonyls may be less volatile than Os carbonyls, while the ΔHads value of Mt carbonyl complexes may still be close to that of Ir carbonyls, which is around −37 kJ mol–1.
Conclusions
Short-lived ruthenium and rhodium carbonyl complexes were synthesized and their gas-phase chemistry was studied using an online isothermal chromatography apparatus coupled to a 252Cf SF source. In this work, the VB theory and the EAN rule were used to deduce the coordination and VE configuration of the 4d element carbonyls formed in gas-phase reactions of 252Cf SF fission fragments and CO gas. As a result, Mo(CO)6, Tc(CO)5, Ru(CO)5, and Rh(CO)4 should be the final products in the current study. Chemical yields of 75% and about 20% were measured for Ru(CO)5 and Rh(CO)4, respectively. The CO concentration of 40% was found to be enough to give the highest yield for Ru and Rh carbonyls in the Ar/CO gas mixture.By fitting the breakthrough curves with the Monte Carlo simulation, the adsorption enthalpies of Ru(CO)5 and Rh(CO)4 on the FEP Teflon surface were deduced to be around ΔHads = −33 kJ mol−1 and −36 kJ mol−1, respectively. Furthermore, a small amount of oxygen gas was found to have no remarkable influence on the chemical yields of Ru and Rh carbonyls, which was totally different from Mo and Tc carbonyls.
In combination with the previous studies, the general chemical behaviors of group VI–IX element carbonyl complexes were discussed. And several important information was provided for the future study on the gas-phase chemistry of SHEs – Bh, Hs and Mt.
Acknowledgements
The present work was supported by the National natural Science Foundation of China (Grant No. 11079006, 11505250, and 11575260) and the West Light Foundation of Chinese Academy of Sciences (CAS).Notes and references
- A. Türler and V. Pershina, Chem. Rev., 2013, 113, 1237–1312 CrossRef PubMed
.
- A. Türler, Radiochim. Acta, 1996, 72, 7–18 CrossRef
.
- S. Hübener, S. Taut, A. Vahle, R. Dressler, B. Eichler, H. Gäggeler, D. Jost, D. Piguet, A. Türler and W. Brüchle, Radiochim. Acta, 2001, 89, 737 CrossRef
.
- B. Eichler, A. Türler and H. Gäggeler, J. Phys. Chem. A, 1999, 103, 9296–9306 CrossRef CAS
.
- R. Eichler, B. Eichler, H. Gäggeler, D. Jost, D. Piguet and A. Türler, Radiochim. Acta, 2000, 88, 87 CAS
.
- M. S. Lin, Z. Qin, F. A. Lei, J. S. Guo, L. N. Zhang, H. J. Ding, F. L. Fan, J. Bai and X. L. Wu, Radiochim. Acta, 2010, 98, 321–326 CrossRef CAS
.
- J. Even, A. Yakushev, C. E. Düllmann, J. Dvorak, R. Eichler, O. Gothe, D. Hild, E. Jäger, J. Khuyagbaatar, J. V. Kratz, J. Krier, L. Niewisch, H. Nitsche, I. Pysmenetska, M. Schädel, B. Schausten, A. Türler, N. Wiehl and D. Wittwer, Inorg. Chem., 2012, 51, 6431–6433 CrossRef CAS PubMed
.
- Y. Wang, Z. Qin, F. L. Fan, F. Y. Fan, S. W. Cao, X. L. Wu, X. Zhang, J. Bai, X. J. Yin, L. L. Tian, L. Zhao, W. Tian, Z. Li, C. M. Tan, J. S. Guo and H. W. Gäggeler, Radiochim. Acta, 2014, 102, 69–76 CAS
.
- J. Even, A. Yakushev, Ch. E. Düllmann, H. Haba, M. Asai, T. K. Sato, H. Brand, A. Di Nitto, R. Eichler, F. L. Fan, W. Hartmann, M. Huang, E. Jäger, D. Kaji, J. Kanaya, Y. Kaneya, J. Khuyagbaatar, B. Kindler, J. V. Kratz, J. Krier, Y. Kudou, N. Kurz, B. Lommel, S. Miyashita, K. Morimoto, K. Morita, M. Murakami, Y. Nagame, H. Nitsche, K. Ooe, Z. Qin, M. Schädel, J. Steiner, T. Sumita, M. Takeyama, K. Tanaka, A. Toyoshima, K. Tsukada, A. Türler, I. Usoltsev, Y. Wakabayashi, Y. Wang, N. Wiehl and S. Yamaki, Science, 2014, 345, 1491–1493 CrossRef CAS PubMed
.
- J. Even, A. Yakushev, C. E. Düllmann, J. Dvorak, R. Eichler, O. Gothe, W. Hartmann, D. Hild, E. Jäger, J. Khuyagbaatar, B. Kindler, J. V. Kratz, J. Krier, B. Lommel, L. Niewisch, H. Nitsche, I. Pysmenetska, M. Schädel, B. Schausten, A. Türler, N. Wiehl and D. Wittwer, Radiochim. Acta, 2014, 102, 1093–1110 CrossRef CAS
.
- Y. Wang, Z. Qin, F. L. Fan, H. Haba, Y. Komori, S. W. Cao, X. L. Wu and C. M. Tan, Phys. Chem. Chem. Phys., 2015, 17, 13228–13234 RSC
.
-
J. E. House, in Inorg. Chem., ed. J. E. House, Academic Press, America, 2nd edn, 2013, ch. 16, pp. 553–590 Search PubMed
.
- F. L. Fan, Y. Wang and Z. Qin, presented in Poster Contributions at 5th International Conference on the Chemistry and Physics of the TransActinide Elements, Japan, May, 2015.
- I. Usoltsev, R. Eichler, Y. Wang, J. Even, A. Yakushev, H. Haba, M. Asai, H. Brand, A. Di Nitto, Ch. E. Düllmann, F. Fangli, W. Hartmann, M. Huang, E. Jäger, D. Kaji, J. Kanaya, Y. Kaneya, J. Khuyagbaatar, B. Kindler, J. V. Kratz, J. Krier, Y. Kudou, N. Kurz, B. Lommel, S. Miyashita, K. Morimoto, K. Morita, M. Murakami, Y. Nagame, H. Nitsche, K. Ooe, T. K. Sato, M. Schädel, J. Steiner, P. Steinegger, T. Sumita, M. Takeyama, K. Tanaka, A. Toyoshima, K. Tsukada, A. Türler, Y. Wakabayashi, N. Wiehl, S. Yamaki and Z. Qin, Radiochim. Acta, 2015 DOI:10.1515/ract-2015-2445
.
- M. Zhou, L. Andrews and C. W. Bauschlicher, Chem. Rev., 2001, 101, 1931–1962 CrossRef CAS PubMed
.
- P. L. Bogdan and E. Weitz, J. Am. Chem. Soc., 1989, 111, 3163–3167 CrossRef CAS
.
- G. A. Ozin and A. L. Hanlan, Inorg. Chem., 1979, 18, 2091–2101 CrossRef CAS
.
-
I. Zvára, The Inorganic Radiochemistry of Heavy Elements: Methods for Studying Gaseous Compounds, Springer Science & Business Media, Germany, 2008 Search PubMed
.
- Fission Product Yields, http://ie.lbl.gov/fission.html, accessed 20 Dec., 2014.
| This journal is © the Owner Societies 2016 |