Open Access Article
Open Access ArticleSynthesis, characterisation, and catalytic evaluation of hierarchical faujasite zeolites: milestones, challenges, and future directions†
D.
Verboekend
*a,
N.
Nuttens
a,
R.
Locus
a,
J.
Van Aelst
a,
P.
Verolme
b,
J. C.
Groen
b,
J.
Pérez-Ramírez
c and
B. F.
Sels
a
aDepartment M2S, K.U. Leuven, Kasteelpark Arenberg 23, 3001 Heverlee, Belgium. E-mail: danny.verboekend@biw.kuleuven.be
bDelft Solids Solutions B.V., Molenweer 2 B, 2291 NR Wateringen, The Netherlands
cInstitute for Chemical and Bioengineering, Department of Chemistry and Applied Biosciences, ETH Zurich, Vladimir-Prelog-Weg 1, CH-8093, Zurich, Switzerland
First published on 27th August 2015
Abstract
Faujasite (X, Y, and USY) zeolites represent one of the most widely-applied and abundant catalysts and sorbents in the chemical industry. In the last 5 years substantial progress was made in the synthesis, characterisation, and catalytic exploitation of hierarchically-structured variants of these zeolites. Hererin, we provide an overview of these contributions, highlighting the main advancements regarding the evaluation of the nature and functionality of introduced secondary porosity. The novelty, efficiency, versatility, and sustainability of the reported bottom-up and (predominately) top-down strategies are discussed. The crucial role of the relative stability of faujasites in aqueous media is highlighted. The interplay between the physico-chemical properties of the hierarchical zeolites and their use in petrochemical and biomass-related catalytic processes is assessed.
1. Introduction
Faujasites comprise aluminosilicate zeolites with the FAU topology (Fig. 1a). The zeolites within this framework combine a 3-dimensional network of accessible micropores (0.74 nm) with an organic-free synthesis. In addition, the framework composition (Si/Al ratio) can be tuned from 1 to infinite by altering the hydrothermal synthesis (ratios 1 to 3) or by application of post-synthetic modifications (ratios 3 to infinite). As a result, they have become the most widely-applied zeolites in adsorption and catalysis. Of the faujasites, X was first conceived with a Si/Al = 1–1.5, featuring a large cation-exchange capacity, and is accordingly industrially used as ion exchanger, molecular sieve, and adsorbent. In catalysis, however, the protonic form of X is unstable. Accordingly, a more stable less-acidic variant was conceived in the form of zeolite Y. This zeolite comprises a Si/Al = 2.5 and accordingly only about 50% of the total acidity of X. Moreover, unlike X, the Si/Al ratio of Y zeolites can be increased and tuned using steam and/or acid treatments, while largely maintaining crystallinity. The resulting zeolites, with a framework Si/Al ratio of ca. 6 and higher, were labeled ‘ultra-stable’ Y (USY) due to their improved catalytic and hydrothermal stability. Accordingly, the Y and USY zeolites excel as catalysts in numerous petrochemical applications, such as (hydro)cracking, where they can display remarkable activity and (shape) selectivity.1–6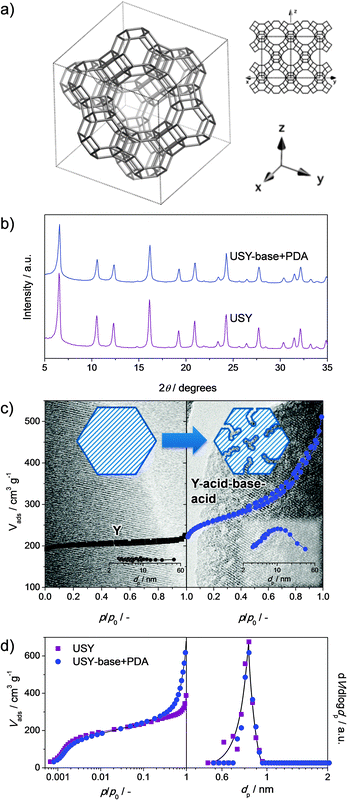 | ||
| Fig. 1 The most common techniques to assess the intrinsic zeolite properties and the nature and quantity of secondary porosity in hierarchical faujasites. The crystallinity is the fingerprint of the faujasite structure2 (a) which is usually assessed using X-ray diffraction (b). Particularly, in post-synthetic modifications, the quantitative determination of the crystallinity is important to assess the influence of mesopore formation and/or the presence of secondary phases. Another key intrinsic zeolite property is the microporosity. The amount of microporosity is often derived by application of the t-plot method to the nitrogen adsorption isotherms at 77 K (c). However, a more accurate assessment of the microporosity is obtained by application of NLDFT methods to argon adsorption isotherms obtained at 87 K (d). The secondary porosity is most routinely assessed by evaluation of the nitrogen isotherms at 77 K. In this case, application of the BJH method to the adsorption isotherms yields the mesopore size (insets in c). Hysteresis on the desorption branch of the isotherm yields valuable information regarding the degree of cavitation of the mesoporosity. Electron microscopy is routinely used to assess the mesopores, resulting in a more sponge-like appearance, which should ideally be combined with the preservation of lattice fringes (c). | ||
Although the micropores in zeolites are responsible for superior properties compared to amorphous aluminosilicates (ASAs), they also cause diffusion limitations inside the zeolite crystals. In addition, the crystal is inaccessible for a wide variety of bulky molecules, which can only react on the external surface. To alleviate these problems, the class of hierarchically structured zeolites originated around the year 2000. This type of materials couples an auxiliary level of meso- or macroporosity to the micropores, enhancing mass transport and diffusion within the crystals, ideally while maintaining the intrinsic zeolitic properties (Fig. 1). In addition, the enhanced external surface of the crystal provides ample active sites for reactants that, due to size constraints, cannot enter the micropores. As a result, hierarchical zeolites have attained superior performance in a wide variety of catalysed reactions like alkylation, isomerisation, and (hydro)cracking.7–13
Although center of attention in the last 5 years, the occurrence of hierarchical faujasites is not entirely novel. By stabilizing the Y zeolites to form USY zeolites by steaming and subsequent acid leaching, meso- and macropores are formed, which have been known for decades.14–16 However, secondary porosity was regarded a side product of the framework stabilizing effect, and its beneficial role in catalysed reactions was perhaps never fully identified. This questionable role of the developed secondary porosity was supported by diffusional studies17 and microscopy studies,18 which highlighted that a large part of the mesopores form isolated cavities in the crystals and therefore do not significantly contribute to enhancing the molecular transport.
The development of synthetic pathways to attain hierarchical faujasites occurred relatively late. The latter may be due to the fact that a large focus on the synthesis of hierarchical zeolites has been on the application of bottom-up methods, involving the modification of the hydrothermal synthesis protocol.8–13 Ironically, the most common zeolite catalyst, viz. USY, cannot be directly made by hydrothermal synthesis, forcing the synthesis of hierarchical faujasites to rely predominately on post-synthetic modifications. As the secondary porosity generated by dealumination remained in question,17,18 the use of acid leaching to generate mesoporosity did not attract further attention. Also, the potential of base treatments to enhance the accessibility of the USY active sites has remained obscure for a relatively long time. Only about a decade after the pioneering work of Ogura et al.19 and Groen et al.20 on the preparation of hierarchical ZSM-5, the potential of hierarchical USY zeolites prepared by base treatment was recognized.21,22 Even more recently, the application of optimized dealumination treatments on X and Y zeolites23 revitalized the use of acid treatments as tool to generate functional mesoporosity.
The key works on the synthesis of hierarchical faujasites have originated a substantial academic and industrial attention. This interest focused primarily on different acid and base leaching techniques to prepare hierarchical X, Y, and predominately USY zeolites. In addition, the resulting materials have attained promising results in existing and novel applications in the petrochemical industry and in biomass-related conversions. Accordingly, synthesis–property–function relationships and descriptors were identified and, moreover, novel types of active sites were generated. Nevertheless, these papers (duly referred to below) have been reported as stand-alone contributions preventing a comprehensive interpretation. Moreover, various pitfalls in materials synthesis and data interpretation have been identified, which, particularly to those outside the field, are crucial for the practical implementation of these attractive materials.
Herein, we provide an overview of the recent publications and patents dedicated to the preparation and catalytic exploitation of hierarchical faujasites. The synthetic pathways are classified based on applied treatments, and the resulting solids are evaluated based on the type, nature, location, and efficiency of the introduced secondary porosity and the remaining zeolitic properties. Often-overlooked environmental and economic concerns are highlighted, which are particularly relevant to methods involving (micelle-forming) organics, such as tetrapropylammonium (TPA+) or cetyltrimethylammonium (CTA+) cations. Stability aspects and related mesopore formation mechanisms are emphasized, which enable to generalize the behavior of X, Y, and USY in post-synthetic modifications primarily based on their composition. We highlight why, compared to the modification of 10 member-ring (MR) zeolites like ZSM-5, ferrierite, and ZSM-22 reviewed in 2011,13 the post-synthetic design of the 12-MR faujasites in aqueous solutions is particularly challenging. Catalytic evaluations of Y and USY zeolites are summarized, confirming the role of the secondary porosity generated by base and acid leaching, and highlighting the nature and potential of novel types of acid and basic sites. Finally, future directions are provided on synthetic, characterisation, and catalytic aspects.
2. Synthesis
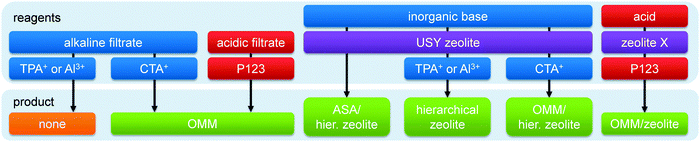
From a material perspective, the aluminosilicates species that form the ‘perfect’ hierarchical faujasite should be purely incorporated in the FAU topology, and its secondary porosity should stem from inter and/or intra-crystalline voids between the crystalline domains. In addition, in order for a mesoporous faujasite to be considered ‘hierarchical’ its secondary porosity should enhance the task of the active sites located in the micropores. This implies that the mesoporosity should be accessible from the outside, and that steamed zeolites, although mesoporous, may therefore not necessarily be considered ‘hierarchical’. A typical hierarchical faujasite combines a substantial mesoporosity (>200 m2 g−1)25 with a maximum preservation of the crystallinity, microporosity, and acidity, which depends on the Si/Al ratio and cation. A brief overview of typical properties of such hierarchical faujasites is presented in Fig. 1. An overview of the different strategies is provided in Fig. 2, whereas the implications of different post-synthetic treatments on X and Y zeolites are summarized in Table 1. Of course, as elaborated in Section 3, the exact properties of the optimal hierarchical faujasite should be dictated by its application. For example, in fluid catalytic cracking (FCC) only a fraction (ca. 10–30%) of the catalyst granule is zeolitic,24 and it may therefore be necessary that the faujasite crystals are accompanied by amorphous species.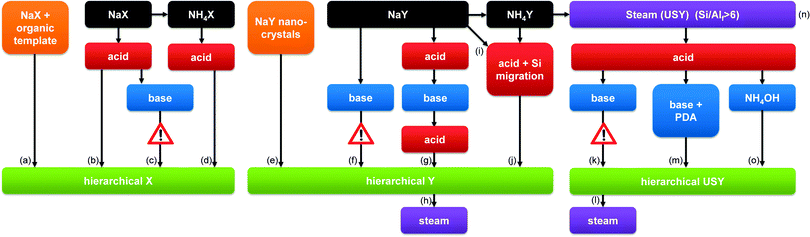 | ||
| Fig. 2 The synthesis of hierarchical faujasites using bottom-up (orange) and top-down strategies. Top-down methods consist of steam (purple), acid (red), and base (blue) treatments. The colour coding is used throughout the figures of the review. The references used to compose this illustration are summarized in Table S1 (ESI†). The exclamation mark in (c) indicates that this strategy has not proven more beneficial than (b) (see similar mesopore volumes in Fig. 7a). The exclamation mark in (f) indicates that base treatment on parent Y zeolites is inefficient (see Fig. 4). The exclamation mark in (k) highlights the severe amorphisation that USY zeolites undergo in inorganic bases (see also Fig. 12a). | ||
| Treatmenta | Crystallinitye | Mesoporosityf | ||||
|---|---|---|---|---|---|---|
| X | Y | USY | X | Y | USY | |
| a In case of a sequence, the effect of the last step (outside of the brackets) is described. b Dealumination of the framework using the Soxhlet procedure reported in ref. 23. c X, Y: dealumination of the framework using standard direct treatment. USY: Removal of extra-framework aluminum produced by steaming. d Removal of alkaline-induced realuminated species. e Ranges from strong reduction (−−), to no significant change in crystallinity (+/−), to strong enhancement (++). f Ranges from negligible (−−), to limited mesopore formation (+/−), to very strong enhancement (++). g Not applicable. | ||||||
| Acid + Si migrationb | nag | +/− | na | na | ++ | na |
| Acidc | − | − | +/− | +/− | − | − |
| Base | +/− | +/− | −− | −− | −− | ++ |
| Base + PDA | +/− | +/− | +/− | −− | −− | ++ |
| (Acidc)–base | +/− | + | na | + | + | na |
| (Acidc–base)–mild acidd | na | ++ | na | na | + | na |
| Steam | na | +/− | na | na | +/− | na |
2.1. Bottom-up strategies
Bottom-up approaches are worthwhile as they often unravel new types of synthetic possibilities and can yield solids with exceptional features, which can be nicely visualized using microscopic techniques (Fig. 3). A recent example is the hierarchical X prepared by including 3-(trimethoxysilyl)propyl hexadecyl dimethyl ammonium chloride (TPHAC) as template in the hydrothermal synthesis (Fig. 3a).26 The degree of mesoporosity introduced was moderate (maximum external surface introduced was ca. 100 m2 g−1, Fig. 4). However, the efficiency of these materials as catalysts or sorbents remains unclear. Besides the synthesis of hierarchical zeolite crystals with intracrystalline cavities, hierarchical faujasites can also be made by reducing the crystal size to the nanometer range, giving rise to intercrystalline voids.7 These nanocrystals are prepared by altering the hydrothermal conditions such that the crystal nucleation is favored over crystal growth. In addition, the prevention of aggregation is a crucial element, which can be achieved using a large number of nucleating crystals (high supersaturation) and/or steric stabilisation of the nucleating crystals (e.g. using organics). Several studies have focused on synthesizing FAU nanocrystals, yielding moderate mesoporosity, but substantial macroporosity.27,28 However, they are often obtained at very low yield (ca. 6 wt% of Si in the gel is incorporated in the solid).27 Moreover, the resulting materials can comprise substantial amorphous (unreacted) species. These contribute to the solid's mesoporosity, but lower the specific micropore volume. In a recent work, Awala et al.28 demonstrated the potential of bottom-up methods by synthesizing, without organic additives, X and Y nanocrystals (Fig. 3e). The external surface is almost double compared to that of the hierarchical X prepared using TPHAC (Table 2, Fig. 4), and the material displayed enhanced activity in the cracking of triisopropylbenzene. However, although promising, an inherent disadvantage of bottom-up approaches is the inability to hydrothermally synthesize highly-siliceous faujasites (with Si/Al > 6). Due to the stability complications of faujasites within this compositional range, their potential in conventional catalytic applications is unclear. It is accordingly not surprising that most bottom-up techniques used to make zeolite catalysts, such as soft and hard templating, focus on zeolites that are hydrothermally synthesized directly with relatively high Si/Al ratios (>10), like ZSM-5, ZSM-12, and MCM-22.7–12| Method | Si/Al gel (mol mol−1) | Si/Al solid (mol mol−1) | Yield zeolitea (g cm−3) | Alumina yieldb (g g−1) | Silica yieldb (g g−1) | Crystal. time (h) | V micro (cm3 g−1) | S meso (m2 g−1) |
|---|---|---|---|---|---|---|---|---|
| a Estimated by dividing the yield of zeolite by the sum of the used liquids. b g of alumina or silica in zeolite vs. alumina or silica in gel, respectively. c Ref. 48. d Ref. 28. e Not mentioned, based on Si/Al ratio difference between the gel and the final product. f Assumed 1.00 for further calculations. | ||||||||
| Conventionalc | 5.0 | 2.43 | 0.11 | 0.98 | 0.47 | 7 | 0.30 | 20 |
| 10 nm nanocrystalsd | 7.1 | 1.60 | 0.07e | 1.00f | 0.22 | 45 | 0.30 | 180 |
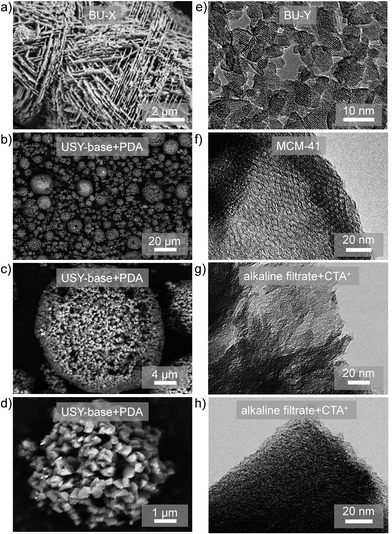 | ||
| Fig. 3 (Left) Scanning electron micrographs of (a) hierarchical X, prepared using a bottom-up (BU) strategy,26 and (b–d) hierarchical USY zeolites prepared by spray drying of the suspension obtained by alkaline treatment.52 (Right) Transmission electron microscopy (TEM) images of (e) Y nanozeolites,28 (f) MCM-41,49 and (g and h) MCM-41 prepared by reacting alkaline filtrates derived from desilication of zeolites with CTABr.49 The materials in (g) and (h) do not display clear MCM-41 features microscopically. This renders it challenging to distinguish hierarchical zeolites from CTA+-derived hierarchical zeolite/OMM composites using TEM. (a–d) Reprinted with permission from Wiley-VCH Verlag. (e) Reprinted with permission from Macmillan Publishers. (f–h) Reprinted with permission from American Chemical Society. | ||
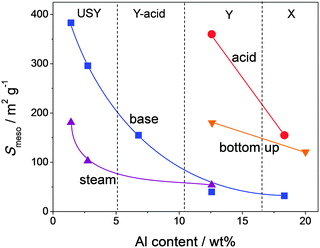 | ||
| Fig. 4 Mesopore surface area (Smeso) of hierarchical faujasites prepared by steam (triangles), acid (circles), and base treatment (squares), and bottom-up strategies (inverted triangles). Alkaline treatment is most efficient for relative low Al contents and acid treatments more efficient for Al-rich faujasites. Steaming and bottom-up strategies yield moderate external surfaces. The references used to compose this figure are summarized in Table S1 (ESI†). | ||
2.2. Top-down strategies
It was recently demonstrated that NH4X is a lot more reactive than the standard NaX in aqueous acid or base treatments.23 This phenomenon has enabled the first-time hierarchisation of zeolite X by post-synthetic modification. The pronounced influence of the type of the exchangeable charge balancing counter cations (CBCCs) was since long established for steam treatment in Y zeolites,16 but also plays a key factor in the modification of high-alumina zeolites by aqueous solutions in general. By acid treatment in aqueous solutions of Na2H2EDTA, NH4X was partially dissolved and external surfaces up to 177 m2 g−1 were achieved (Fig. 4). This was attained while preserving the crystallinity and micropore volume for about 70%. Since Y does not dissolve in aqueous Na2H2EDTA,22 the dissolution of zeolite X emphasizes the stability differences between X and Y. The relative stability aspect of faujasites is further discussed in Section 2.6. The efficiency of the leaching process can be assessed by relating the mesopore surface generated to the weight loss upon the treatment. This efficiency, with unit m2 g−1 %−1, was introduced for the preparation of hierarchical zeolites by desilication, and was accordingly labelled ‘desilication efficiency’.55 However, since dealumination by acid leaching can now also be used to generate functional mesoporosity, it may be more appropriate to refer to it as ‘mesopore efficiency’. The mesopore formation in NH4X was, unlike in the case of H4EDTA-treated NH4Y, considered suboptimal as only 2 m2 g−1 of external surface was introduced per percent of dissolved solid. This stands in great contrast with the dealuminated Y zeolite described above, where about 15 m2 g−1 %−1 was attained. This large difference may be attributed to the partial fragmentation of the crystals, which in turn, finds its origin in the larger content and more unfavorable distribution of aluminum atoms in the FAU framework in the case of X.31,32 Subsequent removal of Al atoms from zeolite X, significantly increases the concomitant removal of Si from the framework.32 However, it must be emphasized that, the reaction time and the manner in which the acid is exposed to the zeolite (direct or controlled) was not optimized. The different susceptibility of zeolite X containing different cations was confirmed in a later work where hierarchical X zeolites were prepared by a partial ion exchange (from Na+ to NH4+ form) followed by calcination.33 This method attained a similar external surface (ca. 200 m2 g−2) as the Na2H2EDTA treatment, and like for the latter treatment, at more severe conditions the zeolitic properties are reduced.
Instead of steaming, the framework Si/Al in Y zeolites can also be increased by acid treatment.22,35 As shown in Section 2.2.1, an optimized treatment alone can render faujasites with well-developed and functional mesoporosity. However, as the framework Si/Al ratio as well as the bulk Si/Al ratio increases, the material can be readily transformed into hierarchical zeolites by base leaching in inorganic bases such as NaOH or KOH. Moreover, unlike its steamed analogue, this alkaline treatment does not strongly amorphize the sample.22,23,35 This is attributed to the fact that non-optimized acid treatments create defects and Al-deficient zones,16,36,37 which are selectively removed by base leaching. This process originates intracrystalline mesoporosity and external surfaces up to around 200 m2 g−1.22,23,35 Importantly, base treatment on Al-rich zeolites gives rise to substantial realumination of the external surface. Although these species are tetrahedrally coordinated, they should not be considered as classical framework species as they lower crystallinity, display a particular acidity, and can detriment catalytic performance.22,23,25 Accordingly, a mild acid wash using Na2H2EDTA, devised not to influence the zeolite framework, can be used to remove those species, enlarging the mesopore size and restoring the microporosity, crystallinity, and acidity (Fig. 2g).22
The sensitivity of dealuminated faujasites in alkaline media becomes more apparent when the Y zeolite is steamed and acid treated reaching bulk Si/Al ratios above 6. This aspect was first demonstrated by NaOH treatment of a USY with bulk Si/Al = 30.21 This USY was treated at room temperature in 0.05 and 0.1 M NaOH developing large amounts of mesoporosity (up to ca. 500 m2 g−1). However, the intrinsic zeolite properties dropped substantially down to about a third of the parent USY zeolite. The incurred severe amorphisation during dissolution can be avoided by addition of organic pore-directing agents like TPA+ to the alkaline solution (Fig. 2m).22,38 In addition, inorganic additives in the form of Al and Ga salts can be used to prevent structural deterioration during alkaline treatment, although they do not facilitate mesopore formation to the same extent.39 The fragile USY crystal facilitates the transformation to the hierarchical form using the weak base NH4OH.40,41 This treatment proved inefficient for ZSM-5 zeolites as this zeolite does not dissolve in NH4OH.42 In the case of USY though, instead of leaching solid from the sample, the treatment partially converts a zeolitic fraction to a denser silica phase (see Section 2.6) hereby generating external surfaces of ca. 400 m2 g−1 and implying very high mesopore efficiencies.40
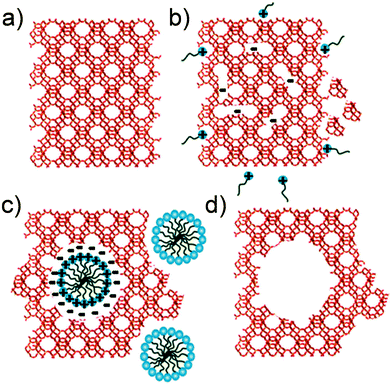 | ||
| Fig. 5 Schematic illustration of the mesopore formation or ‘riving’ in USY zeolites as put forward in ref. 46. (a) original USY zeolite. (b) Si–O–Si bond opening/reconstruction in basic media. (c) Crystal rearrangement to accommodate the surfactant micelles. (d) Removal of the template to expose the mesoporosity introduced. Reproduced with permission of The Royal Society of Chemistry. | ||
Riving was also demonstrated for pristine Y zeolites: in this case, an initial acid leaching step is required, prior to the above-described hydrothermal treatment. The authors described that this ‘acid wash’ is required to ‘loosen up’ the framework.46 The use of sequential acid–base treatments on Y zeolite, shows great resemblance to the method described in Fig. 2g. In addition, Verboekend et al.22 showed that exactly the acid wash as performed in ref. 46 induces a significant leaching and facilitates, like the strategy in Fig. 2g, mesopore formation by subsequent base treatment. Moreover, for these zeolites, instead of NH4OH, NaOH was used. This is not unexpected since, as reported by Van Aelst et al.,40,41 NH4OH is not alkaline enough to dissolve Al-rich Y zeolites.
To more closely assess the striking similarities of riving with conventional base leaching, Verboekend et al. alkaline treated USY zeolites without and with organics like tetrapropylammonium bromide (TPABr) and CTABr.38,49 In addition, strategic experimentation was performed with the filtrates obtained after treatment with only NaOH or NH4OH.49 It was found that, although comprising similar external surface areas, zeolites treated with TPABr are highly crystalline and those treated in base with CTABr are substantially less crystalline. Moreover, the mesostructuring treatments were reproduced on a USY with a Si/Al = 15 using exactly similar and slightly different conditions as patented (Fig. 6). In a first reproduction, CTABr was omitted from the NH4OH solution. This treatment induced a substantial dissolution of the zeolite proving that NH4OH is a strong enough base to leach Al-deficient USY zeolites. The Si-containing alkaline filtrate obtained from this treatment was subsequently complemented with CTABr and exposed to the same hydrothermal treatment. This treatment yielded an MCM-41 with distinct uptake in the nitrogen adsorption isotherm in the range of 0.3 < p/p0 < 0.5. The traditionally ‘rived’ sample possesses the exact same distinct uptake. Conversely, this uptake was not present in a sample exposed to a reproduction with an equimolar amount of TPABr instead of CTABr. This TPABr-prepared sample was concomitantly more crystalline compared to the material obtained by exactly following the patent. Accordingly, it is likely that ‘rived’ samples are composites of (hierarchical) zeolite with ordered mesoporous materials. Importantly, whereas the filtrate-derived OMMs possess the typical porous properties of MCM-41, they lack the typical ordering as can be observed by microscopy (Fig. 3f–h). Accordingly, the absence of clear MCM-41-type ordering as observed by TEM is not a guarantee that the studied sample is purely zeolitic. Therefore, the best way to assess a sample's intrinsic zeolitic properties is by using quantitative XRD, Ar adsorption at 87 K, or acidity assessment by NH3-TPD or IR of pyridine adsorbed.
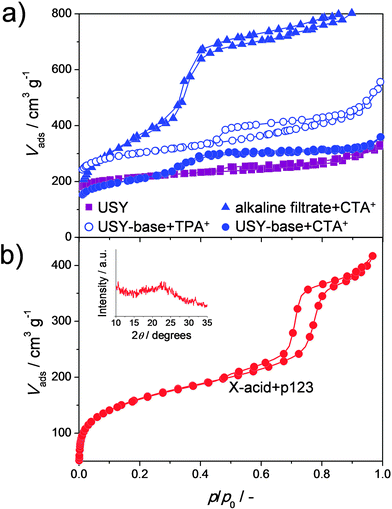 | ||
| Fig. 6 The role of surfactants during the alkaline (a) or acid (b) treatment of USY and X zeolites, respectively. CTA+ facilitates the formation of MCM-41 from dissolved zeolite in alkaline media,49 whereas P123 facilitates the formation SBA-15 in acid media. The inset in (b) displays the X-ray diffraction pattern of sample X-acid+p123. The SBA-15 was obtained at 40% solid yield based on treatment of a NaX following the synthetic protocol of example 2 in ref. 51. Advantages of having ordered amorphous species in the solid, as opposed to non-ordered amorphous species, have yet to be demonstrated. (a) Reprinted with permission from American Chemical Society. | ||
More recently, García-Martínez et al. patented the synthesis of crystalline mesostructured X and Y zeolites prepared by acid treatments.50,51 Remarkably, the approach to yield crystalline hierarchical X and Y zeolites and the properties of the resulting materials closely resemble those associated with previously-established strategies23 (Fig. 7). In one of the patents, a new approach was presented: contacting zeolite NaX with aqueous HCl in the presence of a P123 surfactant.51 This represents a roughly similar strategy as the base leaching using micelle-forming TAAs; an organic template serves to precipitate dissolved zeolitic species forming a zeolite/OMM composite. However, in this case the zeolite is leached using an acid and the template molecule is P123. Reproduction of this treatment yields a highly-porous solid (Fig. 6b). However, this material did not contain any zeolitic crystallinity (Fig. 6b, inset) and can therefore be best described as SBA-15. Accordingly, it is clear that, like in the case of base leaching of USY zeolites with CTA+, this method yields OMM/zeolite composites. However, whereas in base leaching CTA+ also serves to preserve the USY crystals, P123 is not able to exert the same role, and therefore only serves as template for the formation of SBA-15. An overview of the influence of various supplements to the acid or base treatment of faujasites is provided in Fig. 8.
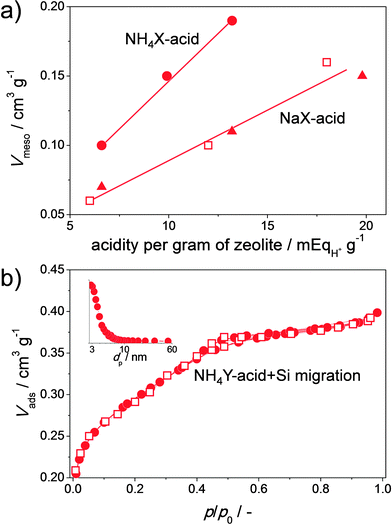 | ||
| Fig. 7 Similarities between the porous properties of hierarchical X (a) and Y (b) zeolites prepared using different strategies. The ‘NH4’ and ‘Na’ indicate the charge-balancing counter cations (CBCC) present in the parent zeolites. (a) Comparison between the mesopore volumes (Vmeso) or hierarchical X zeolites prepared by acid treatment (solid symbols, strategy Fig. 2b)23 and those prepared by sequential acid and base treatment in presence of CTABr (open symbols, strategy Fig. 2c).50 (b) Comparison between the nitrogen isotherms of hierarchical Y zeolites prepared by controlled acid treatment (solid symbols, strategy Fig. 2j)23 and prepared by acid treatment in the presence of P123 (empty symbols)51 Both materials in (b) were obtained by acid treatment of the NH4Y zeolite at elevated temperatures for ca. 72 h. The inset in (b) displays the BJH adsorption pore size distribution. The isotherm of the hierarchical Y with solid symbols in (b) was shifted down by 0.18 cm3 g−1. The similar porosities emphasize that organic additives are not required in the preparation of hierarchical X and Y zeolites. (b, solid symbols) Reprinted with permission from Wiley-VCH Verlag. | ||
2.3. Sustainability aspects in synthetic routes
The need to produce hierarchical zeolites in a sustainable fashion was recently highlighted (Fig. 9a).52 With respect to faujasites, the cost concern is very dominant as the parent material is relatively cheap (3–4 USD kg−1)5 since it is hydrothermally synthesized without organics.2,4,48 As such, many strategies to derive the hierarchical variants are less attractive as they involve costly organics such as TPHAC and tetraalkylammonium cations,26 which need to be removed thermally after the synthesis. In addition, TAAs are often used as Br and Cl salts, which require careful disposal. In the case of hierarchical X prepared by TPHAC this concern was recognized, resulting in a study to replace these organics by inorganic species (Li2CO3 or ZnNO3), yielding similar highly crystalline hierarchical X zeolites.53 However, the degree of mesoporosity was negatively influenced. In addition to the use of organics, a number of aspects in bottom-up strategies persist that constrain commercial prospects. For example, in the case of hierarchical zeolite X, crystallisation times of up to 5 days were reported,26 being an order of magnitude larger than a commercial X.2,54 Also, in the case of Y nanocrystals28 the crystallisation time was about 6 times longer than the typical synthesis (Table 2). Moreover, the reported high yields of nanocrystals relate to roughly 50% compared to that in a conventional Y synthesis.2,48 Finally, the separation needs to be performed using centrifugation, followed by freeze drying to maintain the crystal size. Hence, although bottom-up approaches yield fascinating materials, both the moderate introduction of external surface and the highly-demanding synthetic conditions challenge their practical relevance.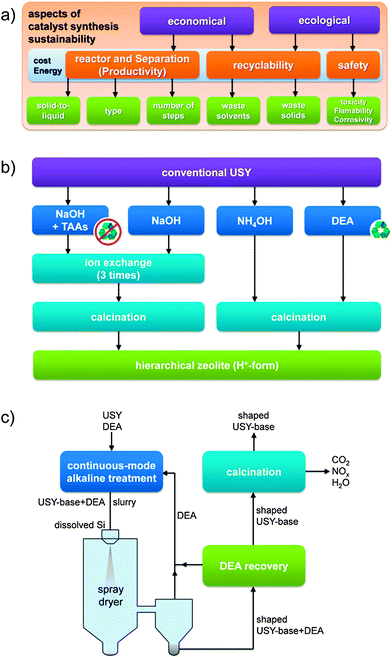 | ||
| Fig. 9 (a) Sustainability aspects in the synthesis of hierarchical faujasites. Many of the green themes were recently addressed to make the manufacture of hierarchical zeolite catalysts more ecologically and economically attractive. Cost and energy concerns represent general underlying aspects.52 (b) Different synthesis pathways to convert a conventional USY into its hierarchical variant.56 The recyclability of the organics (recycling symbols) and the need for an additional ion-exchange are important sustainability gains achieved by using NH4OH or diethylamine (DEA) as bases. (c) Scheme demonstrating the conceptual continuous synthesis of shaped USY catalysts by sequential desilication and spray drying.52 By application of DEA as pore-directing agent and base, a subsequent ion exchange and the combustion of hydrocarbons is prevented. Leached Si species are integrated in the technical catalysts in which they serve as binder/shaping agent. The solids obtained by using the strategy in (c) may resemble those in the images in Fig. 3b–d. (a–c) Reproduced with permission from Wiley-VCH Verlag. | ||
Top-down strategies have the intrinsic advantage of starting with commercial crystals, which implies that they can be separated in the same fashion as conventional faujasite crystals and start with a non-organic fingerprint. However, also many top-down strategies include unfavorable features, such as the loss of solid,55 the use of organics,38,44,46 and even additional hydrothermal steps.44,46 For example, the use of organics as TAAs have been reported to maintain crystallinity in the case of the sensitive USY zeolites.38 Recently, Verboekend et al. showed that the preparation of a highly-crystalline hierarchical USY can also be attainted, instead of using TAAs + NaOH, by leaching in a weak base as diethylamine (DEA) (Fig. 9b).52 This has as advantage that the zeolite remains in the H-form after removal of the DEA, which saves another ion exchange.56 Moreover, it proved that the volatility of DEA enables to recover 80% of the organic molecules applied.52 A similar argumentation holds for the recently explored weak base NH4OH. However, while this approach enables to yield hierarchical USY at high yields (>90 wt%), this approach does not enable to preserve the microporous character to the same extent.41 A summary of the samples resulting from the different techniques on USY zeolites were recently summarized by Van Aelst et al. (Fig. 10).41 Herein, the relation between intrinsic zeolitic properties, the use of organics, and the material yield is highlighted.
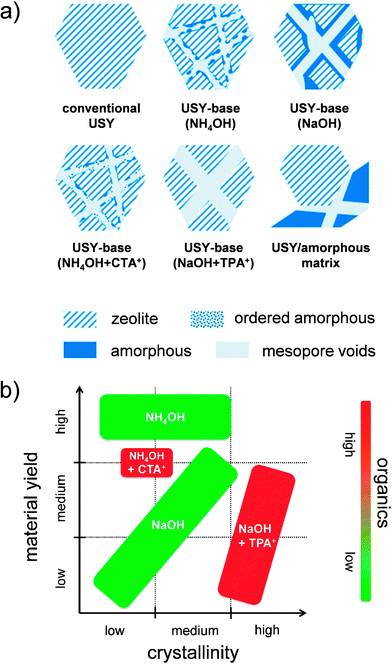 | ||
| Fig. 10 (a) Overview of solids obtained by alkaline treatment of USY (Si/Al > 15) using distinct approaches.41 The use of NH4OH and NaOH lead to (controlled) amorphisation and accordingly amorphous silica-alumina (ASA) zeolite composites. In the case of NH4OH, the amount of solids leached is minimal. When TPA+ is used in the alkaline solution, the crystallinity can be fully preserved during the alkaline treatment (see Fig. 1b). When CTA+ is present during the alkaline treatment crystallinity is preserved to a lower extent compared to with TPA+, which can be attributed to the presence of MCM-41-type ordered amorphous materials (see Fig. 8). Although USY crystals can display reduced zeolite properties, the presence of well-localized intracrystalline mesopores render them superior catalyst compared to mechanical mixtures of USY/ASA, which feature similar bulk crystallinity and porosity. (b) Plot highlighting the relation between the material yield, crystallinity, and used organics.41 Thus far, the synthesis of highly-crystalline hierarchical USY zeolites can only be attained using organics such as DEA or TPA+. On the other hand, highly mesoporous samples with high solid yield are attained using NH4OH treatment. Reprinted with permission from Wiley-VCH Verlag. | ||
Besides the use of organics, other practical aspects of post-synthetically treating faujasites were recently studied. It was demonstrated that species leached during the desilication treatments can be easily recovered and used for additional hydrothermal synthesis of new batches of zeolites.52 Moreover, addition of CTA+ to the filtrates obtained from alkaline-treated zeolites enables to recover MCM-41-type materials (Fig. 8).49 Still, the post-synthetic modification of zeolites are routinely performed in batches, which may limit productivity. Verboekend et al. therefore demonstrated the in-line synthesis of hierarchical zeolites using a high-shear micro-reactor,38 which enables to optimize the stirring and contact time. At constant solid-to-liquid ratio (SLR), the authors managed to produce hierarchical USY zeolites attaining productivities that were 100 times higher. Later, the same authors showed that by choosing the right base (diethylamine) and alkalinity, the reactor productivity could be enhanced another 5 times by raising the zeolite content from 33 to 150 g per L in the preparation of hierarchical USY.52 The in-line preparation combined with the increased SLR enables an hypothetical 500-fold increase of reactor productivity. However, a highly-productive in-line synthesis of hierarchical zeolites remains futile if separation remains performed batch-wise. This urged the authors to explore in-line separation by spray drying of the treated zeolite in its alkaline suspension.52 This has as concomitant advantage that the dislodged Si species could be reintegrated in the solid in the form of amorphous silica. Indeed, this approach enabled to make hierarchical zeolite/silica composites, which should be of particular use in FCC.24 In this application, both the use of spray drying to make granules and the presence of amorphous silica are mandatory. Advantageously, the resulting solid comprised a fully preserved (bulk) microporosity which implies that the precipitated silica is highly porous. Moreover, it was demonstrated microscopically that the leached species were incorporated homogeneously into the solid (Fig. 3), where they fulfill an additional role in shaping.52 Combining this technique with the ability to recover DEA after alkaline treatment, a conceptual in-line synthesis of USY composites was conceived (Fig. 9c).
In case of Y, the same authors showed that the initial dealumination and sequential base treatment (Fig. 2g) can directly be executed without the need for an additional separation.52 This was achieved by adding enough base directly to the reacted acidic suspension to neutralize the acid and provide enough alkalinity to induce a partial dissolution and mesopore formation in the dealuminated zeolite. It should be duly mentioned that the reverse approach (going from alkaline to acidic conditions) is more challenging due to the precipitation of silicates.52 However, as mentioned above, this also offers a means to prepare zeolite/silica composites without the loss of silicon species.
2.4. Generation of active sites
In addition to the alleviation of access and mass transport limitations, the emergence of hierarchical zeolites has sprouted a novel generation of active sites (Fig. 11a). In traditional faujasite zeolites, two main classifications of acid sites exist. The first is formed by the classical Brønsted acid site generated by the exchangeable charge balancing proton within the micropores, the second is the Lewis acidity generated by EFAl on the external surface of the zeolite crystal.57 This EFAl is caused by expulsion of Al from the framework during the steaming process.14 Conversely, the novel types of active sites result from the deposition of metals or alkali cations during the mesopore formation by base treatment.39,58–62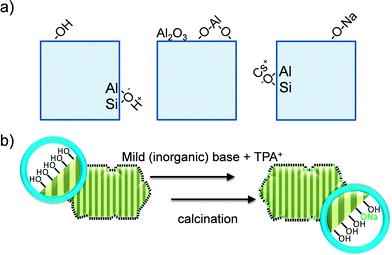 | ||
| Fig. 11 Schematic representation of different types of active sites present in hierarchical zeolites. (a) The typical Brønsted site and surface hydroxyl (left), Lewis acid sites derived from steaming and alkaline-induced surface realumination (middle), and basic sites generated by the framework or by the formation of surface-bound alkali cations (right). (b) The generation of novel surface basic sites by tailored alkaline treatment.59 Adapted with permission Wiley-VCH Verlag. | ||
Like in steam treatment, base treatment (Fig. 2g, k, m and o) results into the removal of aluminum from its traditional framework position and the reincorporation into other places on the solid.13 However, whereas EFAl caused by steaming is predominately in the form of octahedrally-coordinated alumina-type species,57 EFAl caused by base treatment is predominately tetrahedrally-coordinated, giving rise to a distinct Lewis acidity.25,60,61 Especially at low to moderate coverages of alkaline-induced aluminum, the density of Lewis acid sites per aluminum atom approaches unity.60 In addition to the remetallation of framework Al, the addition of external metal salts to alkaline solutions forms a tool to tailor the active site in faujasite zeolites.60 Dapsens et al.39 used this approach to remetallate faujasites with Al and Ga and hereby obtained highly active and selective catalysts (see Section 4.3). This approach was also demonstrated to be effective using Sn on MFI.62 Tin-containing zeolites, particularly Sn-beta, display particularly attractive features in biomass conversions,63–65 and it is likely that also Sn-USY can be made using this strategy. The latter is particularly attractive as Sn-USY cannot be synthesized using bottom-up strategies. Still, although some preliminary studies have been performed,66,67 the exact nature of ‘alkaline-induced’ metal species remains obscure.
A different type of active site was encountered in the synthesis of hierarchical faujasites for application in base-catalysed conversions. In base catalysis, the main way to obtain the basic site is by introducing a large amount of electron donating cations into the framework. This is commonly achieved by placing the largest alkali cation (cesium) within the faujasite of the largest CBCC density, that is, X. Accordingly, CsX was traditionally considered the most active base catalyst, whereas high-silica zeolites were disregarded as potential base catalysts.68–70 Keller et al.58 found that, unlike reported literature prescribes, alkaline-treated high-silica zeolites (Si/Al > 200) displayed exceptional activity. This type of active site stems from the surface coverage of alkali cations (Fig. 11b), which are present in non-framework types of coordination.59 The excellent performance of the derived materials (see Section 4.2) was ascribed to the moderate basicity of the sites.
3. Mechanism and characterisation
3.1. Understanding the mechanism of mesopore formation
The fundamental knowledge of mesopore formation in zeolites in general remains limited. In the case of faujasites, the mesopore formation was traditionally studied for steam treatment.14 The mesopore formation mechanism by base leaching has been studied mostly for ZSM-5 zeolites,60,71,72 which should be related to its relatively early genesis (2000).19,20 In the case of hierarchical faujasites prepared by post-synthetic modification, a discussion on mesopore formation should tackle the relative stability of the framework. The latter is urgent since, in contrast to 10 MR zeolites, which only (partially) dissolve in alkaline solutions,13 faujasites readily dissolve and amorphize in aqueous solutions of either very acidic or very alkaline nature.73The definition of ‘stability’ can be rather confusing as it depends heavily on the conditions to which the zeolite is exposed. For example, high-silica USY zeolites are labeled traditionally as ‘ultra-stable’ Y (USY), since they are relatively stable (compared to Y and X) under steam in atmospheric conditions.16,37 However, in contrast, USY zeolites are extremely sensitive to alkaline solutions.38 Therefore, the label ‘stable’ seems to be inaccurate. For nostalgic purposes, we have not changed the name of ‘USY’ zeolite throughout the paper. However, we emphasize that in the discussion of the stability of a zeolite, the applied conditions need to be carefully specified (Fig. 12b). Several works have focused on studying the relative stability of faujasites under various conditions. In agreement with Briend et al.,74 Verboekend et al.73 showed that faujasites with a low Si/Al ratio (parent X and Y zeolites) are relatively stable in alkaline solutions (in atmospheric liquid water), whereas those with Si/Al > 4 are very sensitive, as they dissolve and amorphize under the same conditions. It should be highlighted that once controlled (using NH4OH), this amorphisation enables to introduce intracrystalline mesoporosity, while virtually no silica is leached from the solid based on a partial densification of the solid (Fig. 12c).40 In acidic conditions, the stability trends are reversed, showing that X and Y zeolites are the most unstable (Fig. 12a). With the eye on the application of faujasites in biomass-related reactions, the stability of faujasites in water at T > 100 °C and super-atmospheric pressures (‘hot liquid water’) is highly relevant. Ravenelle et al.75 and later Ennaert et al.76 revealed that especially high-silica USY zeolites are more sensitive in hot liquid water. Under those conditions, the zeolites behave in a similar fashion compared to zeolites in alkaline solutions at atmospheric pressure and moderate temperatures. Ennaert et al.76 related this to the changing water equilibrium becoming more basic under increased pressure and temperature.
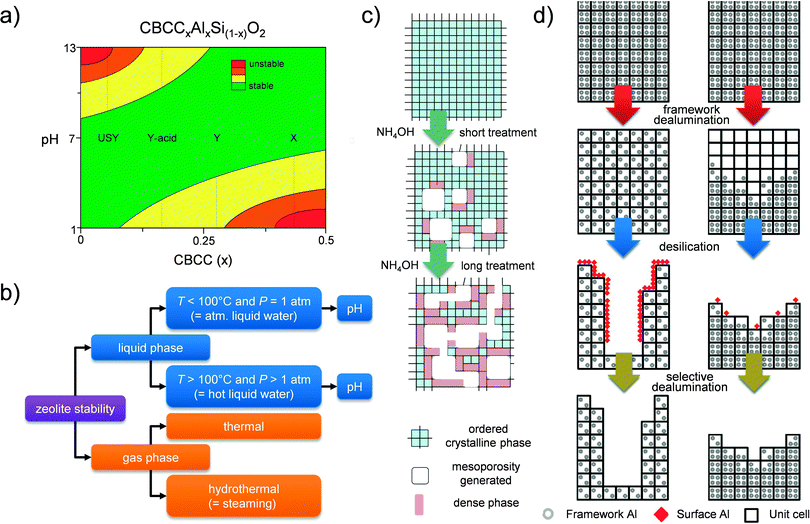 | ||
| Fig. 12 Advances on the understanding of faujasite stability and mesopore formation. (a) Plot relating the relative stability of faujasites to the relative amount of charge balancing counter cations (CBCC), in atmospheric liquid water (P = 1 atm, T < 100 °C).73 The relative stability refers to the occurrence of (i) dissolution and (ii) amorphization. In alkaline media, these events take place simultaneously for faujasites. In contrast, for many 10 MR zeolites (ZSM-5, ferrierite, ZSM-22),13 the amorphization taking place during alkaline dissolution is limited. (b) Overview of different conditions in which faujasites display distinct relative stabilities. (c) The controlled mesopore formation in USY by partial amorphisation and associated formation of dense silica phases.40 (d) The framework dealumination has a major influence on the efficiency of the subsequent alkaline treatment (desilication), and illustrates the need for a homogeneous dealumination.77 (a, c and d) Adapted with permission from American Chemical Society. | ||
Whereas the bulk Si/Al ratio is typically referred to in the modification of faujasites, the distribution of Al in the zeolite crystals is of key importance too. For example, the unfavorable Al distribution in X zeolites relates directly to its crystallinity loss during dealumination.23 Similarly, acid treatment of Y zeolites gives rise to pronounced Si/Al gradients inside the crystals, yielding Al-rich and Al-depleted zones.16 Such gradients have a very pronounced influence on the subsequent alkaline treatment (Fig. 12d).77 In the case of a highly inhomogeneous dealumination, the Al-deficient zones are readily removed from the solid, while Al-rich zones will not be affected much. This implies that the degree of inhomogeneity predicts the efficiency of a subsequent alkaline treatment. In turn, this is probably the reason why acid-treated Y zeolites can be alkaline-treated in the absence of organics: the Al-free zones may initially amorphize, but are eventually completely removed. Al-rich zones are stable in alkaline conditions and not affected. Moreover, during direct dealumination, the crystallinity is often strongly reduced, as the dislodged Si species are not given enough time to be reintegrated into the framework.29 In this case, the desilication treatment can enhance crystallinity by removing the amorphous Si-rich zones.23,35 Hence, the mechanism of mesopore formation by base treatment of acid-treated Y zeolites should be distinctly different compared to the mesopore formation in highly-siliceous USY zeolites. This likely also relates to the lower external surface area introduced by base leaching in acid-treated Y zeolites with relatively high Al content (Fig. 4).
3.2. Advanced characterisation of hierarchical zeolites
The advanced characterisation of hierarchical faujasites as synthesized using the strategies in Fig. 2 is at its infancy. One of the most routine exercises in the characterisation of the faujasites is porosity assessment using nitrogen adsorption isotherms. Although the isotherms should always be central, a common focal point is the use of the t-plot model, which is used to distinguish between (ideally zeolitic) microporosity and the introduced secondary porosity.78 Although this method has proved of instrumental value to the characterisation of conventional mostly microporous zeolites, its application on hierarchical zeolites should be performed with caution. The latter is particularly true in the case secondary pores in the size range of 2–4 nm are present. Such pores are formed easily when USY zeolites are reacted with mild alkaline solutions, especially with micelle forming TAAs, like CTA+ (Fig. 6a).49 In the latter case, a hierarchical zeolite/MCM-41 composite is formed comprising pores in the size range typical of faujasites (0.74 nm), MCM-41 (2–4 nm) and mesoporous zeolites (2–20 nm). Fajula et al.79 showed that indeed the t-plot has strong limitations for such composite materials and provided an abacus to correct for the obtained Vmicro and Smeso (Fig. 13a). However, a doubtful application of the original t-plot over the parent microporous material, which is an important correction factor in the abacus, obscures the implication of the proposed methodology. Besides the complication of the presence of 2–4 nm pores in MCM-41 type materials, another intrinsic difficulty is that OMMs often comprise substantial microporosity.49,79,80 This means that the assessment of the micropore volume, as derived by nitrogen is of limited value, and a more trustworthy alternative is the adsorption of argon at 87 K.78,81 Such analysis, in combination with quantitative crystallinity assessment and acidity characterisation may be the best way to analyze the intrinsic zeolitic properties. Besides in zeolite/OMM composites, 2–4 nm pores can also occur in highly-crystalline mesoporous faujasites. For example, such porosity occurs after the (optimized) dealumination of NH4Y zeolites using H4EDTA (Fig. 7b).22,23 Also, in this case, the incorrect application of the t-plot can, instead of large external surface areas (ca. 400 m2 g−1), yield potentially unrealistic micropore volumes exceeding 0.40 cm3 g−1.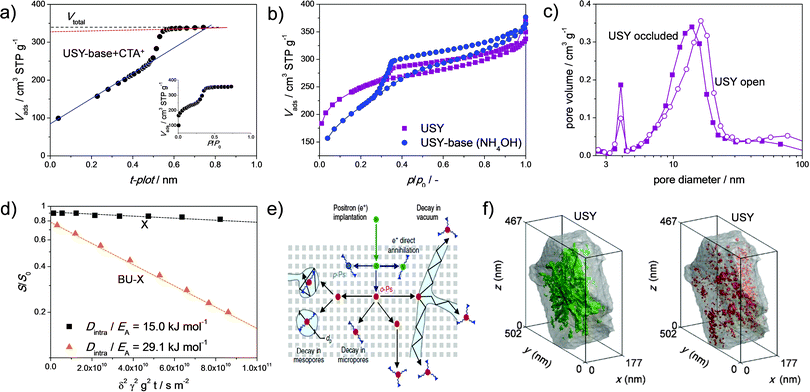 | ||
| Fig. 13 Advanced characterisation of hierarchical faujasites. (a) Difficulties of non-linearity in the application of the t-plot on zeolite/OMM materials.79 (b) Ar adsorption at 77 K reveals hysteresis, hence occluded mesopores, in the parent and NH4OH treated USY zeolites.41 (c) The BJH desorption mesopore size distributions for steamed Y zeolites comprising occluded (larger 4 nm peak) and more accessible mesopores (smaller 4 nm peak). (d) enhanced diffusivity in a hierarchical X prepared using a bottom-up (BU) strategy.88 (e) Positron annihilation lifetime spectroscopy: the relative life time of implanted positrons gives an indication of the accessibility of mesopores.87 (f) Accessibility of the mesopores in USY quantified using processing of transmission electron micrographs.82 3D representations of the open mesopores in green (left) and the closed mesopores in red (right). (a) Reprinted with permission from American Chemical Society. (b, d and f) Reprinted with permission from Wiley-VCH Verlag. (e) Reprinted with permission from Macmillan Publishers. | ||
An important piece of information that can be derived from nitrogen (and argon) isotherms is the degree of pore blockage.78 Based on the adsorbate properties, the amount of blocked pores can be evaluated using the degree of hysteresis in the desorption isotherm. In the case of nitrogen at 77 K, this can be performed by application of the BJH model to the desorption isotherm. Therein, the sudden closure of the hysteresis loop, for N2 at 77 K this occurs at p/p0 ∼ 0.45, is observed as a sharp peak at 4 nm (Fig. 13c).78 The relative size of this peak can be used to quantify the degree of pore blockage. De Jong et al.82 used this approach to quantify the pore blockage in a USY and found that about 22% of the mesopores are occluded. This was successfully related to the amount of cavitated mesopores found by electron tomography (18%, Fig. 13f). However, they did not reveal the influence of any hierarchisation by the alkaline treatments. Garcia-Martinez et al.83 demonstrated a novel interpretation of Ar adsorption measurements performed at different temperatures, that is 77 K and 65 K, to study the same effect. Due to the different nature of the adsorbate compared to nitrogen, pores down to size range of 2–5 nm can be probed. They concluded that 64% of the mesopore volume of an alkaline-treated USY is accessible. Interestingly enough, this value was not compared to the parent zeolite. Van Aelst et al.41 used the same technique to assess and compare the porosity of an NH4OH-treated USY with the parent zeolite (Fig. 13b). They observed, like Garcia-Martinez et al.,83 that about 70% of the porosity in the relevant size range was accessible in the alkaline-treated USY. However, they also observed that the percentage of accessible mesoporosity in the parent zeolite was higher (80%), agreeing well with the results from De Jong et al.82 Importantly, this means that, of the mesopores generated by alkaline treatment, a significant fraction may not be accessible. Further study will need to reveal whether this is also the case for more crystalline alkaline-treated zeolites treated with TPA+ or diethylamine. Mesopore accessibility of hierarchical Y zeolites prepared by acid–base treatment was confirmed by Verboekend et al. using mercury intrusion.22 This technique enables to selectively probe connected mesopores down to sizes of 5 nm. The mesoporous samples displayed a strongly enhanced uptake of pores smaller than 10 nm, proving that the bulk of the pores of size 10–5 nm are accessible from the exterior of the crystal.
The introduced porosity ultimately aims at enhanced catalysis either by increasing the number of accessible active sites, in the case of access limited reactions, or by reducing mass-transfer limitations. In the latter case, the role of diffusion is eminent to quantify the functionality of the secondary porosity more accurately. Related studies have focused primarily on 10 MR zeolites, like ZSM-5,84–87 as the mass transfer limitations can be assessed in the gas phase using common molecules, such as xylene or neopentane. However, in the case of faujasite, studies are fewer, which may be due to the necessity for larger probe molecules to study mass transfer limitations. These molecules are less volatile, which is experimentally challenging as experiments need to be performed in the liquid phase. Still, Kortunov et al.17 used pulsed field gradient (PFG) nuclear magnetic resonance (NMR) spectroscopy with n-octane and triisopropylbenzene as probe molecules to study mass transfer limitations in parent Y zeolites and their steamed analogs. Large differences in intracrystalline diffusivity were not observed, which led the authors to conclude that mesopores introduced via steaming are of little use. It should however be emphasized that the studied crystals comprised only 32 m2 g−1 (parent) and 62 m2 g−1 (steamed) external surface, which is little compared to the commercial steamed (and acid leached) USY zeolites (ca. 200 m2 g−1, Fig. 4). Later, Kärger and coworkers88 used the same technique to quantify mass transfer limitations of conventional and hierarchical zeolite X (prepared using strategy Fig. 2a), and found large enhancements of transport rates (Fig. 13d). However, in this case the hierarchical sample did not exceed an external surface of 84 m2 g−1, and it therefore remains unclear how these diffusivities relate to those in highly mesoporous (Smeso > 200 m2 g−1) faujasites. In addition, since these studies focused primarily on faujasites with Si/Al < 3.4, it is unclear how the diffusivity is affected in the commonly-used high-silica faujasites (Si/Al > 10).
The influence of acidity of hierarchical faujasites was on several occasions assessed,21–23 but only rarely studied in depth. In the case of hierarchical faujasites, study of acidity is not straightforward due to the relatively low overall stability of X and Y in the protonic form.16 In addition, total acidities measured on such faujasites are often much lower than what could be expected based on their framework Al content.73 Accordingly, it is not surprising that acidity changes were mostly focused on the implications of the alkaline treatment on USY zeolites in the compositional range of Si/Al = 15–40.21,49,56,66,89,90 The influence of such treatments on the total acidity is commonly measured using IR of pyridine adsorbed. As expected, based on the total loss of bulk crystallinity, treatment in NaOH alone leads to a significant reduction in Brønsted acidity.21,49,56 Methods that enable to preserve intrinsic zeolitic properties, such as TPABr + NaOH (Fig. 2m), yield solids with Brønsted acidities varying from 60–110% compared to that of the parent (Fig. 14b).49,56,66,90 Rac et al.66 studied the total acidity and acid strength of hierarchical USY by NH3 desorption and PEA adsorption. They found that the total acidity was largely maintained, the acid strength was reduced by 50% (Fig. 14a and c). The latter trend was confirmed using NH3-TPD for a USY with Si/Al = 15 by Pérez-Ramírez and coworkers.89
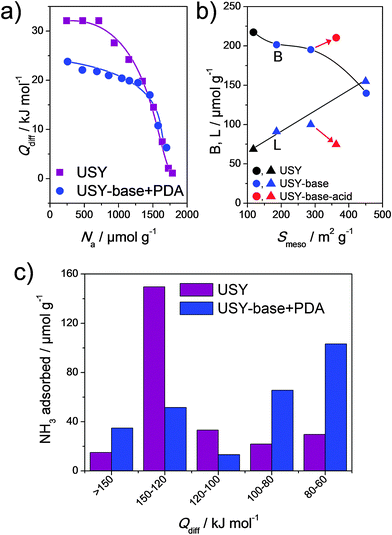 | ||
| Fig. 14 Advanced acidity characterisation of parent USY and its hierarchical analogue (prepared using strategy in Fig. 2m). (a) Differential heats of phenylethylamine adsorption.66 (b) Interdependence between the Brønsted (B) and Lewis (L) acidity and the mesopore surface area (Smeso).90 The influence of washing an alkaline-treated zeolite with Na2H2EDTA is indicated by the arrows. (c) Distribution in strength of acid sites using temperature-programmed desorption of NH3.66 (a–c) Reproduced with permission from Elsevier. | ||
4. Catalytic evaluation
4.1. (Hydro)cracking of vacuum gas oil
The catalytic evaluation of hierarchical faujasites in the hydrocracking of vacuum gas oil (VGO) has been studied on several accounts. De Jong et al.21 studied conventional and a mildly-NaOH leached USY zeolite, both as part of an alumina-supported NiMoS2 catalyst, in the cracking of vacuum gas oil (Table 3). The hierarchical variant attained a higher activity, and displayed a pronounced selectivity shift from lower-boiling point fractions (up to 140 °C) towards higher-boiling point fractions (140–375 °C). Accordingly, more kerosene and gasoline were formed at the expense of light gases and naphtha. In addition, the catalysts displayed a lower degree of coking. Also, García-Martínez et al.46 evaluated a parent and a single alkaline-treated USY zeolite in the catalytic cracking of VGO. They observed an increase in gasoline and a higher olefinicity in the C3 and C4 fraction and a reduced occurrence of cokes and bottoms. Martínez et al.91 studied hierarchical stabilized Y zeolites and evidenced a higher C3–C4 olefinicity and diesel yields at the expense of bottoms and gas formation. The similar results (Table 3) highlight the value of secondary porosity in catalytic cracking. Moreover, they indicate that the presence of OMMs, as facilitated by the CTA+-assisted synthesis, does not yield any additional advantage.The catalytic results as described in Table 3 were attributed to a higher accessibility of the crystal domains. Such crystals enable a better access of larger molecules and moreover, facilitate the timely evacuation of cracked products from these microporous domains. Whereas the former leads to an enhanced bottom-conversion capacity and reduced coke formation, the reduced over-cracking leads to a lower gas formation and higher olefinicity. A very important aspect in cracking is that the three mesoporous samples comprised largely inferior acidity properties. The latter is not only a coincidence, but seems to be mandatory to maintain an optimal cracking behavior. For example, Martínez et al.91 showed that a highly mesoporous and highly acidic faujasite leads to strong over-cracking and accordingly more gas formation and lower olefinicity values. Only after moderating its acidity by steaming, the optimal performance was achieved. Accordingly, both activity and selectivity in catalytic cracking are a function of the acidity and the external surface. This concept is schematically illustrated in the case of squalane cracking (Fig. 15).
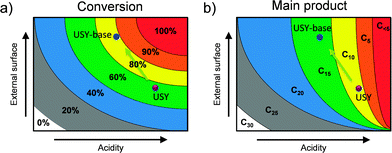 | ||
| Fig. 15 In the design of hierarchical faujasite catalysts the balance between external surface and the acidity is crucial. The catalytic activity (a) and main products formed (b) in the (hydro)cracking of squalane (C30) as a function of acidity and external surface. The points highlight the performance of a parent and alkaline-treated USY as presented in ref. 21. In the case of cracking, the moderation of the acidity of hierarchical zeolites is important to steer selectivity. For example, as indicated in (b), should the acidity be maintained upon hierarchisation, significantly more lower-value gases would be obtained (C5). Therefore, the partial amorphisation of USY zeolites by base treatment (in the absence of organics), is not detrimental and may be even mandatory. | ||
4.2. Carbon–carbon couplings
Frequently-applied model reactions to evaluate the performance of hierarchical USY zeolites are carbon couplings in the form of acid-catalysed alkylations or base-catalysed condensations. Various acid-catalysed couplings were tested on post-synthetically modified Y zeolites (Fig. 16). Verboekend et al.22 showed that a dealuminated and alkaline treated Y zeolite yielded a similar performance as the parent zeolite. Although this implies an increase of the TOF based on the lower Si/Al ratio in the treated sample, an overall enhancement was not achieved. Only when the zeolite was treated in a mild acid, to remove the Al-rich deposits (Fig. 2g), an enhanced performance was achieved. The latter performance related to an estimated 4-fold increase in the TOF (Fig. 16d). Jiao et al.35 studied the acetalisation of cyclohexanon with glycol and pentaerythritol, on pristine, acid-treated, and sequentially acid–base treated Y zeolites. They observed, like Verboekend et al. that the catalytic performance was not enhanced by the acid–alkaline treatment (Fig. 16f). Only when a significantly larger substrate was used (Fig. 16e), did the hierarchical porous zeolites yield enhanced performance. This suggests that the value of the external surface increases with the size of the products. Unfortunately, the efficiency of a mild acid washing of the alkaline-treated sample was not demonstrated.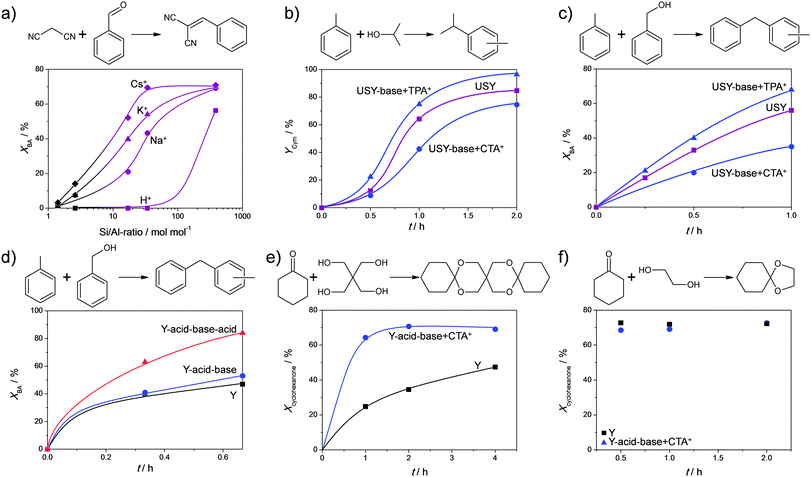 | ||
| Fig. 16 Performance of hierarchical faujasites in various carbon–carbon coupling reactions. (a) X (Si/Al = 1.4), Y (Si/Al = 2.6), and USY (Si/Al > 10) zeolites with different charge-balancing counter cations in a Knoevenagel condensation.58 The enhanced performance at high Si/Al ratios indicates the potential of surface alkali sites (see Fig. 11). (b and c) The performance of USY zeolites in alkylation.49 Enhanced performance is only achieved when USY is base treated in the presence of TPA+. (d) Performance of Y zeolites in alkylation.22 The role of the acid wash (From strategy Fig. 2g) is clearly demonstrated. (e and f) Performance of Y zeolites in acetalisations.35 The hierarchical alkaline-treated Y is only superior in the case the formed product is large enough (e). The role of a subsequent acid wash in (e and f) may help to further enhance the catalytic performance. (a, e and f) Reproduced with permission of The Royal Society of Chemistry. (b and c) Reprinted with permission from American Chemical Society. (d) Reprinted with permission Wiley-VCH Verlag. | ||
Although the synthesis and characterisation of TPA+- and CTA+-treated USY zeolites has been systematically compared on several occasions, the differences in catalysis has been limited to a single study. Verboekend et al.49 studied alkaline-treated USY zeolites in the conversion of alkylate benzyl alcohol or isopropyl alcohol with toluene, forming either cymenes (C10) or dibenzenes (C13, Fig. 16b and c). It was demonstrated that only a zeolite treated with TPABr + NaOH leads to superior performance. Moreover, the CTA+-treated sample displays an activity inferior to that of the starting USY zeolite. This suggests that, unlike in cracking reactions where acidity is best moderated (Fig. 15), reactions requiring high Brønsted acid densities benefit from maintaining the intrinsic zeolitic properties. Accordingly, in such reactions, there is a clear benefit in using non-micelle forming organics, such as DEA or TPA+, as they enable to preserve these properties to the largest extent during mesopore formation.
Base-catalysed coupling reactions were studied mostly in the form of Knoevenagel condensations. Cesium-exchanged hierarchical X (Fig. 2b), Y (Fig. 2g), and USY (Fig. 2m) were evaluated in a classic Knoevenagel condensation of malononitrile with benzaldehyde.23 Unlike reported previously,92 a pronounced near-linear relationship between the activity of the catalysts and the external surface was established (Fig. 17b). Since the studied Cs-faujasites comprised different quantities of basic sites, it was concluded that the external surface displays a dominant influence on the activity. This relationship sprouted the authors to evaluate the activity of a larger number of alkali-exchanged hierarchical faujasites, including a much wider range of Si/Al ratios. Since mesopore formation is generally more efficient for high-silica faujasites (Fig. 4), and a higher density of alkali cations (hence active sites) is present in X and Y, an optimum activity was expected. However, instead of an optimum, the highest activity was obtained for all-silica, hence virtually alkali-free, USY zeolites (Fig. 16a), which was attributed to the establishment of a novel site by base treatment (see Section 2.4). The potential of these hierarchical all-silica faujasites was evaluated as a function of the kinetic diameter of the aldehyde (Fig. 17e), where, as observed earlier in Fig. 16e and f, the utilisation of the mesopore surface area increased significantly with the size of the reactants.
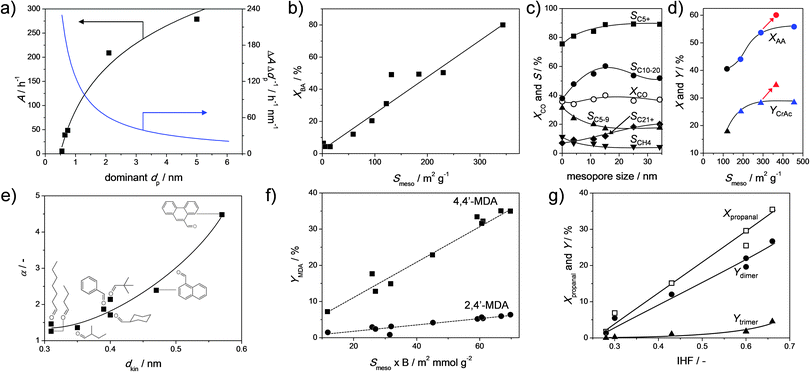 | ||
| Fig. 17 Synthesis–property–function relationships determined for hierarchical faujasites prepared by alkaline treatment. (a) The activity in the valorisation of α-pinene as a function of the dominant pore diameter of alkaline-treated USY.56 (b) The influence of the external surface (Smeso) on the activity in a Knoevenagel reaction (see Fig. 16a).23 (c) Influence of mesopore size on the catalytic behavior of Co-loaded hierarchical Y catalysts in Fischer–Tropsch synthesis.94 (d) The yield of cresol acetate (YCrAc) in the esterification of acetic acid (AA) with o-cresol as a function of Smeso in USY.90 The influence of washing an alkaline-treated zeolite with Na2H2EDTA is indicated by the arrows. (e) Activity amplification (α) through introduction of Smeso in USY zeolites for Knoevenagel condensations with malononitrile as a function of the kinetic diameter (dkin) of the aldehyde.58 (f) Correlation between the yield of two distinct 2-ring methylenedianiline (MDA) isomers and the product of Brønsted acidity (B) and mesopore surface of USY zeolites.89 (g) Conversion and product yields to dimers and trimers (Ydimer, Ytrimer, respectively) in the self-condensation of propanal as a function of the indexed hierarchy factor (IHF) of alkaline-treated USY zeolites.58 (a–c) Reprinted with permission from Wiley-VCH Verlag GmbH. (d) Reprinted with permission from Elsevier. (e) Reproduced with permission of The Royal Society of Chemistry. (f) Adapted with permission from American Chemical Society. | ||
Another reaction where carbon couplings are made is in Fischer–Tropsch chemistry. Here, both hierarchical USY and Y zeolites have been evaluated in the form of bi-functional catalysts. Sartipi et al.93 used cobalt-loaded hierarchical USY zeolites (Fig. 2m) and found active and stable catalytic performance. However, they did not directly compare it to a conventional USY zeolite and the role of hierarchical porosity remained therefore obscure. Peng et al.94 recently demonstrated the role of secondary porosity in Fischer–Tropsch catalysis more explicitly. They used cobalt-supported hierarchical Y (Fig. 2g) zeolites to attain 20% activity gains over conventional Co-supported Y zeolites. Moreover, they exploited the potential of post-synthetic design by synthesizing hierarchical Y zeolites of varying mesopore sizes. Hereby, they managed to tune the selectivity by reducing, like in the case of VGO cracking, gas formation and increasing the selectivity to C10 to C20 alkanes.
4.3. Novel catalytic applications
Motivated by the eminent transition to more sustainable production processes, biomass is nowadays frequently studied as alternative to oil-derived feedstocks. Zeolites have the potential to catalyse the transition to biomass conversion, owing to their excellent adaptability to meet the specific demands of chemical transformations.95–97 Based on the different nature of the biomass streams, such as higher oxygen content, the use and therefore the design of zeolites will differ. Moreover, the more bulky and reactive nature of biomass-derived molecules implies that, like in oil-derived streams, the potential of hierarchical zeolites in the valorisation of biomass is vast.95,98The application of hierarchical zeolites in biomass has not yet been very well studied. The latter may be due to the relatively recent establishment of both research fields. In biomass, hierarchical faujasites were applied thus far in bio-oil upgrading,58,90,99 biofuel production,93,100 hydrolysis73,101 and isomerisation reactions.41,56,102 The applied hierarchical zeolites were prepared predominately by post-synthetic modifications, that is, base leaching of dealuminated Y or USY zeolites. The main reported advantage of the developed mesoporosity is a relative increase of the catalyst activity.
Arias et al.100 used a hierarchical USY (prepared using strategy Fig. 2g and h), in the alkylation of toluene with bio-derived platform molecule 5-hydroxymethylfurfural (HMF). Whereas a conventional USY displayed poor conversion (less than 10%), introduction of mesoporosity boosted the conversion fourfold. Nuttens et al.56 studied the conversion of α-pinene, a by-product of the wood industry, and observed large activity improvements using hierarchical USY zeolites. The latter was obtained using sustainable mild leaching techniques (Fig. 9b), which concomitantly highlighted that the largest relatively activity gains are obtained at the initial stages of leaching (Fig. 17a). To address the higher oxygen content in bio oils, Milina et al.90 studied the use of hierarchical faujasites in the acid-catalysed esterification of o-cresol with acetic acid. They applied hierarchical USY zeolites and observed a 50% conversion increase, and a doubled yield to cresol acetate (Fig. 17d). In addition, they showed that the acid wash can be important to increase the external surface and to thereby also enhance the catalytic performance. The potential of hierarchical porosity was also demonstrated in the valorisation of cellulose using Ru-loaded USY zeolites.76 Although the role of mesoporosity was not explicitly studied, it was shown that upon reuse of the catalyst, an enhanced activity was achieved. The latter was attributed to the mesopore formation incurred by the first catalytic run. This mesopore formation was ascribed to the alkaline nature of the water at elevated temperatures (see Section 3.1).
Using the novel type basic sites described in Section 2.4 (Fig. 16b), hierarchical high-silica faujasites were used to catalyse the self-condensation of propanal, attaining a fourfold activity gain.58 In addition, the need to optimize the porosity of the hierarchical USY can be displayed by relating the performance to the porous properties of zeolites (Fig. 17g). This is achieved by plotting the conversion and yields to dimers and trimers as a function of the indexed hierarchy factor (IHF) of the used catalysts.25 The IHF relates the normalized Smeso to the normalized Vmicro, and the obtained linear relationship indicates that, upon introducing secondary porosity, microporosity should not be lost. On the other hand, Dapsens et al.39 used the novel ‘alkaline induced’ Al and Ga Lewis sites on hierarchical USY zeolites in the acid-catalysed conversion of dihydroxyacetone to ethyl lactate. Whereas the role of the external surface was not explicitly studied, they found a linear relationship between the selectivity to ethyl lactate and the Lewis acidity of the hierarchical zeolite.
Besides novel applications in biomass, the more open structure of hierarchical faujasites also facilitates the use in acid-catalysed reactions, that are traditionally only performed using organic acids, such as HCl or HNO3.103 Keller et al.89 prepared a variety of hierarchical Y and USY zeolites using post-synthetic strategies (Fig. 2g and m) and evaluated their performance in the synthesis of polyurethane intermediates. In the conversion of formaldehyde with aniline, they achieved a doubled conversion, and were able to combine that with a high selectivity to the desired isomer 4,4′-methylenedianiline (4,4′-MDA) relative to the unwanted 2,4′-MDA. Moreover, the performance of the hierarchical USY zeolite was successfully attributed to the Brønsted acidity multiplied by the external surface (Fig. 17f). Like the IHF,25 this parameter relates the intrinsic zeolitic properties to the external surface. However, unlike the IHF, it has the potential to correlate the catalytic performance of zeolites of different acidities. Finally, since the trend indicates that only acid sites on the external surface are active in this reaction, it could be expected that the performance of hierarchical USY zeolites in this conversion relates linearly to the acidity as measured using more bulky probe molecules, such as 2,6-di-tert-butylpyridine.104
5. Industrial interest in hierarchical faujasites
A great obstacle in the design of superior hierarchical zeolites in academia is the need of companies to protect their intellectual property. Although from a corporate perspective this is understandable, it also implies that many aspects regarding technical zeolite catalyst design, that is, going beyond the powder, remain obscure for academia. Nevertheless, the availability of commercial faujasite catalysts to academia by companies as UOP, Tosoh, Zeochem, Clariant, and Zeolyst have been instrumental to the development of the field of zeolite catalysts. A good example is the comprehensive list of Y and USY zeolites that is provided by Zeolyst (Table 4).105 Based on the wide variation of properties and their long-lasting availability, their zeolite products have been the starting point for many zeolite studies. Unsurprisingly, they are routinely used as parent zeolites in the synthesis of hierarchical zeolites by post-synthetic modifications.22,23,52,89,106| CBV code | Si/Al [mol mol−1] | Cation | Unit cell size [Å] | Treatment |
|---|---|---|---|---|
| 100 | 2.6 | Na+ | 24.65 | Freshly synthesized |
| 300 | 2.6 | NH4+ | 24.68 | Ion exchanged |
| 400 | 2.6 | H+ | 24.50 |
Ion exchanged
Mildly steamed |
| 500 | 2.6 | NH4+ | 24.53 |
Ion exchanged
Mildly steamed |
| 600 | 2.6 | H+ | 24.35 |
Ion exchanged
Moderately steamed |
| 712 | 6 | NH4+ | 24.35 |
Ion exchanged
Moderately steamed Acid treated |
| 720 | 15 | H+ | 24.28 |
Ion exchanged
Severely steamed Acid treated |
| 760 | 30 | H+ | 24.24 |
Ion exchanged
Severely steamed Acid treated |
| 780 | 40 | H+ | 24.24 |
Ion exchanged
Severely steamed Acid treated |
| 901 | 40 | H+ | 24.24 |
Ion exchanged
Severely steamed Acid treated Heat treated |
Besides the attention in academia, also a substantial attention of hierarchical faujasites has occurred in patent literature (Table 5). Prior to the establishment of secondary porosity as key tool to enhance catalytic performance (around 2000), the patent literature of mesoporous faujasites was limited. The occurrence of only one patent, by PQ on the generation of mesopores by hydrothermal treatment, may be due to the dubious role mesopores were perceived to play at that time. Only after the realisation of the potential of secondary porosity, a wave of industrial interest occurred (2009–2012). Unsurprisingly, the majority of those focus on the synthesis of hierarchical Y and USY zeolites by post-synthetic modification, and more specifically, by (acid- and) base treatments (patents by Total, Eni, and IFP). As may be expected, the application of these hierarchical faujasites focused on hydro-conversions and specifically on hydrocracking (patents by Total, ExxonMobil, IMP).
| Patent number | Company | Filing date | Zeolite | Main observations | Application | Strategy in Fig. 2 |
|---|---|---|---|---|---|---|
| a Final acid wash not performed. b Renewable feedstocks (see Section 4.3). c As judged by the 4 nm peak in the BJH desorption mesopore size distribution (see Fig. 13c). d Not applicable. | ||||||
| US5601789 | PQ | 1994 | Y | Mesopores generated by hydrothermal treatments | nad | n |
| US2012/0018349 | Total | 2009 | USY | Small mesopore formation and severe amorphisation by alkaline treatment | Hydrocracking | k |
| US2014/0249344 | Total | 2012 | USY | Crystallinity alkaline-treated USY zeolites partially restored by ammonia treatment | Hydrocracking | na |
| US2012/0227584 | ExxonMobil | 2011 | Y | Smaller aggregates obtained by aging of the synthesis gel, external surface limited to 20 m2 g−1 | Adsorption | e |
| US8513150 | ExxonMobil | 2012 | Y | Mesopores formed by steam treatments | na | n |
| US2013/0118954 | ExxonMobil | 2012 | Y | Low-occlusionc mesopores formed by steam treatment | Hydrocracking | n |
| US2013/0171058 | IMP | 2011 | Y | Mesopore formation, crystallinity and microporosity largely preserved by using hydrothermal treatment in polyols and ammonium salts | Catalytic cracking | na |
| US2011/0108460 | IFP | 2010 | Y | Superior catalyst are obtained by sequential acid–base treatment | Hydrotreatmentb hydro-isomerisationb | ga |
| US8679323 | ENI, IFP | 2010 | Y | Superior catalyst are obtained by sequential acid–base treatment | Production of middle distillates from waxes | ga |
| US2012/0205286 | IFP | 2012 | USY | Superior catalysts obtained by base treatment | Hydrocracking | k |
The majority of the patents can be ratified using the strategies in Fig. 2. However, a number of interesting observations deserve extra notice. First, Total showed that the partial (10%) recovery of the crystallinity of USY zeolites, that were completely amorphized by base treatment, could be restored by ammonia treatment.107 Although an additional (gas-phase) NH3 treatment is required, this provides a tool to obtain highly mesoporous and (partially) crystalline USY zeolites without the need for organics, such as TPA+ or diethylamine, to preserve the FAU structure during the alkaline attack. At the same time, ExxonMobil patented an approach to adjust the synthesis of Y zeolites to increase the external surface.108 Although the external surface is limited to 20 m2 g−1, it is essential to note that the influence of the crystal aggregate size is an important part of the patent. This stresses the importance of zeolite design beyond the zeolite crystal. Moreover, since the targeted application concerns adsorption, the potential of hierarchical faujasites may go beyond catalysis.
Another interesting work by ExxonMobil regards the characterisation of hierarchical Y zeolites prepared by steam treatment.109 Like De Jong et al.82 they applied the BJH model to the desorption branches of N2 isotherms at 77 K to quantify the degree of pore blockage (Fig. 13c). Moreover, they claimed a method to produce low-occlusion mesopores in Y zeolites (Table 5). These zeolites feature an exceptionally-low 4 nm peak in the BJH pore size distribution, and accordingly yielded an enhanced selectivity in hydrocracking. This patent further stresses that besides the quantity of external surface, also the quality of the generated mesopores plays a crucial role in zeolite design. IMP (Mexican institute of petroleum) featured a novel hydrothermal technique to generate mesopores, preserve zeolitic properties, and lower the sodium content simultaneously by 75%. This is achieved by single step hydrothermal treatments at an elevated temperature using aqueous solutions of polyols and ammonium salts. The different components in this synthetic procedure make it hard to assess what the purpose of each component is. Nevertheless, it does provide with a potential new strategy to synthesize hierarchical faujasites.
An interesting observation is the absence of major zeolite manufacturers Grace and UOP. Since it is obvious that both multi-nationals routinely apply post-synthetic modifications to optimize the performance of their zeolite catalysts,3,5,6 they may have opted to keep their post-synthetic strategies undisclosed. However, their absence may also be explained by the fact that the use of base treatments on zeolites to enhance micropore accessibility, enhance adsorption, and attain superior catalytic performance was already claimed in 1967 by Young.110 In this patent, the main claim elaborates on a hydrocarbon conversion catalysts, which is predominantly based on a crystalline aluminosilicate (Si/Al = 3–6, micropore size 0.3–0.8 nm), which has been leached using alkali metal hydroxides of pH >10.5. Although the compositional range of this claim is limited and the patent deals exclusively with mordenite, it remains unclear whether or not this patent indicates that the use of base leaching was already effectively demonstrated for decades.
6. Outlook and future directions
Since the breakthrough contributions on their synthesis, hierarchical faujasites have rapidly become one of the most promising class of hierarchical zeolites, based on a variation of advantages. The combination of a cheap and scalable synthesis with accessible 3D micropores and a tunable mesoporosity, composition, stability, and nature of the active site, make them superior catalysts in many established and novel applications in the petrochemical industry as well as in the valorisation of biomass. However, a variety of challenges and opportunities exists in order to secure their position as superior porous material (Fig. 18).A first concern regarding post-synthetic modifications is that, of the applied acid or base treatment, mostly the concentration was varied. It is imaginable that, by variation of the time and temperature, a certain solution may give rise to completely different materials. Next, although post-synthetic modifications have enabled a full compositional flexibility of the synthesis of hierarchical faujasites (Fig. 2), several questions concerning the actual mechanism of mesopore formation persist. For example, though the alkaline treatment of zeolites has received a substantial attention, major progresses on the actual mechanism on a molecular scale remain lacking. It is for example not entirely clear where the leaching starts: Does it start on the outside of the crystal, or homogenously throughout the crystal? Microscopic observations and a linear relation between the degree of leaching and the total surface area of the parent materials suggest a leaching throughout the crystal. However, this was never unambiguously demonstrated. Another interesting aspect, particularly in USY zeolites, is the different nature and degrees of heterogeneity (Al gradients or Si- or Al-rich amorphous species), that can occur in faujasite samples. This is highly relevant as in sequences of treatments the outcome of the initial treatment directly influences the productivity of the next, and thereby all other treatments that follow. Of course, fundamental differences between framework topologies (for example FAU vs. MFI) represent an important pathway for future study.
Hierarchical faujasites derived from bottom-up strategies face different challenges. Besides the obvious sustainability and scalability difficulties regarding their synthesis, an important concern is the apparent impossibility to hydrothermally synthesize highly siliceous (Si/Al > 6) USY zeolites, that is, the most used faujasite catalysts. The difficulty of doing so, implies that an important future focal point may constitute the dealumination of bottom-up prepared hierarchical Y faujasites. In the case of Y nanocrystals this may be particularly challenging as their Si/Al ratio is approaching that of zeolite X (Table 2), and therefore may not be stable upon dealumination. In addition, it remains unclear if the accessible nature of the nanocrystals can be preserved upon post-synthetic treatments.
In terms of characterisation, we expect that substantial headway can be made using systematic studies featuring distinct hierarchical zeolites. This can be achieved by either evaluating the properties of hierarchical zeolites using a single method comprising different degrees of mesoporosity (Fig. 17), or by using hierarchical faujasites with similar external surfaces but prepared by different strategies. Especially in studies contacting USY zeolites with CTA+-containing alkaline solutions, such systematic approaches should help to more precisely unravel the relationships between synthesis, properties, and function, crucial to the application-oriented design of hierarchical faujasites (Fig. 18). Although several works have reported the superior performance of bifunctional faujasite catalysts,21,93,94 the role of external surface in the preparation of bifunctional catalysis is relatively unclear. For example, the large external surface may yield enhanced dispersion of deposited metals, hereby attaining superior catalytic performance.
More specifically, key challenges in the advanced study of hierarchical faujasites regard diffusion and accessibility studies. For example, the role of steam treatments on the diffusional properties and accessibility deserves to be revisited based on the newly obtained synthetic possibilities. Together with the industrial attention to steam treatments (Table 5), this may even rejuvenate the use of steam treatment as a tool to introduce secondary porosity. Also, diffusion studies using positron annihilation lifetime spectroscopy (Fig. 13e) may prove a powerful tool, as it is not limited by the experimental difficulties related to the limited volatility of bulky probe molecules. The use of bulky pyridines, larger than in the case of 10 MR zeolites as ZSM-5, may be used to systematically map the accessibility of the acid sites. Finally, a special point is the relative stability of faujasite zeolites under the different conditions. We believe it is important to highlight the need to more clearly indicate the type of stability (Fig. 12b), and we identify the zeolite composition (Si/Al ratio, type of cation, amount of CBCCs, and presence of EFAl species) as an important tool to tailor that relative stability.
Also in catalytic evaluations a more systematic study should strengthen the synthesis–property–function relationships. This process can be facilitated by the supermarket of strategies developed within the last several years (Fig. 2). For example, in addition to evaluating activity gains as a function of external surface introduced, more links between the porosity, acidity, and the selectivity patterns may be established (Fig. 17). Although strong selectivity benefits are obtained in catalytic cracking (Table 3), the involved studies typically include a limited amount of samples, that is, one conventional and one hierarchical sample. Using a larger set of samples should enable to more precisely steer a reaction to a desired product. This approach may concomitantly lead to better insights in the reaction mechanisms. The latter is important as, in contrast to homogeneous catalysis in the liquid phase, the reaction mechanisms in zeolites are often not entirely clear. This may be due to access limitations, competitive adsorption, or other (often energetic) reasons.
Besides a more optimized use in established reactions, the substantially enhanced secondary porosity and the generation of novel types of actives sites may enable to successfully apply hierarchical faujasites in novel catalytic applications. Particularly the conversions of bulky reagents, traditionally performed using mineral acids, may be replaced with these accessible solid acids. In addition, by careful design, hierarchical faujasites may be prepared comprising in addition to the classic active sites, a secondary level of active sites. These could even be, besides the reactivity differences, location specific (being either in micropore or mesopore). Such principles were already devised in catalytic cracking of gas oil.24 Next, a large market is the application in biomass-related conversions. Here, insights in the relative stability and adsorptive properties are crucial based on the more polar feedstocks and more polar solvents, such as water or alcohols. Particularly based on their large tunability of the relative stability and the active site, hierarchical faujasites may become widely applied in biomass related conversions.
Apart from the application of faujasites in catalytic conversions, the market for ion exchangers, molecular sieves, and adsorbents is significant. In these fields, the presence of hierarchical porosity has not been established. Here particularly, the strategies regarding the synthesis of cation-rich hierarchical zeolite X should play a decisive part. It is an important question whether the added level of mesoporosity is able to instigate an enhanced performance in these fields. Of course, independent of application, scale up represents a key challenge; a topic for which thus far little fundamental knowledge exists.111 The latter may simply be explained by the lack of the need to scale up in academia, however this does not justify it. We are convinced that, by combining scalable synthesis strategies with smart experimentation and collaboration with industrial partners, it must be possible to gather fundamental understanding on the scale-up and shaping of heterogeneous catalysts in general.
It is remarkable that, although one of the oldest families of synthetic zeolites, faujasites remain such a widely-applied and highly-interesting material. Their continued success story may be related to a Darwinistic ability of these zeolites to adjust to new application-based demands, such as its conversion from microporous to hierarchical porous materials (described in this review). In addition, their organic-free synthesis in combination with advanced post-synthetic designs, seems to keep them a step ahead from competitive large-micropore frameworks, such as LTL, EMT, and DON. Therefore, based on the abovementioned challenges and opportunities, we perceive that the synthesis and application of hierarchical faujasites provide ample room for high quality and innovative future studies for years to come.
Acknowledgements
D.V. acknowledges the Flanders Research Foundation (FWO) for a post-doctoral fellowship. N.N. thanks the KU Leuven for financial support (FLOF). J.V.A. acknowledges the Agency for Innovation by Science and Technology in Flanders (IWT) for financial support.References
- D. W. Breck, J. Chem. Educ., 1964, 41, 678–689 CrossRef CAS
.
- C. Baerlocher and L. B. McCusker, Database of Zeolite Structures, http://www.iza-structure.org/databases/, accessed April 2015.
-
R. W. Broach, D.-Y. Jan, D. A. Lesch, S. Kulprathipanja, E. Roland and P. Kleinschmit, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2000, pp. 1–35 Search PubMed
.
- C. S. Cundy and P. A. Cox, Microporous Mesoporous Mater., 2005, 82, 1–78 CrossRef CAS
.
-
E. M. Flanigen, R. W. Broach and S. T. Wilson, in Zeolites in Industrial Separation and Catalysis, ed. S. Kulprathipanja, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2010, pp.1-26 Search PubMed
.
-
C. V. McDaniel and P. K. Maher, Molecular Sieves, Soc. Chem. Ind., London, 1968 Search PubMed
.
- L. Tosheva and V. P. Valtchev, Chem. Mater., 2005, 17, 2494–2513 CrossRef CAS
.
-
(a) J. Pérez-Ramírez, C. H. Christensen, K. Egeblad, C. H. Christensen and J. C. Groen, Chem. Soc. Rev., 2008, 37, 2530–2542 RSC
; (b) S. Lopez-Orozco, A. Inayat, A. Schwab, T. Selvam and W. Schwieger, Adv. Mater., 2011, 23, 2602–2615 CrossRef CAS PubMed
.
- W. J. Roth, P. Nachtigall, R. E. Morris and J. Čejka, Chem. Rev., 2014, 114, 4807–4837 CrossRef CAS PubMed
.
- K. Egeblad, C. H. Christensen, M. Kustova and C. H. Christensen, Chem. Mater., 2008, 20, 946–960 CrossRef CAS
.
- K. Na, M. Choi and R. Ryoo, Microporous Mesoporous Mater., 2013, 166, 3–19 CrossRef CAS
.
-
(a) R. Chal, C. Gérardin, M. Bulut and S. van Donk, ChemCatChem, 2011, 3, 67–81 CrossRef CAS
; (b) K. Möller and T. Bein, Chem. Soc. Rev., 2013, 42, 3689–3707 RSC
.
- D. Verboekend and J. Pérez-Ramírez, Catal. Sci. Technol., 2011, 1, 879–890 CAS
.
- S. van Donk, A. H. Janssen, J. H. Bitter and K. P. de Jong, Catal. Rev.: Sci. Eng., 2003, 45, 297–319 CAS
.
-
T. Tatsumi, Handbook of Porous Solids, Wiley-VCH Verlag GmbH, 2008, pp. 903–935 Search PubMed
.
-
H. K. Beyer, in Molecular Sieves, Science and Technology, ed. H. G. Karge and J. Weitkamp, Springer-Verlag, Berlin, 2002, vol. 3, pp. 203–256 Search PubMed
.
- P. Kortunov, S. Vasenkov, J. Kärger, R. Valiullin, P. Gottschalk, M. Fé Elía, M. Perez, M. Stöcker, B. Drescher, G. McElhiney, C. Berger, R. Gläser and J. Weitkamp, J. Am. Chem. Soc., 2005, 127, 13055–13059 CrossRef CAS PubMed
.
- A. H. Janssen, A. J. Koster and K. P. de Jong, J. Phys. Chem. B, 2002, 106, 11905–11909 CrossRef CAS
.
- M. Ogura, S.-Y. Shinomiya, J. Tateno, Y. Nara, E. Kikuchi and M. Matsukata, Chem. Lett., 2000, 882–883 CrossRef CAS
.
- J. C. Groen, L. A. A. Peffer, J. A. Moulijn and J. Pérez-Ramírez, Colloids Surf., A, 2004, 241, 53–58 CrossRef CAS
.
- K. P. de Jong, J. Zečević, H. Friedrich, P. E. de Jongh, M. Bulut, S. van Donk, R. Kenmogne, A. Finiels, V. Hulea and F. Fajula, Angew. Chem., Int. Ed., 2010, 49, 10074–10078 CrossRef CAS PubMed
.
- D. Verboekend, G. Vilé and J. Pérez-Ramírez, Adv. Funct. Mater., 2012, 22, 916–928 CrossRef CAS
.
- D. Verboekend, T. C. Keller, S. Mitchell and J. Pérez-Ramírez, Adv. Funct. Mater., 2013, 23, 1923–1934 CrossRef CAS
.
- R. Mann, Catal. Today, 1993, 18, 509–528 CrossRef
.
- D. Verboekend, S. Mitchell, M. Milina, J. C. Groen and J. Pérez-Ramírez, J. Phys. Chem. C, 2011, 115, 14193–14203 CAS
.
- A. Inayat, I. Knoke, E. Spiecker and W. Schwieger, Angew. Chem., Int. Ed., 2012, 51, 1962–1965 CrossRef CAS PubMed
.
- P. Morales-Pacheco, J. M. Domínguez, L. Bucio, F. Alvarez, U. Sedran and M. Falco, Catal. Today, 2011, 166, 25–38 CrossRef CAS
.
- H. Awala, J.-P. Gilson, R. Retoux, P. Boullay, J.-M. Goupil, V. Valtchev and S. Mintova, Nat. Mater., 2015, 14, 447–451 CrossRef CAS PubMed
.
- G. Kerr, A. Chester and D. Olson, Catal. Lett., 1994, 25, 401–402 CrossRef CAS
.
- G. T. Kerr, J. Phys. Chem., 1968, 72, 2594–2596 CrossRef CAS
.
- S. Ramdas, J. M. Thomas, J. Klinowski, C. A. Fyfe and J. S. Hartman, Nature, 1981, 292, 228–230 CrossRef CAS
.
- R. L. Hartman and H. S. Fogler, Langmuir, 2007, 23, 5477–5484 CrossRef CAS PubMed
.
- S. Lee, H. Kim and M. Choi, J. Mater. Chem. A, 2013, 1, 12096–12102 CAS
.
- Z. Qin, B. Shen, X. Gao, F. Lin, B. Wang and C. Xu, J. Catal., 2011, 278, 266–275 CrossRef CAS
.
- W. Q. Jiao, W. H. Fu, X. M. Liang, Y. M. Wang and M.-Y. He, RSC Adv., 2014, 4, 58596–58607 RSC
.
-
D. W. Breck and G. W. Skeels, US Pat., 4503023A, 1985 Search PubMed
.
- W. Lutz, Adv. Mater. Sci. Eng., 2014, 2014, 1–20 CrossRef
.
- D. Verboekend, G. Vilé and J. Pérez-Ramírez, Cryst. Growth Des., 2012, 12, 3123–3132 CAS
.
- P. Y. Dapsens, M. J. Menart, C. Mondelli and J. Pérez-Ramírez, Green Chem., 2014, 16, 589–593 RSC
.
- J. Van Aelst, M. Haouas, E. Gobechiya, K. Houthoofd, A. Philippaerts, S. P. Sree, C. E. A. Kirschhock, P. Jacobs, J. A. Martens, B. F. Sels and F. Taulelle, J. Phys. Chem. C, 2014, 118, 22573–22582 CAS
.
- J. Van Aelst, A. Philippaerts, N. Nuttens, D. Verboekend, M. Kurttepeli, E. Gobechiya, M. Haouas, S. P. Sree, J. F. M. Denayer, J. A. Martens, C. E. A. Kirschhock, F. Taulelle, S. Bals, G. V. Baron, P. A. Jacobs and B. F. Sels, Adv. Funct. Mater., 2015 DOI:10.1002/adfm.201502772
.
- J. C. Groen, J. A. Moulijn and J. Pérez-Ramírez, Ind. Eng. Chem. Res., 2007, 46, 4193–4201 CrossRef CAS
.
-
J. Y. Ying and J. García-Martínez, US Pat., 7589041B2, 2009 Search PubMed
.
- R. Chal, T. Cacciaguerra, S. van Donk and C. Gérardin, Chem. Commun., 2010, 46, 7840–7842 RSC
.
- I. I. Ivanova and E. E. Knyazeva, Chem. Soc. Rev., 2013, 42, 3671–3688 RSC
.
- J. García-Martínez, M. Johnson, J. Valla, K. Li and J. Y. Ying, Catal. Sci. Technol., 2012, 2, 987–994 Search PubMed
.
-
J. Pérez-Pariente and T. Álvaro-Muñoz, in Mesoporous Zeolites: Preparation, Characterisation and Applications, ed. J. Pérez-Pariente and K. Li, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 1st edn, 2015, pp. 1–29 Search PubMed
.
-
D. M. Ginter, A. T. Bell and C. J. Radke, in Synthesis of Microporous Materials, Molecular Sieves, ed. M. L. Occelli and H. E. Robson, Van Nostrand Reinhold, New York, 1992, vol. 1, p. 6 Search PubMed
.
- D. Verboekend, M. Milina, S. Mitchell and J. Pérez-Ramírez, Cryst. Growth Des., 2013, 13, 5025–5035 CAS
.
-
K. Li, J. García-Martínez and M. G. Beaver, US Pat., 20130183230A1, 2013 Search PubMed
.
-
J. García-Martínez, US Pat., 20130183229A1, 2013 Search PubMed
.
- D. Verboekend and J. Pérez-Ramírez, ChemSusChem, 2014, 7, 753–764 CrossRef CAS PubMed
.
- A. Inayat, C. Schneider and W. Schwieger, Chem. Commun., 2014, 51, 279–281 RSC
.
- H. Lechert and H. Kacirek, Zeolites, 1991, 11, 720–728 CrossRef CAS
.
- D. Verboekend, A. M. Chabaneix, K. Thomas, J.-P. Gilson and J. Pérez-Ramírez, CrystEngComm, 2011, 13, 3408–3416 RSC
.
- N. Nuttens, D. Verboekend, A. Deneyer, J. Van Aelst and B. F. Sels, ChemSusChem, 2015, 8, 1197–1205 CrossRef CAS PubMed
.
- R. A. Beyerlein, C. Choi-Feng, J. B. Hall, B. J. Huggins and G. J. Ray, Top. Catal., 1997, 4, 27–42 CrossRef CAS
.
- T. C. Keller, S. Isabettini, D. Verboekend, E. G. Rodrigues and J. Pérez-Ramírez, Chem. Sci., 2014, 5, 677–684 RSC
.
- T. C. Keller, E. G. Rodrigues and J. Pérez-Ramírez, ChemSusChem, 2014, 7, 1729–1738 CrossRef CAS PubMed
.
- D. Verboekend and J. Pérez-Ramírez, Chem. – Eur. J., 2011, 17, 1137–1147 CrossRef CAS PubMed
.
- P. Y. Dapsens, C. Mondelli and J. Pérez-Ramírez, ChemSusChem, 2013, 6, 831–839 CrossRef CAS PubMed
.
- P. Y. Dapsens, C. Mondelli, J. Jagielski, R. Hauert and J. Pérez-Ramírez, Catal. Sci. Technol., 2014, 4, 2302–2311 CAS
.
- M. Moliner, Y. Román-Leshkov and M. E. Davis, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 6164–6168 CrossRef CAS PubMed
.
- M. S. Holm, S. Saravanamurugan and E. Taarning, Science, 2010, 328, 602–605 CrossRef CAS PubMed
.
- J. Dijkmans, D. Gabriëls, M. Dusselier, F. de Clippel, P. Vanelderen, K. Houthoofd, A. Malfliet, Y. Pontikes and B. F. Sels, Green Chem., 2013, 15, 2777–2785 RSC
.
- V. Rac, V. Rakić, D. Stošić, O. Otman and A. Auroux, Microporous Mesoporous Mater., 2014, 194, 126–134 CrossRef CAS
.
- M. S. Holm, S. Svelle, F. Joensen, P. Beato, C. H. Christensen, S. Bordiga and M. Bjørgen, Appl. Catal., A, 2009, 356, 23–30 CrossRef CAS
.
- H. Hattori, Chem. Rev., 1995, 95, 537–558 CrossRef CAS
.
-
A. Corma and S. Iborra, in Fine Chemicals through Heterogeneous Catalysis, ed. R. A. Sheldon and H. van Bekkum, Wiley-VCH, New York, 2002, vol. 1, p. 309 Search PubMed
.
- Y. Ono, J. Catal., 2003, 216, 406–415 CrossRef CAS
.
- J. C. Groen, L. A. A. Peffer, J. A. Moulijn and J. Pérez-Ramírez, Chem. – Eur. J., 2005, 11, 4983–4994 CrossRef CAS PubMed
.
- D. Zhai, L. Zhao, Y. Liu, J. Xu, B. Shen and J. Gao, Chem. Mater., 2015, 27, 67–74 CrossRef CAS
.
- D. Verboekend, M. Milina and J. Pérez-Ramírez, Chem. Mater., 2014, 26, 4552–4562 CrossRef CAS
.
- M. Briend-Faure, O. Cornu, D. Delafosse, R. Monque and M. J. Peltre, Appl. Catal., 1988, 38, 71–87 CrossRef CAS
.
- R. M. Ravenelle, F. Schüβler, A. D'Amico, N. Danilina, J. A. van Bokhoven, J. A. Lercher, C. W. Jones and C. Sievers, J. Phys. Chem. C, 2010, 114, 19582–19595 CAS
.
- T. Ennaert, J. Geboers, E. Gobechiya, C. M. Courtin, M. Kurttepeli, K. Houthoofd, C. E. A. Kirschhock, P. C. M. M. Magusin, S. Bals, P. A. Jacobs and B. F. Sels, ACS Catal., 2015, 5, 754–768 CrossRef CAS
.
- D. Verboekend, T. C. Keller, M. Milina, R. Hauert and J. Pérez-Ramírez, Chem. Mater., 2013, 25, 1947–1959 CrossRef CAS
.
- J. C. Groen, L. A. A. Peffer and J. Pérez-Ramírez, Microporous Mesoporous Mater., 2003, 60, 1–17 CrossRef CAS
.
- A. Galarneau, F. Villemot, J. Rodriguez, F. Fajula and B. Coasne, Langmuir, 2014, 30, 13266–13274 CrossRef CAS PubMed
.
- A. Sayari, M. Kruk and M. Jaroniec, Catal. Lett., 1997, 49, 147–153 CrossRef CAS
.
-
M. Thommes, in Nanoporous Materials: Science and Engineering, ed. G. Q. Lu and X. S. Zhao, Imperial College Press, London, 2004, vol. 4, pp. 317–364 Search PubMed
.
- J. Zečević, C. J. Gommes, H. Friedrich, P. E. de Jongh and K. P. de Jong, Angew. Chem., Int. Ed., 2012, 51, 4213–4217 CrossRef PubMed
.
- J. Garcia-Martinez, C. Xiao, K. A. Cychosz, K. Li, W. Wan, X. Zou and M. Thommes, ChemCatChem, 2014, 6, 3110–3115 CrossRef CAS
.
- J. C. Groen, T. Bach, U. Ziese, A. M. Paulaime-van Donk, K. P. de Jong, J. A. Moulijn and J. Pérez-Ramírez, J. Am. Chem. Soc., 2005, 127, 10792–10793 CrossRef CAS PubMed
.
- L. Gueudré, M. Milina, S. Mitchell and J. Pérez-Ramírez, Adv. Funct. Mater., 2014, 24, 209–219 CrossRef
.
- F. C. Meunier, D. Verboekend, J.-P. Gilson, J. C. Groen and J. Pérez-Ramírez, Microporous Mesoporous Mater., 2012, 148, 115–121 CrossRef CAS
.
- M. Milina, S. Mitchell, P. Crivelli, D. Cooke and J. Pérez-Ramírez, Nat. Commun., 2014, 5, 3922 Search PubMed
.
- D. Mehlhorn, A. Inayat, W. Schwieger, R. Valiullin and J. Kärger, ChemPhysChem, 2014, 15, 1681–1686 CrossRef CAS PubMed
.
- T. C. Keller, J. Arras, S. Wershofen and J. Pérez-Ramírez, ACS Catal., 2015, 5, 734–743 CrossRef CAS
.
- M. Milina, S. Mitchell and J. Pérez-Ramírez, Catal. Today, 2014, 235, 176–183 CrossRef CAS
.
- C. Martínez, D. Verboekend, J. Pérez-Ramírez and A. Corma, Catal. Sci. Technol., 2013, 3, 972–981 Search PubMed
.
- A. Corma, V. Fornés, R. M. Martín-Aranda, H. Garcia and J. Primo, Appl. Catal., 1990, 59, 237–248 CrossRef CAS
.
- S. Sartipi, M. Alberts, M. J. Meijerink, T. C. Keller, J. Pérez-Ramírez, J. Gascon and F. Kapteijn, ChemSusChem, 2013, 6, 1646–1650 CrossRef CAS PubMed
.
- X. Peng, K. Cheng, J. Kang, B. Gu, X. Yu, Q. Zhang and Y. Wang, Angew. Chem., Int. Ed., 2015, 54, 4553–4556 CrossRef CAS PubMed
.
- P. A. Jacobs, M. Dusselier and B. F. Sels, Angew. Chem., Int. Ed., 2014, 53, 8621–8626 CrossRef CAS PubMed
.
- D. Kubička and O. Kikhtyanin, Catal. Today, 2015, 243, 10–22 CrossRef
.
- D. Kubička, I. Kubičková and J. Čejka, Catal. Rev., 2013, 55, 1–78 Search PubMed
.
- P. Y. Dapsens, C. Mondelli and J. Pérez-Ramírez, ACS Catal., 2012, 2, 1487–1499 CrossRef CAS
.
- J. R. García, M. Bertero, M. Falco and U. Sedran, Appl. Catal., A, 2015, 503, 1–8 CrossRef
.
- K. S. Arias, M. J. Climent, A. Corma and S. Iborra, Energy Environ. Sci., 2015, 8, 317–331 CAS
.
- L. Zhou, M. Shi, Q. Cai, L. Wu, X. Hu, X. Yang, C. Chen and J. Xu, Microporous Mesoporous Mater., 2013, 169, 54–59 CrossRef CAS
.
- A. Philippaerts, S. Goossens, W. Vermandel, M. Tromp, S. Turner, J. Geboers, G. Van Tendeloo, P. A. Jacobs and B. F. Sels, ChemSusChem, 2011, 4, 757–767 CrossRef CAS PubMed
.
- A. de Angelis, P. Ingallina and C. Perego, Ind. Eng. Chem. Res., 2004, 43, 1169–1178 CrossRef CAS
.
- A. Corma, V. Fornés, L. Forni, F. Márquez, J. Martínez-Triguero and D. Moscotti, J. Catal., 1998, 179, 451–458 CrossRef CAS
.
- Zeolyst International, Zeolite Y, http://www.zeolyst.com/our-products/standard-zeolite-powders/zeolite-y.aspx, accessed July 2015.
- M. J. Remy, D. Stanica, G. Poncelet, E. J. P. Feijen, P. J. Grobet, J. A. Martens and P. A. Jacobs, J. Phys. Chem., 1996, 100, 12440–12447 CrossRef CAS
.
-
D. Minoux and N. Danilina, US Pat., 20140249344, 2012 Search PubMed
.
-
K. Wang, R. C. Lemon, J. S. Buchanan, C. E. Kliewer and W. J. Roth, US Pat., 20120227584, 2011 Search PubMed
.
-
J. J. Wu, A. B. Dandekar and C. G. Oliveri, US Pat., 20130118954, 2012 Search PubMed
.
-
D. A. Young, US Pat., 3326797, 1967 Search PubMed
.
- S. Mitchell, N.-L. Michels and J. Pérez-Ramírez, Chem. Soc. Rev., 2013, 42, 6094–6112 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cs00520e |
| This journal is © The Royal Society of Chemistry 2016 |