A quantitative microbial risk assessment of wastewater treatment plant blending: case study in San Francisco Bay
Edmund Y.
Seto
*a,
Jon
Konnan
b,
Adam W.
Olivieri
*b,
Richard E.
Danielson
c and
Donald M. D.
Gray
d
aUniversity of Washington, USA. E-mail: eseto@uw.edu
bEOA, Inc., USA. E-mail: jkonnan@eoainc.com; awo@eoainc.com
cBioVir, Inc., USA. E-mail: red@biovir.com
dEBMUD, USA. E-mail: dgabb@ebmud.com
First published on 2nd October 2015
Abstract
An investigation was carried out to evaluate the impacts of blending practices (i.e., a practice used to manage wet weather flows) on the effluent from the East Bay Municipal Utility District's (EBMUD) wastewater treatment plant in Oakland, California and water quality in the receiving water (San Francisco Bay). A static based quantitative microbial risk assessment (QMRA) was used to estimate the incremental risk to public health from recreational exposure to adenovirus and the protozoan Giardia spp. in San Francisco Bay for wet season (generally between October and March) blending and non-blending events. The mean risks of infection per recreational exposure event during the wet season for all of the modeled scenarios were more than an order-of-magnitude below the USEPA's illness level (36 illnesses per 1000 contact events) associated with recreational water quality. While the QMRA results showed discernible differences in per event estimated risks between blending and non-blending scenarios, the estimated incremental increase in the annual number of infections due to blending (based on median estimates) resulted in an estimated combined increase of less than one infection annually. These estimates are subject to various uncertainties, including the potential for secondary transmission, assumptions on the extent of exposures, and the number of blending days required in the future due to climate change, which are discussed in this paper.
Water impactThe manuscript contains an initial relative microbial risk assessment for exposure to adenovirus and the Giardia spp. during wastewater treatment plant blending and non-blending events at three recreational sites in San Francisco Bay. Findings indicate that the estimated mean microbial risk associated with blending during wet weather events are an order-of-magnitude less than EPA recreational water quality criteria. |
Introduction
Wastewater treatment plant effluent blending has been used since the 1970s to prevent overflows in a wastewater collection system or washout of a treatment plant's biological secondary process during peak wet weather flows. The infectious waterborne agents associated with municipal wastewater are those found in the domestic sanitary waste of the population. These microbial pathogens include bacteria, viruses, and parasites. From a public health perspective it is important to assume that raw wastewater contains pathogenic organisms. Although a wide range of pathogens have been identified in raw wastewater, relatively few types of pathogens appear to be responsible for the majority of the waterborne illnesses caused by pathogens of wastewater origin.2 Typical concentrations of some of the key pathogenic microorganisms found in raw wastewater are: enteric viruses range of 103–104 MPN per 100 mL, Cryptosporidium parvum oocysts range of 101–105 MPN per 100 mL, and Giardia lamblia cysts range of 101–104 MPN per 100 mL.3 Additional pathogen data characterizing raw wastewater, secondary treated effluent, filtered and disinfected effluents are available in Cooper et al., 2012,4 and Rose et al., 2004.5 Unfortunately, limited data exist regarding effluent pathogen concentrations during blending, and the additional potential risk of infection and disease resulting from the exposure to receiving waters subject to discharges from blending practices.To help ensure that decisions regarding wastewater infrastructure improvements to properly manage peak wet weather wastewater flows are optimized based on quantifiable water quality and public health benefits a Water Environment Research Foundation (WERF) funded study was conducted by the East Bay Municipal Utility District (EBMUD) for the collection of additional data on peak wet weather events in San Francisco.6,7 The WERF investigation evaluated the impacts of blending practices at a municipal wastewater treatment plant on effluent and receiving water quality, and estimated public health risks associated with recreation in surface waters receiving blended flows. Field samples were collected at the EBMUD municipal wastewater treatment plant for in-plant processes and receiving waters during wet weather blending and non-blending events.
Laboratory analyses for Giardia, Cryptosporidium, viruses (adenovirus, enteric viruses, rotavirus, norovirus), pathogen indicator organisms (fecal coliform, Escherichia coli, enterococcus, and male-specific coliphage), and other water quality parameters were performed on many of these samples. Field sample results for the East Bay Municipal Utility District's (EBMUD) Main Wastewater Treatment Plant (MWWTP) served as the basis for developing hydrodynamic and water quality computer models to estimate receiving water exposure as part of developing quantitative estimates of the human health risk associated with microbial pathogens present in the treatment plant effluent. As described below, because we found evidence of elevated Giardia and adenovirus concentrations between blending and non-blending periods, the focus of our analysis was on the incremental risk associated with increased concentrations of these pathogens during wet season exposure. Data on the other microbial pathogens and indicators are contained in the final WERF6 report.
Quantitative Microbial Risk Assessment (QMRA) is a well-established, formal approach for quantifying the human health risks associated with exposure to infectious pathogens.1 As its name implies, QMRA relies upon and integrates quantitative information on human exposures to certain pathogens (exposure assessment) and the likelihood that these exposures might result in infection and/or illness (dose–response relationships). QMRA has been applied to a variety of water-related issues, to evaluate risks, management strategies, and identify important uncertainties and knowledge gaps. The US EPA recognizes the importance of QMRA, allowing for its use by individual states to inform alternative recreational water quality criteria that meet local environmental conditions and exposure scenarios.1 QMRAs for recreational water have helped to characterize important factors that may affect health risks, including the importance of enteric viruses in crowded human recreational water,8 the potential for non-human sources of infection (e.g., cattle and bird sources of protozoa and bacteria) for some recreational waters,9–11 which are cited in the most recent US EPA water quality criteria recommendations.
Here, we apply the QMRA methodology to evaluate the risk of gastrointestinal infection (which may result in gastroenteritis in some of those infected) to people participating in water contact recreation (e.g., swimming and wind surfing) in central San Francisco Bay during periods when blended wastewater is discharged into the bay. The blended wastewater effluent pathogen concentration data are presented, along with an estimate of the attributable risk of infection for recreationalists at nearby beaches in the San Francisco area. The uncertainties in these risk estimates are discussed as well as their implications, and the cost-benefits of increasing wastewater treatment capacity to address these risks.
Material and methods
Study area
A study was conducted of the blending practices of EBMUD, which operates a 636 ML d−1 disinfected secondary wastewater treatment plant that discharges into the San Francisco Bay (Fig. 1). The plant collects wastewater from nine cities in the East Bay region. The plant normally operates primary, and secondary treatment along with chlorine disinfection/dechlorination of wastewater before discharging effluent, one mile off the shore through a deep-water outfall located below the Bay Bridge near to Treasure Island. Primary treatment can be provided for up to 1212 ML d−1, while short-term storage capacity can handle up to 360 ML d−1. The average annual dry weather flow for the plant is approximately 284 ML d−1. During peak wet weather flow conditions, up to 576 ML d−1 of primary effluent may be diverted around the secondary treatment, disinfected, and blended with 636 ML d−1 of disinfected secondary effluent prior to discharge.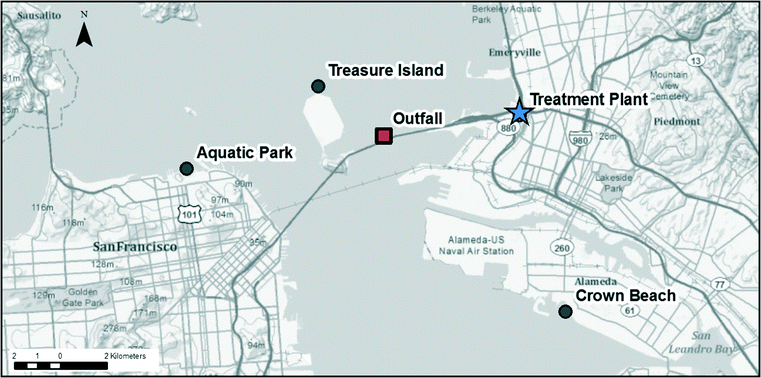 | ||
| Fig. 1 Locations of treatment plant (star), outfall (square), and exposure assessment sites (circle). | ||
Four recreational exposure sites were selected at and near to the outfall for risk assessment: the outfall location (worst case), northern tip of Treasure Island, Aquatic Park Beach, and Robert Crown Memorial State Beach. The San Francisco Bay climate is very similar to coastal areas on the Mediterranean with temperatures generally moderate and ranging between 24 °C and 7 °C. There are two seasons—wet and dry—with more than 80 percent of annual precipitation taking place between November and March. Hence with Bay recreational activities occurring year-around potential public health impacts associated with blending activities are important to consider.
The outfall location was included as a worst case exposure location, though recreation at this location is likely minimal. Treasure Island (northern tip) in San Francisco (Treasure Island) is one of the most popular locations in central San Francisco Bay for wind surfing during the winter (Voss 2009 personal communication). Robert Crown Memorial State Beach (Crown Beach) in Alameda is a popular location for swimming, wind surfing and kite boarding (East Bay Regional Parks District, Avalos 2009 personal communication). Aquatic Park Beach, Maritime National Historic Park on the northern shore of San Francisco (Aquatic Park) serves members of two clubs, who swim at this location year round.12
Pathogen measurements
Samples of plant influent and effluent (at the plant and at the outfall) were collected over a 3 year period from the fall of 2005 to the spring of 2008. Representative dry weather (influent flow ≤246 ML per day), wet weather non-blending (influent flow >246 ML d−1 no blending) and blended (influent flow >246 ML per day during period of blending) grab samples were analyzed for the presence of fecal coliform, Escherichia coli, enterococcus, male specific coliphage, adenovirus, enterovirus, Giardia spp., and Cryptosporidium spp. (BioVir Laboratories, Benicia, California). Effluent samples were analyzed for both total Giardia spp. and a subset of the total concentration, the concentration of Giardia spp. that did not retain the propidium iodide stain (referred to as PI−) and therefore presumably had intact cell membranes. Risk estimates for Giardia spp. were then developed based on both types of Giardia measurements as output from the water quality model. All samples were grab samples and represent concentrations for a singular point in time. Table 1 contains a summary of the measured parameters as well as the analytical method and associated sample volume and applicable minimum detection limit.6Table 2 contains a summary of the pathogen monitoring results for Giardia spp., Cryptosporidium spp., and adenovirus at a number of plant locations (influent, and primary, secondary effluent and final (disinfected) effluents), and limited data at the outfall. Pathogen data collected in the receiving waters are limited, therefore effluent concentration data were used as input to characterize exposure as part of the receiving water modeling.| Wastewater samples (plant) | Receiving water samples | |||||
|---|---|---|---|---|---|---|
| Analyte | Method | MDLa | Volume (L) | Method | MDLa | Volume (L) |
| a MDL = Minimum Detection Limit. b VOCs analysis scans for 31 compounds, including disinfection byproducts. c Metals analysis includes arsenic, cadmium, chromium, copper, lead, mercury, nickel, selenium, silver, and zinc. A field blank is included for mercury analysis. | ||||||
| Water quality parameters | ||||||
| cBOD5 | SM 18 5210B | 2 mg L−1 | 1.0 | |||
| TSS | EPA 160.2 | 6 mg L−1 | 1.0 | |||
| VOCsb | EPA 624 | Varies | 1.0 | |||
| Metalsc | EPA 200.8 (filtered) | Varies | 0.5 | |||
| Particle size distribution | ASTM D4464M | — | 1.0 | |||
| Pathogens and indicator organisms | ||||||
| Adenovirus | Virus assay (SM18 9510 modified) | ~1 MPN L−1 | 1.0 | Virus assay (SM18 9510 modified) | ~1 MPN per 100 L | 100 |
| Enterovirus | Virus assay (SM18 9510 modified) | ~1 MPN L−1 | 1.0 | |||
| Norovirus | Polymerase chain reaction (PCR) assay (Jothikumar, 2005, modified; Bae and Schwab, 2008) | 1.0 | ||||
| Rotavirus | Virus assay (SM18 9510 modified) | ~1 MPN L−1 | 1.0 | |||
| Giardia & Crypto | Giardia & Crypto enumeration (McCuin and Clancy, 2005) | ~1 L−1 | 1.0 | Giardia & Crypto enumeration (EPA 1623) | ~1/10 L | 10 |
| Giardia characterization | DAPI/PI cyst cell wall characterization with propidium iodide staining (McCuin and Clancy, 2005, modified for PI staining) | ~1 L−1 | 1.0 | DAPI/PI cyst cell wall characterization with propidium iodide staining (McCuin and Clancy, 2005, modified for PI staining) | ~1/10 L | 10 |
| Crypto infectivity | Infectivity assay (Slifko, 1997; Slifko, 1999) | ~1 L−1 | 1.0 | Infectivity assay (Slifko, 1997; Slifko, 1999) | ~1/10 L | 10 |
| Male specific coliphage | Double agar layer plaque assay (Adams, 1959) | 1/10 mL | 0.01 | Single agar layer plaque assay (EPA 1602) | 1/250 mL | 0.25 |
| Fecal coliform | Multiple tube fermentation (SM 9221 E) | 2 MPN per 100 mL | 0.10 | Multiple tube fermentation (SM 9221 E) | 2 MPN per 100 mL | 0.10 |
| E. coli | Multiple tube fermentation – EC MUG (EPA 40 CFR 136.3) | 2 MPN per 100 mL | 0.10 | Multiple tube fermentation – EC MUG (EPA 40 CFR 136.3) | 2 MPN per 100 mL | 0.10 |
| Enterococcus | Membrane filtration – 2 stage mE–EIA agar (SM 18 9230 C/EPA 1106.1) | 10 CFU per 100 mL | 0.10 | Membrane filtration – 2 stage mE–EIA agar (SM 18 9230 C/EPA 1106.1) | 10 CFU per 100 mL | 0.10 |
| Field measurements | ||||||
| pH | CTD probe or other | |||||
| DO | ||||||
| Conductivity | ||||||
| Temperature | ||||||
| Salinity | Indirect calculation | |||||
| Location | Date | Giardia spp. (# per L) | Giardia spp. (PI−) (# per L) | Adenovirus (MPN per L) |
|---|---|---|---|---|
| N = number of samples. | ||||
| Wet season non-blending | ||||
| Influent | 2/9/07 | 14.6 | — | 96.3 |
| Influent | 2/10/07 | 228 | — | 122.1 |
| Influent | 12/20/07 | 557 | 128 | 166.4 |
| Influent | 1/26/07 | 1050 | 315 | 66.9 |
| Influent | 2/1/08 | 2930 | 2050 | 23.9 |
| N | 5 | 4 | 5 | |
| Effluent | 1/14/06 | 588 | 174 | 25 |
| Effluent | 2/27/06 | 60 | 29 | 12.4 |
| Effluent | 2/9/07 | 148 | 28 | <2.4 |
| Effluent | 2/10/07 | 120 | 25 | <2.1 |
| Effluent | 12/20/07 | 48 | 27 | 16.0 |
| Effluent | 1/26/07 | 40 | — | <2.8 |
| Effluent | 2/1/08 | 30 | 20 | <2.8 |
| N | 7 | 6 | 7 | |
| Outfall | 2/9/07 | <0.1 | <0.1 | <0.02 |
| Outfall | 2/10/07 | 6.5 | 1 | <0.02 |
| Outfall | 12/20/07 | <0.1 | <0.1 | <0.03 |
| Outfall | 1/26/07 | 0.2 | 0.06 | <0.03 |
| Outfall | 2/1/08 | 0.2 | <0.2 | <0.03 |
| N | 5 | 5 | 5 | |
| Wet season blending | ||||
| Influent | 12/12/06 | 4424 | 1229 | 15.6 |
| Influent | 1/4/08 | 3056 | 2139 | 89.4 |
| Influent | 1/25/08 | 750 | — | 166.4 |
| N | 3 | 2 | 3 | |
| Effluent | 3/6/06 | 868 | 260 | 128 |
| Effluent | 3/25/06 | 59 | 41 | 124.8 |
| Effluent | 3/29/06 | 74 | 59 | 112.1 |
| Effluent | 4/3/06 | 556 | 256 | <2 |
| Effluent | 12/12/06 | 1637 | 887 | <1.9 |
| Effluent | 1/4/08 | 1729 | 346 | <2.8 |
| Effluent | 1/25/08 | 2640 | 2640 | <2.8 |
| N | 7 | 7 | 7 | |
| Outfall | 12/12/06 | 50.7 | 13 | <0.02 |
| N | 1 | 1 | 1 | |
Plant operational and field sampling objectives
All in-plant samples were grab samples and were analyzed for 5 day carbonaceous biochemical oxygen demand (cBOD5) and total suspended solids (TSS). In-plant samples were taken from seven locations: plant influent, primary effluent (prior and post disinfection), secondary effluent (prior and post disinfection), downstream of the blending point for blending events, and final effluent. Primary effluent (prior to disinfection) and final effluent samples were also analyzed for volatile organic compounds (including disinfection byproducts) and heavy metals (arsenic, cadmium, chromium, copper, lead, mercury, nickel, selenium, silver, and zinc). Blending events in the study had plant influent flows ranging from 681 to 1143 ML d−1 (equivalent to plant peaking factors of 2.6 to 4.3), durations of 7 to 19 hours and blend ratios of 0.20 to 0.78 (diverted primary effluent flow to secondary effluent flow. Primary effluent (prior to disinfection), secondary effluent (prior to disinfection), and final effluent (dechlorinated) grab samples were also analyzed for particle size distribution.The goal of the field sampling program was to compare blended effluent and receiving water quality during peak wet weather blending events to two different “baseline” conditions:
1) dry weather, which is defined by a minimum of 72 hours of no rainfall prior to sampling, and 2) wet weather non-blending, which is defined as a storm event that causes a minimum 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 peaking factor at the MWWTP, but does not require blending. Water samples were collected from San Francisco Bay (Fig. 1) directly above the midpoint of the diffuser section of the deep water outfall from the EBMUD MWWTP.
1 peaking factor at the MWWTP, but does not require blending. Water samples were collected from San Francisco Bay (Fig. 1) directly above the midpoint of the diffuser section of the deep water outfall from the EBMUD MWWTP.
Samples collected from San Francisco Bay, were not analyzed for any of the parameters described above, because contributions from other point and non-point sources would significantly limit the usefulness of this data. See Table 1 for identification of plant analytical methods, sample volumes, and minimum detection limits. During the study period, from fall of 2005 to the spring of 2008, samples were taken for seven wet weather non-blending and seven blending events, as summarized in Table 3. The blending ratio, as shown in Table 3, is defined as the ratio of the diverted primary effluent flow to the secondary effluent flow.
| Year | Event no. | Event type | Date | Plant flow rates (MGD)b | Blend ratioa | % diverted primary effluent | Event duration prior to bay sampling (h) | ||
|---|---|---|---|---|---|---|---|---|---|
| Influent | Diverted primary effluent | Secondary effluent | |||||||
| DW = dry weather; WW = wet weather.a Blend ratio = ratio of diverted primary effluent flow (MGD) to secondary effluent flow (MGD).b Plant flow was the average of the 15-minutes flow during the sampling durations plant flow was continuously measured by the distributed control system and stored in a data historian (PI).c Six hours of blending, followed by four hours without blending (6:00–10:00); samples collected two hours after restart of blending (10:15). | |||||||||
| 1 | 1 | DW no. 1 | 9/21/05 | 65 | 0 | 65 | — | 0% | — |
| 2 | WW non-blend no. 1 | 1/14/06 | 135 | 0 | 135 | — | 0% | — | |
| 3 | WW non-blend no. 2 | 2/27/06 | 135 | 0 | 135 | — | 0% | — | |
| 4 | Blend no. 1 | 3/6/06 | 210 | 42 | 168 | 0.25 | 20% | 13.0 | |
| 5 | Blend no. 2 | 3/25/06 | 215 | 65 | 150 | 0.43 | 30% | 7.3 | |
| 6 | Blend no. 3 | 3/29/06 | 180 | 30 | 150 | 0.20 | 17% | 5.5 | |
| 7 | Blend no. 4 | 4/3/06 | 200 | 40 | 160 | 0.25 | 20% | 2.8c | |
| 2 | 8 | DW no. 2 | 12/4/06 | 65 | 0 | 65 | — | 0% | — |
| 9 | Blend no. 5 WW | 12/12/06 | 235 | 67 | 168 | 0.40 | 29% | 2.8 | |
| 10 | WW non-blend no. 3 | 2/9/07 | 150 | 0 | 150 | — | 0% | — | |
| 11 | WW non-blend no. 4 | 2/10/07 | 168 | 0 | 168 | — | 0% | — | |
| 3 | 12 | WW non-blend no. 5 | 12/20/07 | 170 | 0 | 170 | — | 0% | — |
| 13 | Blend no. 6 (in-plant) | 1/4/08 | 302 | 132 | 170 | 0.78 | 44% | — | |
| 14 | Blend no. 7 (in-plant) | 1/25/08 | 286 | 118 | 168 | 0.70 | 41% | — | |
| 15 | WW non-blend no. 6 | 1/26/08 | 164 | 0 | 164 | — | 0% | — | |
| 16 | WW non-blend no. 7 | 2/1/08 | 144 | 0 | 144 | — | 0% | — | |
Microbial risk assessment framework
A static risk assessment modeling approach13 was used to assess the attributable risk of gastrointestinal infection associated with blending events. The approach consisted of a linked process of hazard characterization produced through water quality modeling, exposure assessment to identify ingestion rates and population at risk, and the use of a dose–response relationship to characterize the risk of infection for a given dose of microbial pathogen.14–16 Monte Carlo simulations were conducted to characterize the uncertainty in risk. The static model has commonly been used as a generic framework for conducting MRAs of food and waterborne pathogens.16–20 Assessments using a static model typically focus on estimating the probability of infection or disease to an individual as a result of a single exposure event. Multiple or recurring exposures are assumed to constitute independent events that increase the risk of infection over a period of time.21 The static approach does not consider secondary transmission or the development of immunity (e.g., person-to-person transmission) (which might be considered in a dynamic epidemiologic transmission model), under the assumption that they are negligible or largely cancel each other out.22Of the measured indicators and pathogens, Giardia spp. (total and PI−) and adenovirus were chosen for risk assessment based on evidence of elevated concentrations at the final effluent and outfall locations between blending/non-blending periods, and the availability of a dose–response relationship. Pathogen concentrations were modeled using an existing San Francisco Bay water quality model (Bay model) developed and maintained by Resource Management Associates (RMA).6 Input into the water quality model included flow and microbial pathogen concentration field sampling data to characterize treatment plant effluent under blending and non-blending conditions. The model was used to produce a 15 minute interval time-series of concentrations at each of the four exposure sites for a 35 day period from December 1, 2005 to January 5, 2006, when there was most available measured pathogen concentration data. At each site modeled pathogen concentrations were extracted for the periods of exposure, which were assumed to occur between the hours of 10 AM to 5 PM, and within that time period, only when the rainfall rate was less than light rain (0.04 inches per hour) for at least four consecutive hours.23 The highest mean pathogen concentrations occurred for two days-locations (December 31 from 10 AM to 5 PM for the outfall and Treasure Island and January 1 from 10 AM to 3 PM for Aquatic Park and Crown Beach). All of the modeled 15 minute blending and non-blending concentrations for these two days and locations were selected for risk assessment, and were used to fit a log-normal concentration distribution. For the outfall location, an additional worst case assessment was performed that used the highest modeled concentration observed during the period.
In addition, the Visual Plumes UM3 model was run under various conditions to estimate plume movement and dilution ratios. To estimate the minimum dilution values (maximum surface effluent concentrations), conditions including high effluent discharge flow, low (near zero) current speed and a weekly stratified receiving water density profile were considered. Weak stratification is conservative because the plume is more likely to surface during these conditions. Comparison of the initial dilution results calculated by each model indicated that they were similar6 and the dates selected for running the RMA bay model coincide with the conservative assumptions to provide exposure input for the QMRA.
The survival of Giardia spp. and adenovirus in receiving waters is represented by a simplified first-order exponential equation in the Bay model.6 For this investigation, the lowest die-off rates found as part of a literature review in sea water at temperatures ranging from 8–18 °C (receiving water adjacent to the EBMUD outfall) for Giardia (Kb 0.45 per day) and adenovirus (Kb 0.054 per day) were utilized in the receiving water Bay model.6
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Monte Carlo simulations were conducted by randomly sampling a concentration from the log-normal distribution, and multiplying this by a randomly sampled ingestion rate to obtain a dose of pathogen ingested per exposure event. The ingestion rate was assumed to be lognormal distributed with arithmetic mean of 50 mL water ingested per recreational event (median of 18 mL per event),24 which is generally consistent with World Health Organization (WHO) guidelines of 20–50 ml of water ingested per hour of swimming related activity.25
000 Monte Carlo simulations were conducted by randomly sampling a concentration from the log-normal distribution, and multiplying this by a randomly sampled ingestion rate to obtain a dose of pathogen ingested per exposure event. The ingestion rate was assumed to be lognormal distributed with arithmetic mean of 50 mL water ingested per recreational event (median of 18 mL per event),24 which is generally consistent with World Health Organization (WHO) guidelines of 20–50 ml of water ingested per hour of swimming related activity.25
Each resulting dose was input into a dose response relationship to estimate a risk of gastrointestinal infection. The dose–response relationship for Giardia spp. was assumed to follow that of Giardia lamblia. Data from a feeding study,26 in which healthy prisoner volunteers ingested varying doses of Giardia lamblia cysts, and were examined for infection after a prepatent period, has been used to define a dose–response relationship27 that has been used in other risk assessment studies.28 The dose–response relationship follows an exponential model:
| P = 1 − e−rd |
In the feeding study, cysts fed to volunteers were isolated from infected humans and washed with a saline solution in an attempt to preserve viability. However, no mention was made of a reliable method used in that study to determine the viability of the cysts in vitro. Because of the uncertainty surrounding the viability of the cysts used in the feeding study, we used the above dose–response relationship for all Giardia spp. scenarios (i.e., regardless of whether concentrations at the exposure site were modeled using total or PI- Giardia spp. effluent concentrations).
For adenovirus, Crabtree et al. (1997)18 estimated an exponential dose–response parameter for infection of r = 0.4172 based on data from human inhalation of aerosolized adenovirus particles.29 As exemplified by the Crabtree et al. study, this dose–response parameter has generally been used in drinking water and recreational water risk assessments.
In all, 30 sets of Monte Carlo simulations were run for all combinations of pathogen, blending versus non-blending, at the four exposure sites, and an additional worst case outfall scenario to produce estimates of gastrointestinal infection risk per recreational event.
Annualized risks
For each exposure site, the estimated annual number of infections due to blending was estimated based on the number of recreational exposure events per day, the previous estimates of risk per recreational event under the blending and non-blending scenarios, and the annual number of blending days. To assess sensitivity to climate change, we varied the number of blending days in a year from 0 to 30. Exposure events were assumed to be independent. The annual number of infections was estimated as follows:| IAnnual = (RiskB × EventsW × DaysB) + (RiskNB × EventsW × (DaysW − DaysB)) + (RiskD × EventsD × DaysD) |
I Annual is the estimated annual number of infections.
RiskB is the risk of infection per exposure event during blending conditions (Tables 4–6).
| Exposure location | Treatment plant blending status | Mean adenovirus concentration (MPN per L) | Estimated infection risk per recreation event | |||
|---|---|---|---|---|---|---|
| Median | Mean | Standard deviation | 95th percentile | |||
| Concentrations were estimated from effluent measurements n = 7 for non-blending and blending and not from outflow data, and were then mathematically assessed using the various hydrologic modeling assumptions to characterize exposure. | ||||||
| Outfall (worst case) | No blending | 5.00 × 10−2 | 3.81 × 10−4 | 1.10 × 10−3 | 3.09 × 10−3 | 4.12 × 10−3 |
| Blending | 6.30 × 10−1 | 4.62 × 10−3 | 1.30 × 10−2 | 3.00 × 10−2 | 5.00 × 10−2 | |
| Outfall (geometric mean) | No blending | 3.22 × 10−2 | 2.40 × 10−4 | 6.95 × 10−4 | 1.97 × 10−3 | 2.61 × 10−3 |
| Blending | 6.63 × 10−2 | 4.91 × 10−4 | 1.42 × 10−3 | 4.01 × 10−3 | 5.33 × 10−3 | |
| Crown Beach | No blending | 9.94 × 10−3 | 7.68 × 10−5 | 2.13 × 10−4 | 5.48 × 10−4 | 8.05 × 10−4 |
| Blending | 1.57 × 10−2 | 1.21 × 10−4 | 3.36 × 10−4 | 8.64 × 10−4 | 1.27 × 10−3 | |
| Aquatic Park | No blending | 4.68 × 10−3 | 3.61 × 10−5 | 1.00 × 10−4 | 2.59 × 10−4 | 3.79 × 10−4 |
| Blending | 6.65 × 10−3 | 5.13 × 10−5 | 1.43 × 10−4 | 3.68 × 10−4 | 5.39 × 10−4 | |
| Treasure Island | No blending | 1.13 × 10−2 | 8.25 × 10−5 | 2.50 × 10−4 | 7.56 × 10−4 | 9.38 × 10−4 |
| Blending | 2.02 × 10−2 | 1.40 × 10−4 | 4.52 × 10−4 | 1.45 × 10−3 | 1.67 × 10−3 | |
| Exposure location | Treatment plant blending status | Mean Giardia spp. concentration (# per L) | Estimated infection risk per recreation event | |||
|---|---|---|---|---|---|---|
| Median | Mean | Standard deviation | 95th percentile | |||
| Concentrations were estimated from effluent measurements n = 7 for non-blending and blending and not from outflow data, and were then mathematically assessed using the various hydrologic modeling assumptions to characterize exposure. | ||||||
| Outfall (worst case) | No blending | 7.11 × 10−1 | 2.49 × 10−4 | 7.29 × 10−4 | 2.11 × 10−3 | 2.73 × 10−3 |
| Blending | 8.02 | 2.78 × 10−3 | 7.96 × 10−3 | 2.00 × 10−2 | 3.00 × 10−2 | |
| Outfall (geometric mean) | No blending | 4.16 × 10−1 | 1.45 × 10−4 | 4.27 × 10−4 | 1.24 × 10−3 | 1.60 × 10−3 |
| Blending | 2.57 | 8.92 × 10−4 | 2.61 × 10−3 | 7.31 × 10−3 | 9.83 × 10−3 | |
| Crown Beach | No blending | 6.50 × 10−2 | 2.40 × 10−5 | 6.66 × 10−5 | 1.73 × 10−4 | 2.50 × 10−4 |
| Blending | 2.97 × 10−1 | 1.10 × 10−4 | 3.04 × 10−4 | 7.91 × 10−4 | 1.14 × 10−3 | |
| Aquatic Park | No blending | 1.74 × 10−2 | 6.45 × 10−6 | 1.79 × 10−5 | 4.64 × 10−5 | 6.76 × 10−5 |
| Blending | 7.88 × 10−2 | 2.91 × 10−5 | 8.08 × 10−5 | 2.10 × 10−4 | 3.05 × 10−4 | |
| Treasure Island | No blending | 1.05 × 10−1 | 3.24 × 10−5 | 1.16 × 10−4 | 4.13 × 10−4 | 4.22 × 10−4 |
| Blending | 6.05 × 10−1 | 1.76 × 10−4 | 6.79 × 10−4 | 2.54 × 10−3 | 2.48 × 10−3 | |
| Exposure location | Treatment plant blending status | Mean Giardia spp. PI− concentration (# per L) | Estimated infection risk per recreation event | |||
|---|---|---|---|---|---|---|
| Median | Mean | Standard deviation | 95th percentile | |||
| Concentrations were estimated from effluent measurements n = 7 for non-blending and blending and not from outflow data, and were then mathematically assessed using the various hydrologic modeling assumptions to characterize exposure. | ||||||
| Outfall (worst case) | No blending | 5.31 × 10−1 | 1.86 × 10−4 | 5.45 × 10−4 | 1.59 × 10−3 | 2.04 × 10−3 |
| Blending | 3.49 | 1.21 × 10−3 | 3.53 × 10−3 | 9.70 × 10−3 | 1.30 × 10−2 | |
| Outfall (geometric mean) | No blending | 2.57 × 10−1 | 9.01 × 10−5 | 2.65 × 10−4 | 7.74 × 10−4 | 9.90 × 10−4 |
| Blending | 5.91 × 10−1 | 2.06 × 10−4 | 6.06 × 10−4 | 1.77 × 10−3 | 2.27 × 10−3 | |
| Crown Beach | No blending | 4.02 × 10−2 | 1.49 × 10−5 | 4.12 × 10−5 | 1.07 × 10−4 | 1.55 × 10−4 |
| Blending | 7.61 × 10−2 | 2.82 × 10−5 | 7.80 × 10−5 | 2.03 × 10−4 | 2.93 × 10−4 | |
| Aquatic Park | No blending | 1.08 × 10−2 | 3.99 × 10−6 | 1.11 × 10−5 | 2.87 × 10−5 | 4.18 × 10−5 |
| Blending | 2.03 × 10−2 | 7.49 × 10−6 | 2.08 × 10−5 | 5.40 × 10−5 | 7.85 × 10−5 | |
| Treasure Island | No blending | 6.52 × 10−2 | 2.01 × 10−5 | 7.18 × 10−5 | 2.56 × 10−4 | 2.61 × 10−4 |
| Blending | 1.43 × 10−1 | 4.25 × 10−5 | 1.59 × 10−4 | 5.90 × 10−4 | 5.76 × 10−4 | |
RiskNB is the risk of infection per exposure event during non-blending conditions (Tables 4–6).
RiskD is the estimated risk of infection per exposure event during the dry season.
EventsW is the estimated number of exposure events per wet season day (see below text).
EventsD is the estimated number of exposure events per dry season day.
DaysB is the estimated number of blending days per year (specified from zero to 30).
DaysW is the estimated number of wet season days per year.
DaysD is the estimated number of dry season days per year.
The attributable number of infections due to blending versus zero blending days can be reformulated to the following calculation:
| IAttributable = (RiskB − RiskNB)(EventsW × DaysB) |
| Parameter | Value | Assumptions/basis |
|---|---|---|
| Key model assumptions (conservative assumptions marked with *). All exposure events involve full body contact*. Wet weather does not deter entry into water for recreation*. Modeled risk at the Outfall (where recreational use is unlikely) represents the worst case exposure scenario*, risk of Giardia spp. infection estimated for PI− (likely infectious) and for total*, risk of infection are modeled vs. US EPA standards which are based on illness*, no immunity protection from infection*, secondary infections are not considered. | ||
| Parameter r for exponential dose–response | 0.0199 (Giardia spp.) | Based on Giardia lamblia |
| 0.4172 (adenovirus) | Based on human inhalation studies | |
| Ingestion rate | Lognormal with arithmetic mean of 50 mL and median of 18 mL | |
| Estimated number of exposure events per wet season day, EventsW | 147 (October–April) | (See text) |
| 107 (November–February) | ||
| 30 (for Aquatic Park) | ||
| 43 (for Treasure Island) | ||
| Estimated number of exposure events per dry season day, EventsD | 344 | (See text) |
| Number of blending days per year, DaysB | Varying from 0 to 30 | For sensitivity analysis |
Exposure events (EventsW)
Approximately 420![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 recreationalists of all types visited Crown Beach in 2008, of which approximately 20% entered the water for activities such as swimming, wading, kite boarding, and wind surfing. Assuming roughly equal numbers of visitors on each day during a given month, fewer water contact recreationalists visited the site during the wet season (October–April, average of 147 per day) than the dry season (May–September, average of 344 per day). The number of water contact recreationalists during November to February, when blending normally occurs, was lower (average of 107 per day), and was used to approximate the level of exposure at Crown Beach. Further, the QMRA conservatively assumed that all entry into the water involved full body contact (swimming exposure).
000 recreationalists of all types visited Crown Beach in 2008, of which approximately 20% entered the water for activities such as swimming, wading, kite boarding, and wind surfing. Assuming roughly equal numbers of visitors on each day during a given month, fewer water contact recreationalists visited the site during the wet season (October–April, average of 147 per day) than the dry season (May–September, average of 344 per day). The number of water contact recreationalists during November to February, when blending normally occurs, was lower (average of 107 per day), and was used to approximate the level of exposure at Crown Beach. Further, the QMRA conservatively assumed that all entry into the water involved full body contact (swimming exposure).
The number of water contact recreationalists during winter days at Aquatic Park was approximately 10 to 20 swimmers, based on an estimate provided by a San Francisco swim club local to the park. This club confers “polar bear” status on swimmers that complete a certain number of swims during the winter months when San Francisco Bay is coldest (48–52 °F). People engaging in this activity are at risk of hypothermia, and so it is likely that only this select group of experienced swimmers would be in the water at Aquatic Park during the winter. To be conservative, we assumed 30 exposure events per wet season day at this site.
Like Aquatic Park, Treasure Island serves a community of dedicated enthusiasts, but for wind surfing instead of swimming. An estimated 300 wind surfing sessions occur per week at this site during peak periods, which was equivalent to about 43 daily sessions; this estimate was used to approximate the level of exposure at Treasure Island.
Results
During the study period, from fall of 2005 to the spring of 2008, field pathogen samples were taken for two dry weather events, seven wet weather non-blending, and seven blending events (see Table 3). As shown in Table 3, the average blending ratio (ratio of diverted primary effluent flow to secondary effluent flow) was 0.43 (range 0.2–0.78), with average influent plant flows on blending days of 878 ML d−1 (range 681–1143 ML d−1) compared to 500 ML d−1 (range 246–644 ML d−1) during dry weather and wet weather non-blending sampled days. Blending events on average lasted 13 hours (range 7 to 19 hours).Not all pathogens were measured at each site on each sample day. However, of the pathogens measured and for which dose response relationships are available, Giardia spp. tended to have elevated concentrations at the effluent and outfall location during blending events compared to non-blending conditions (see Table 2). Additionally, adenovirus concentrations tended to be elevated at the plant effluent location. Relatively small to no differences were seen for Cryptosporidium spp., enterovirus between treatment conditions. In almost all cases, concentrations were lower at the outfall location compared to the sampled effluent from the plant during wet season non-blending, and blending conditions. Overall, pathogen die-off rates had little if any impact on the computed pathogen concentrations in the receiving water.
Giardia
Plant influent Giardia concentration during wet season was within the range of other literature values as well as compared to the single dry weather sample. Giardia concentrations in the primary effluent were slightly lower than those in the plant influent, and decreased further after the secondary treatment (i.e., secondary effluent). Significantly higher Giardia cyst concentrations were measured in the final effluent during wet weather blending (553 cysts L−1 (enumeration) and 126 cysts L−1 (PI−)) compared to non-blending (86 cysts L−1 (enumeration) and 54 cysts L−1 (PI−)). Very limited data (only one field sampling event on 2/12/2006) was collected for Giardia cyst concentrations in the receiving waters during blending conditions. Results from the December 12, 2006 blending event showed that the Giardia cyst concentration at the outfall location (51 cysts L−1 (enumeration) and 32 cysts L−1 (PI−)) was higher than the geometric mean concentrations of 0.4 cyst L−1 (enumeration) and 0.32 cyst L−1 (PI−) for wet weather non-blending events (Table 5). Only one sampling event was conducted for dry weather at receiving water locations and all results were below the MDL of 0.1 cyst L−1.6Adenovirus
The plant influent adenovirus concentrations (geometric mean) were similar for blending events compared to non-blending events. Adenovirus concentrations were in the same order-of-magnitude for the primary effluent and secondary effluent as to the plant influent. Final effluent adenovirus results during blending were highly variable with four results below the MDL (<2.8 MPN L−1) and three results in the range of 112–128 MPN L−1. The geometric mean final effluent adenovirus result is higher during blending than non-blending events. However, adenovirus concentrations were below the detection limit (0.02 or 0.03 MPN L−1) in all of the receiving water samples collected at the outfall location during five non-blending events, and one blending event (on 12/12/2006).6TSS and cBOD5
Final effluent cBOD5 and TSS were significantly higher during blending events than dry weather or wet weather non-blending events. Both cBOD5 and TSS showed a reduction in arithmetic mean (average) concentrations through the treatment process for wet and dry weather events, but increased visibly after the blend point in wet weather blending events. Final effluent average concentrations were significantly higher during 3 blending events (cBOD5: 38 mg L−1 and TSS: 70 mg L−1) compared to 5 non-blending events (cBOD5: 11.5 mg L−1 and TSS: 13.6 mg L−1).6Note that data collected for the WERF study were grab samples, and thus, are not directly comparable to the weekly and monthly average limits stated in the NPDES permit. However, evaluation of secondary effluent grab samples (post chlorination) taken during two of the blending events (on 3/25/06 and 12/12/06) had TSS values of 80 and 90 mg L−1 and were considered to be the equivalent of wet weather non-blending samples since the entire sampled flow received secondary treatment. The daily composited final effluent samples for those blending events, were in compliance for both cBOD5 and TSS (i.e., 7 day average of less than 40 and 45 mg L−1 for both cBOD5 and TSS, respectively).6
Particle size distribution data
Samples for particle size distribution analysis were collected at in-plant locations only (primary effluent and secondary effluent prior to disinfection and final plant effluent) for blending and non-blending events. Results of median particle size, mean particle size, and particle number concentration indicate that the mean and median particle size values and particle number concentrations were higher at the plant effluent for wet weather blending events than for the non-blending events. Results show an increase in median particle size, mean particle size and particle number concentrations from the secondary effluent to the final effluent after blending in wet weather events. Also, these parameters were higher in the final effluent for wet weather blending events in comparison to wet weather non-blending and dry weather events.6Further investigation of plant and process performance parameters (e.g., hydraulic retention time) in addition to the collection of additional pathogen data during blending and non-blending would be useful to better assist characterize unit and plant performance as it relates to exposure and risk estimates.
Wet season estimated risks per single exposure event
The mean concentrations based on the water quality model, and resulting risk of gastrointestinal infection for results of the risk assessment for the three pathogens under blending versus non-blending conditions are shown in Tables 4–6. The risks shown are “per recreational event”. Risks were generally highest at the outfall site (which had the highest mean concentration) followed by the Treasure Island, Crown Beach and Aquatic Park sites. While the risks decrease approximately inversely with distance from the outfall, there is a tendency for tides to carry pathogens in a northwest and southeast direction from the outfall, hence resulting in slightly higher concentrations at Crown Beach than at Aquatic Park.Generally, the estimated risks of infection were higher under blending conditions compared to non-blending conditions. The estimated risk per a single exposure event under blending conditions exceeded non-blending risk by approximately an order-of-magnitude at the outfall site, but by a lesser extent at the other exposure sites. In addition, with the exception of the worst case outfall location during blending events, mean risks per recreational exposure event were more than an order-of-magnitude below the USEPA water quality criteria acceptable level of illness of 36 cases per 1000 for recreational exposure at marine beaches.1
Mean concentrations of Giardia spp. were greater than adenovirus at all sites, however, the risks of infection were greater for adenovirus due to its more intense dose–response relationship. Considering only the viable PI− fraction of the total Giardia, risks were reduced by 23% to 75%.
Annualized wet season risks based on multiple exposure events
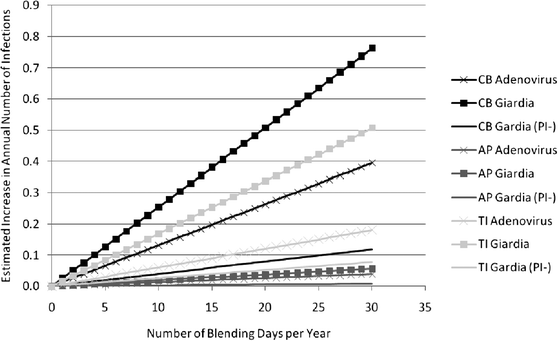
Fig. 2 illustrates the increased annual number of infections as a function of the number of annual blending days (zero to 30) at each of the three exposure locations based on the mean risks of infection during blending. The estimates are based on 107, 30, and 43 recreational exposure events per wet season day for the Crown Beach, Aquatic Park, and Treasure Island sites, respectively.Discussion
This investigation illustrates a quantitative approach for assessing the potential impacts of wastewater management decisions on gastrointestinal infections in an exposed population based on measurements at the treatment plant and outfall, water quality modeling, and microbial risk assessment. The approach has been applied to investigate the public health implications associated with the practice of wastewater treatment plant blending, which has received considerable regulatory and public attention in the U.S.30In the analysis of the EBMUD's blending practice, the added risk of gastrointestinal infection per exposure event was approximately an order-of-magnitude higher at the outfall under blending versus non-blending conditions. The added risk from blending was relatively lower at the exposure sites (blending producing risks 1.4 to 5.9 times higher than non-blending). Although these estimated risks are for infection rather than illness, other studies have assumed a 50% illness rate from infection by adenovirus (Crabtree et al., 199718), while for Giardia lamblia, the reported probability of illness has been found to range from 20% to 70%.31–34
Despite quantifiable estimates of increased risk of infection, the absolute impact in numbers of people affected was found to be small. The attributable impact scales linearly with increasing blending days. Thus, increased numbers of peak wet weather days that may occur with climate change may linearly impact numbers of infection. However, even with as many as 30 blending days per year, we found that given the reported numbers of recreationalists, the attributable increase in infections amounts to less than one additional infection. Even for the 95 percentile risk estimates, the greatest increase with 30 days of blending per year was found to be approximately only three Giardia spp. infections.
In the analysis we chose a static risk assessment approach to estimate the risk of infection and the attributable number of infections. This approach does not account for a number of factors that govern how infectious disease moves through a population (e.g., immunity and secondary transmission), but is useful for developing a first-order approximation. There would be concern with this approach if a large number of exposed recreationalists are repeat visitors, such as in the case of the members-only Aquatic Park swim club, in which case our computed risks would likely be overestimates if there is appreciable acquired immunity. However, this concern is reduced given the somewhat low number of expected infections. Moreover, attributable secondary transmission is likely negligible given the low numbers of infection.
As noted, final plant effluent was used to characterize receiving water exposures at the bathing sites. Additional pathogen receiving water monitoring data at the outfall would be useful to better characterize exposure. In addition, further investigation of plant and process performance parameters (e.g., hydraulic retention time) in addition to the collection of additional pathogen data during blending and non-blending would be useful to better assist characterize unit and plant performance as it relates to exposure and risk estimates.
In many cases, we have chosen conservative (biasing towards higher risk estimates) parameter values in our assessment. For instance, for annual attributable infections, we chose the mean per exposure event infection risks, rather than the median risks since the distribution has a long tail. Also, in the case of the Crown Beach estimate, the figure of 20% of the beachgoers contacting water includes those that may only have hands and feet exposure from wading in the shallow water, as opposed to those that are swimming, and thus more likely to ingest water.
Our modeled findings fall in the middle of conflicting epidemiologic evidence. While we are not aware of an epidemiologic study of blending on recreational water use, Colford & Tager et al., (1999)35 assessed work absenteeism for 449 U.S. Postal Service letter carriers in relationship to rainfall and potential exposure to increased pathogen concentrations due to excess flows, comparing those that served an area with combined sewer and stormwater collection, while the others served an area with separated systems. Using three separate statistical methods, they found no statistically significant findings of an association between rainfall and absenteeism. Yet, another study by Auld & MacIver et al. (2004)36 found increased cases of E. coli O157:H7 and Campylobacter several days following a heavy rainfall event in a community in Ontario, Canada. The authors point to the need for a system to identify and project the impacts of extreme weather conditions.
Concern over the public health impacts of this practice are likely to increase with climate change and more frequent and severe wet weather events. While long-range projections of climate scenarios may inform the future potential for mean temperature change, predicting climate variability and the frequency of extreme weather events is a challenge. Yet, over the past several decades there has been a growing trend toward more severe wet weather events in the Bay Area. Historical data from the rainfall monitoring stations in the East Bay have indicated that since the 1940s the region has had from 6–14 days per decade of greater than 2 inch per day rainfall (Fig. 3). And, since the 1960s and 1980s the region has begun to experience greater than 3 inch and 4 inch per day rainfall events, respectively.
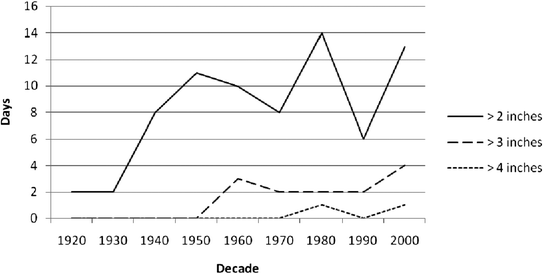 | ||
| Fig. 3 Historical precipitation by decade for the Bay Area. Lines show number of days in the decade with extreme rainfall events (greater than 2, 3, and 4 inches per day).37 | ||
The uncertainty and relative infrequency of extreme wet weather events warrant careful consideration of the cost-benefits of increased short-term storage capacity and/or treatment capacity to deal with excess flow conditions. The EBMUD's system already includes overflow structures, a one million gallon (3.8 ML) wet weather storage basin, and three additional wet weather treatment facilities. Additionally, the District employs emergency procedures for responding to sewage overflow events, which include regulatory and public notifications.
Conclusions
Water managers must anticipate, assess, and mitigate the potential health risks of blending practices that by nature are site-specific. Based on a microbial risk assessment model applied to a specific case study of exposure to blended effluent in San Francisco Bay it was found that the practice of wastewater blending results in a small excess risk of gastrointestinal infection to individuals recreating at nearby beaches during the wet season. Given the relative infrequency of wet weather events, low numbers of persons affected, low valuation of gastrointestinal illness, and high costs of improving treatment and storage capacity, strategies such as beach warnings may be a more cost-effective measure than infrastructure improvements to protect public health.References
- USEPA 2012, 2012 Recreational Water Quality CrieriaAmbient Water Quality Criteria, EPA820-F-12-061, Retrieved from http://water.epa.gov/scitech/swguidance/standards/criteria/health/recreation/upload/RWQC2012.pdf Search PubMed.
- P. S. Mead, L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin and R. V. Tauxe, Emerging Infect. Dis., 1999, 5, 607–625 CrossRef CAS PubMed.
- T. Asano, F. L. Burton, H. L. Leverenz, R. Tsuchihashi and G. Tchobanoglous, Water Reuse: Issues, Technologies, and Applications, McGraw-Hill, New York, NY, 2007 Search PubMed.
- R. C. Cooper, A. W. Olivieri, M. D. Chan, J. Colford, J. Crook, J. Debroux, R. Mandrell, T. Suslow and G. Tchobanoglous, Review of California's water recycling criteria for agricultural irrigation, Recommendations of an NWRI independent advisory panel, National Water Research Institute, Fountain Valley, CA, 2012 Search PubMed.
- J. B. Rose, S. R. Farrah, V. J. Harwood, A. D. Levine, J. Lukasik, P. Menendez and T. M. Scott, Reduction of Pathogens, Indicator Bacteria, and Alternative Indicators by Wastewater Treatment and Reclamation Processes Final Project Report, Water Environment Research Foundation, Project 00-PUM-2T, Alexandria, VA, 2004 Search PubMed.
- M. D. Gray, Y. Shang, J. Hake, V. P. DeLange, M. H. Chien, E. R. Gardner, J. Konnan and S. Grinbergs, Characterizing the Quality of Effluent and Other Contributory Sources during Peak Wet Weather Events, WERF, 03-CTS-12PP/PPa, Alexandria, VA, 2009 Search PubMed.
- J. Konnan, A. W. Olivieri and E. Seto, Microbial Risk Assessment of Human Health Impacts Associated with Water Contact Recreation During Wastewater Treatment Plant Blending, EOA, Inc., Oakland, CA, 2009 Search PubMed.
- J. A. Soller, T. Bartrand, N. J. Ashbolt, J. Ravenscroft and T. J. Wade, Water Res., 2010, 44(16), 4736–4747 CrossRef CAS PubMed.
- D. Roser and N. Ashbolt, Water Sci. Technol., 2006, 54, 245–252 CrossRef CAS PubMed.
- M. E. Schoen and N. J. Ashbolt, Environ. Sci. Technol., 2010, 44(7), 2286–2291 CrossRef CAS PubMed.
- J. A. Soller, M. E. Schoen, T. Bartrand, J. Ravenscroft and T. J. Wade, Water Res., 2010, 44(16), 4674–4691 CrossRef CAS PubMed.
- SFPUC, San Francisco Public Utilities Commission website, 2009, http://www.sfwater.org Search PubMed.
- National Research Council, Risk Assessment in the Federal Government: Managing the Process, National Academy Press, Washington, D.C, 1983 Search PubMed, Authors.
- C. N. Haas, J. - Water Pollut. Control Fed., 1983, 55, 1111–1116 Search PubMed.
- Lawrence Livermore National Laboratory, Evaluation of military field-water quality, volume 5: infectious organisms of military concern associated with consumption: assessment of health risks and recommendations for establishing related standards, 1986, Livermore, CA Search PubMed, Authors.
- C. N. Haas, J. B. Rose and C. P. Gerba, Quantitative Microbial Risk Assessment, John W. Wiley, Inc., New York, 1999 Search PubMed.
- J. M. Farber, W. H. Ross and J. Harwig, Int. J. Food Microbiol., 1996, 30(1-2), 145–156 CrossRef CAS PubMed.
- K. D. Crabtree, C. P. Gerba, J. B. Rose and C. N. Haas, Water Sci. Technol., 1997, 35(11-12), 1–6 CrossRef CAS.
- M. Sanaa, N. Bemrah, S. Meyer, O. Cerf and H. Mohammed, Rev. Epidemiol. Sante Publique, 2000, 48(Suppl 2), 2S11–24 Search PubMed.
- P. A. Voysey and M. Brown, Int. J. Food Microbiol., 2000, 58(3), 173–179 CrossRef CAS PubMed.
- S. Regli, J. B. Rose, C. N. Haas and C. P. Gerba, J. - Am. Water Works Assoc., 1991, 83(11), 76–84 CAS.
- J. A. Soller, A. W. Olivieri, J. N. S. Eisenberg, R. Sakaji and R. Danielson, Evaluation of Microbial Risk Assessment Techniques and Applications, Water Environment Research Foundation, Project 00- PUM-3, 2004 Search PubMed, Final Project Report.
- CDWR, California Department of Water Resources web page, Rain gauge station in Oakland, California (Oakland North), 2009, http://www.water.ca.gov Search PubMed.
- A. P. Dufour, O. Evans, T. Behymer and R. Cantu, J. Water Health, 2006, 4(4), 425–430 Search PubMed.
- WHO, Guidelines for Safe Recreational Water Environments. Volume 1, Coastal and Fresh Waters, 2003, Geneva, Switzerland Search PubMed.
- R. C. Rendtorff, Am. J. Hyg., 1954, 59(2), 196–208 CAS.
- National Institute of Public Health and the Environment, The dose-response relation in human volunteers for gastro-intestinal pathogens, 1996, Bilthoven, The Netherlands Search PubMed, Authors.
- J. B. Rose, C. N. Haas and S. Regli, Am. J. Public Health, 1991, 81(6), 709–713 CrossRef CAS PubMed.
- R. B. Couch, T. R. Cate, R. G. Dougla, P. J. Gerone and V. Knoght, Bacteriol. Rev., 1966, 30(3), 517–529 CAS.
- J. Tibbetts, Environ. Health Perspect., 2005, 113(7), A464–467 CrossRef PubMed.
- H. L. Dupont and P. S. Sullivan, Pediatr. Infect. Dis., 1986, 5(1 Suppl), S131–138 CrossRef CAS.
- G. Birkhead and R. L. Vogt, Am. J. Epidemiol., 1989, 129(4), 762–768 CAS.
- P. A. Flanagan, Epidemiol. Infect., 1992, 109(1), 1–22 CAS.
- E. D. Mintz, M. Hudson-Wragg, P. Mshar, M. L. Cartter and J. L. Hadler, J. Infect. Dis., 1993, 167(1), 250–253 CrossRef CAS PubMed.
- J. M. Colford Jr. and I. Tager, J. Air Waste Manage. Assoc., 1999, 49(4), 454–462 CAS.
- H. Auld, D. MacIver and J. Klaassen, J. Toxicol. Environ. Health, Part A, 2004, 67(20-22), 1879–1887 CrossRef CAS PubMed.
- NCDC, 2010, National Climate Data Center, Surface Data, Daily - US. from http://www.ncdc.noaa.gov/oa/climate/climatedata.html#daily Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |