A novel multi-stage microbial desalination cell for simultaneous desalination and enhanced organics and nitrogen removal from domestic wastewater†‡
Kuichang
Zuo
,
Fubin
Liu
,
Shiting
Ren
,
Xiaoyuan
Zhang
 ,
Peng
Liang
* and
Xia
Huang
*
,
Peng
Liang
* and
Xia
Huang
*
State Key Joint Laboratory of Environment Simulation and Pollution Control, School of Environment, Tsinghua University, Beijing 100084, PR China. E-mail: liangpeng@tsinghua.edu.cn; xhuang@tsinghua.edu.cn; Fax: +(86)10 62771472; Tel: +(86)10 62796790; +(86)10 62772324
First published on 22nd August 2016
Abstract
Conventional microbial desalination cells (MDCs) can extract organic energy from wastewater for in situ utilization in saline water desalination, but are mostly unable to achieve enhanced treatment and desalination for one stream of wastewater. In this study, a multi-stage MDC (M-MDC) with two alternating anodes and cathodes was fabricated, and operated with domestic wastewater in two operational modes. In ACAC operational mode, with wastewater flowing serially from anode-1 → cathode-1 → anode-2 → cathode-2, the M-MDC realized current production of 11.4 mA and desalination efficiency of 52.4%, effluent chemical oxygen demand and total nitrogen removed 92.5% and 87.0% respectively, due to cooperative biological nitrification/denitrification and electrical migration. As a contrast in AACC mode, with wastewater flowing serially from anode-1 → anode-2 → cathode-1 → cathode-2, a higher current generation (17.2 mA) and desalination efficiency (56.4%) were achieved, due to enhanced utilization of wastewater organics in the anode chambers. The M-MDC realized simultaneous self-driven desalination and organics/nitrogen removal for the same stream of domestic wastewater, and various operation modes were proposed for enhanced wastewater treatment (ACAC mode) or energy recovery (AACC mode), indicating a promising potential for the M-MDC in simultaneous treatment and desalination of domestic wastewater.
Water impactConventional microbial desalination cells (MDCs) can extract organic energy from wastewater for in situ utilization in saline water desalination, but are mostly unable to achieve enhanced treatment and desalination for one stream of wastewater. In this study, a multistage MDC was fabricated and realized simultaneous desalination and organics/nitrogen removal from domestic wastewater, providing a promising potential in municipal or industrial reclamation. |
Introduction
The world is increasingly focused on water and energy conservation. It is estimated that the chemical energy contained in the organic fraction of domestic wastewater is nearly 1.93 kW h m−3,1 which is five to ten times the energy required for wastewater treatment using traditional activated sludge processes (0.2–0.4 kW h m−3 of treated wastewater2). However, organic energy in the wastewater is often lost in traditional treatment processes as it is either oxidized to CO2 (released in the form of heat), or transformed into excess sludge. The microbial fuel cell (MFC) is a newly developed technology that produces electricity from the chemical energy contained in wastewater through oxidation using bioelectrochemically active bacteria.3 During the past decade, the configuration and performance of MFCs have improved significantly with volume increasing from a few microliters4 to over 250 L,5 and maximum power density increasing to 3.3 W m−2.6 However, MFCs produce power with low voltage, current, and stability. Further, the anaerobic anode biofilm is inefficient in producing high quality wastewater effluent.7Microbial desalination cells (MDCs) are MFC-derived technology that offer simultaneous wastewater treatment, power generation, desalination, realizing in situ utilization of the chemical energy of wastewater for salt water desalination.8 As they do not require extra energy input besides energy for pumping, MDCs have drawn attention and undergone significant development. Stacked MDCs were invented to improve the desalination rate and current efficiency.9,10 Ion exchange resins were packed in the desalination chamber to decrease internal resistance.11–14 Some extended functions, such as MDCs for nutrients recovery,15 heavy metal removal,16 forward osmosis combined MDCs for seawater desalination,17,18 and expansion to 10 L for practical application,13 were also reported. However, despite their multi-functionality, traditional MDCs (T-MDCs) face various limitations: (i) mostly not feasible to realize simultaneous organics removal and desalination for the same one type of wastewater, as wastewater and salt water are often introduced separately as feed water of anode and desalination chambers;14,19 the organics removal and desalination are realized for different water types in different chambers; (ii) inefficient nitrogen removal of the produced anode effluent due to insufficient nitrification and denitrification in the MDC anode;20–24 (iii) desalination of only pure salt water not containing organics is possible, as the presence of organics in the millimeter-thick desalination chamber causes membrane fouling affecting desalination performance.25 In addition, despite that T-MDCs can achieve a desalination efficiency of over 90%, the organics removal efficiency in the produced anode effluent is often less than 85%,21,22,24 and the recovered salt from the desalination chamber to the anolyte is also not effectively removed, which may cause environmental issues if directly discharged.
To address the disadvantages of T-MDCs, a multistage MDC (M-MDC) was fabricated to achieve simultaneous enhanced treatment and self-driven desalination of wastewater. The M-MDC had two alternating anode and biocathode chambers, with the anion exchange membrane (AEM) and cation exchange membrane (CEM) at opposite positions compared to T-MDCs.8 During operation, real domestic wastewater was utilized as feed water for the M-MDC and performance in terms of organics and nitrogen removal, desalination efficiency, and current production was evaluated under various operating conditions.
Materials and methods
M-MDC construction
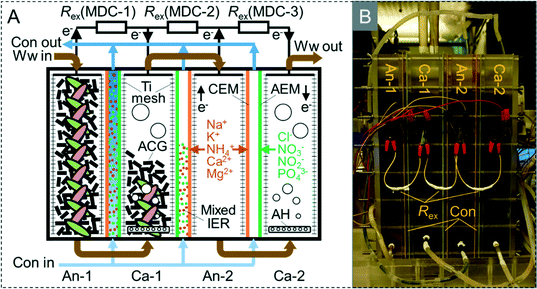
The M-MDC was made of polycarbonate with two anode chambers, two cathode chambers, and three concentrate chambers (Fig. 1A and B). The dimensions of the anode, cathode, and concentrate chambers were 3.5 × 8 × 20 cm (560 mL), 4.5 × 8 × 20 cm (720 mL), and 0.5 × 8 × 20 cm (80 mL), respectively. The total volume of the MDC was 2800 mL, with a sectional area of 160 cm2. In the anode and cathode chambers, conductive activated carbon granules (diameter around 5 mm, length around 10 mm) were packed as electrode material and carriers for bioelectrochemically active bacteria (Fig. S1A‡), they were taken from the anode and biocathode of a mature MFC that was initially inoculated with sludge from the anaerobic (anode) and oxic (biocathode) tank of a wastewater treatment plant. Two titanium meshes (screen mesh size 2 × 2 mm) were assembled in each electrode chamber as current collectors (Fig. 1A and S1C‡). The anode, cathode, and concentrate chambers were separated by CEMs (2.0 mol kg−1, Shanghua, China, Fig. S1B‡) and AEMs (1.8 mol kg−1, Shanghua, China, Fig. S1D‡). The CEMs were set adjacent to anode chambers and AEMs were set adjacent to cathode chambers. Mixed cation exchange resins (Na form, 4.2 mmol g−1, Sinopharm, China) and anion exchange resins (Cl form, 3.0 mmol g−1, Sinopharm, China) were packed in the concentrate chambers (weight ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4) as ion conductors to decrease internal resistance.11–14 An aeration head was embedded at the bottom of each cathode chamber to supply air (Fig. 1A). Each anode and cathode was connected to the adjacent cathode and anode respectively with an external resistance of 1 Ω, forming MDC-1, MDC-2, and MDC-3 (Fig. 1A and S2‡).
1.4) as ion conductors to decrease internal resistance.11–14 An aeration head was embedded at the bottom of each cathode chamber to supply air (Fig. 1A). Each anode and cathode was connected to the adjacent cathode and anode respectively with an external resistance of 1 Ω, forming MDC-1, MDC-2, and MDC-3 (Fig. 1A and S2‡).
M-MDC solution and operation
Domestic wastewater was acquired from downstream of the grid in a wastewater treatment plant in the Tsinghua campus, and utilized as feed water for the anode. It had a chemical oxygen demand (COD) of 393.8 ± 80.0 mg L−1, NH4+-N of 92.3 ± 8.7 mg L−1, total nitrogen (TN) of 99.7 ± 11.4 mg L−1, conductivity of 1673.3 ± 39.2 μS cm−1, and pH of 7.66 ± 0.02. Deionized water was utilized as feed water for the concentrate chamber.During operation, the M-MDC was operated continuously in two operational modes. In one operational mode, the wastewater flowed serially from anode-1 (anaerobic) → cathode-1 (oxic) → anode-2 (anaerobic) → cathode-2 (oxic) (ACAC mode, Fig. 1A), which was a multistage anaerobic/oxic process facilitating the biological removal of nitrogen and organics. In the second operational mode, the wastewater flowed serially from anode-1 → anode-2 → cathode-1 → cathode-2 (AACC mode, Fig. S2‡). Anode-2 utilized residual organics of the anode-1 effluent for electricity production, improving coulombic efficiency, current generation, and desalination performance. In both operational modes, the concentrate was operated in batch mode by circulating in an external reservoir at a flow rate of 12 mL min−1. The concentrate replacement cycle time was the same as the hydraulic retention time (HRT) of the wastewater, which was calculated based on the total empty bed volume of the four electrode chambers. The volume of concentrate was set to 10% of the wastewater volume during each concentrate replacement cycle to decrease the production of concentrate. All experiments were conducted at room temperature.
As the CEMs and AEMs were adjacent to anode and cathode chambers, respectively in this configuration, cations from the anode and anions from the cathode could migrate to the concentrate chambers under an electric field. This facilitated simultaneous desalination and removal of organics and nutrients in the electrode chambers of the M-MDC.
Analysis and calculations
The performance of the M-MDC was evaluated in terms of current production, coulombic efficiency, current efficiency, and water quality, including conductivity, COD, NH4+-N, TN and so on. Detailed analysis and calculations can be seen in the ESI.‡Results and discussion
M-MDC performance in ACAC mode
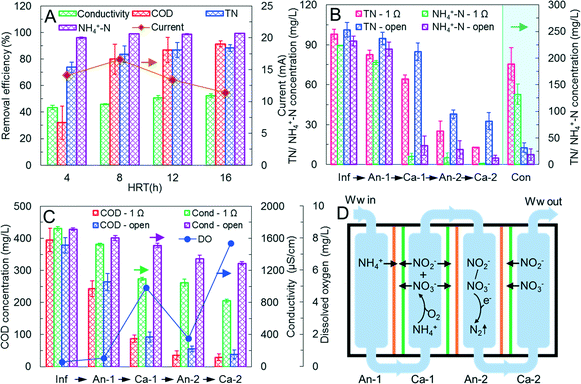
The performance of the M-MDC was evaluated by varying the HRT of the wastewater to 4 h, 8 h, 12 h, and 16 h. As shown in Fig. S3,‡ the effluent concentration of COD, NH4+-N, and TN decreased significantly with increased HRT, implying increased pollutants removal efficiency (Fig. 2A). The M-MDC achieved the best performance at an HRT of 16 h, where the concentration of effluent COD, NH4+-N, and TN were 29.1 ± 10.9 mg L−1, 1.02 ± 0.15 mg L−1, and 12.7 ± 0.4 mg L−1 respectively. This was much lower than the class A level municipal wastewater discharge standard of China, indicating efficient treatment of wastewater in the M-MDC operated in ACAC mode.When the HRT was increased from 4 h to 16 h, total current production of the MDC-1, MDC-2, and MDC-3 exhibited an initial increase followed by a decrease (11.4–16.6 mA, Fig. 2A). The low current production at an HRT of 4 h was probably due to the introduction of O2 from the influent into anode-1 chamber or from cathode-1 to anode-2 chambers with a high wastewater flow rate. The gradual decrease between HRT of 8 h to 16 h was probably due to decreased organic loading with extended HRT. When the HRT was increased from 4 h to 16 h, the desalination efficiencies increased from 43.4% to 52.4% (Fig. 2A), and the coulombic efficiency increased from 1.8% to 6.0% (Fig. S4‡). The coulombic efficiency at the HRT of 16 h was 6.0%, comparable to T-MDC efficiencies of 5–10%.20 As a contrast, the current efficiencies for wastewater ranged from 250.4% to 392.2%, which was higher than the current efficiencies calculated based on the concentrate (144.4–98.3%, Fig. S4‡), and even higher than the theoretical maximum of 100%. This can be attributed to two factors: (i) concentration diffusion of salt from electrode to concentrate chambers as deionized water was utilized as the initial concentrate; (ii) conductivity decrease during the biological treatment of the wastewater, such as nitrification/denitrification of nitrogen, degradation of ionic organics (e.g. carboxylate) and so on.
At an HRT of 16 h, a control experiment with an open circuit was conducted to evaluate the performance of the M-MDC. As shown in Fig. 2B and C, the desalination efficiency with a closed circuit was 52.1%, the nitrogen removal efficiency was 87.0% (final TN was 12.7 mg L−1), and the conductivity of the concentrate was 2898.3 μS cm−1, which recovered 23.4% of nitrogen with TN and NH4+-N of 189.2 mg L−1 and 132.4 mg L−1 respectively. As a contrast, the removal efficiency of conductivity and TN were 24.4% and 67.8% respectively with an open circuit (effluent conductivity and TN were 1264.8 μS cm−1 and 32.6 mg L−1), and the conductivity, TN, and NH4+-N of the concentrate were 430.0 μS cm−1, 12.6 mg L−1, and 7.5 mg L−1 respectively. The conductivity removal in wastewater and the recovered conductivity in the concentrate were only 46.8% and 14.8% respectively of that with the closed circuit, indicating efficient desalination and nitrogen removal of the M-MDC by electrical migration. As to the 24.4% conductivity removal in wastewater effluent with the open circuit, detailed calculations showed that the concentrate only recovered ∼3.0% of the wastewater conductivity, indicating that ∼21.4% of the conductivity removal was resulted from biological removal of nitrogen and ionic organics.
Detailed analysis at an HRT of 16 h showed that nitrogen was removed in all chambers of the M-MDC with the closed circuit: 15.6% in anode-1, 18.6% in cathode-1, 40.2% in anode-2, and 12.5% in cathode-2 (Fig. 2B and S5‡). The removal of nitrogen in anode-1 was mainly driven by the electric field in the form of NH4+, as influent nitrogen existed mainly in the form of NH4+, and the decreased 14.4% of NH4+-N concentration in anode-1 was comparable with that recovered in the concentrate (Fig. 2B). The decreased TN concentration in oxic cathode-1 and cathode-2 effluents can be attributed to electrical removal (migration) in the form of NO3− and NO2−, as most of the NH4+-N was nitrified into NO3− and NO2− in the cathode-1 chamber (Fig. 2B and D). The efficient NH4+-N oxidation in cathode-1 was mainly realized by oxic nitrification but not through autotrophic ammonia/nitrite oxidation, as dissolved oxygen (DO) was 5.07 mg L−1 (Fig. 2C) and it was not conducive for the growth of anaerobic bacteria. In anode-2, the TN removal efficiency was 40.2%, which was mainly realized by biological denitrification in anaerobic circumstances, as the C/N ratio of 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the cathode-1 effluent was sufficient for denitrification of nitrogen. In addition, since NH4+ might migrate from the second and third concentrate chambers to the anode-2 chamber under concentration diffusion, anaerobic ammonium oxidation might also happen in the anode-2 chamber, facilitating nitrogen removal. Compared to traditional multistage anaerobic/oxic activated sludge processes that only removed nitrogen in the anaerobic stages through denitrification of NO3− and NO2−,26 the M-MDC accomplished nitrogen removal in all stages through biological denitrification and electric removal of NH4+, NO3−, and NO2−, indicating the efficient nitrogen removal by the M-MDC in the ACAC mode.
1 in the cathode-1 effluent was sufficient for denitrification of nitrogen. In addition, since NH4+ might migrate from the second and third concentrate chambers to the anode-2 chamber under concentration diffusion, anaerobic ammonium oxidation might also happen in the anode-2 chamber, facilitating nitrogen removal. Compared to traditional multistage anaerobic/oxic activated sludge processes that only removed nitrogen in the anaerobic stages through denitrification of NO3− and NO2−,26 the M-MDC accomplished nitrogen removal in all stages through biological denitrification and electric removal of NH4+, NO3−, and NO2−, indicating the efficient nitrogen removal by the M-MDC in the ACAC mode.
Detailed analysis of conductivity showed that the desalination efficiency in each chamber was 11.6% (anode-1), 24.8% (cathode-1), 2.7% (anode-2), and 13.0% (cathode-2) (Fig. 2C), it was consistent with the current generation of 7.4 mA (MDC-1), 0.5 mA (MDC-2), and 4.3 mA (MDC-3). The pH values of the wastewater in each chamber of the M-MDC were stabilized at 7.04–7.81 (Fig. S6‡), thus the low desalination efficiency and current generation in MDC-2 and MDC-3 were probably due to low COD concentration and high DO in anode-2. As shown in Fig. 2C, the serial flow of wastewater from anode-1 → cathode-1 → anode-2 caused low COD concentration of cathode-1 effluent (87.6 ± 11.2 mg L−1) and a higher DO in anode-2 (1.67 mg L−1) than anode-1 (0.44 mg L−1), both of which were not conducive to current generation for anode-2 in MDC-2 and MDC-3. Furthermore, 39.3% of COD was lost in cathode-1 through aerobic oxidation (Fig. 2C), resulting in low current production and coulombic efficiency.
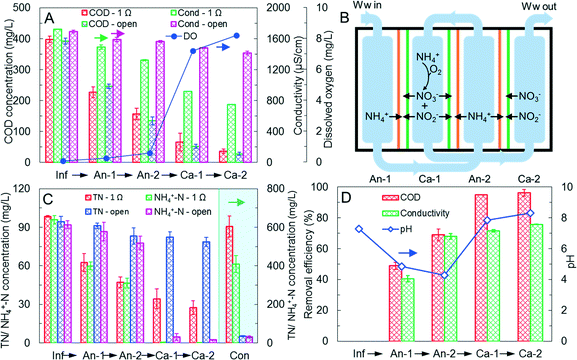
M-MDC performance in AACC mode
To more efficiently utilize organics contained in wastewater and decrease DO in anode-2 for higher current generation, the M-MDC was changed to the AACC operational mode at an HRT of 16 h with the external circuit kept open or closed (1 Ω). As shown in Fig. 3A, anode-1 and anode-2 achieved a total COD removal efficiency of 60.6% (43.0% for anode-1 and 17.6% for anode-2) with the closed circuit, higher than the ACAC mode (38.4% for anode-1 and 13.1% for anode-2). The DO concentrations in anode-2 and cathode-1 were also lower and higher (Fig. 3A), respectively than the ACAC mode, despite that the pH fluctuation (6.78–8.13, Fig. S8‡) was bigger than that in ACAC mode. Consequently, the M-MDC generated a total current of 17.2 mA (8.7 mA for MDC-1, 2.2 mA for MDC-2, and 6.3 mA for MDC-3) and a coulombic efficiency of 8.4%, both of them were higher than the ACAC mode (11.4 mA, 6.0%). The desalination efficiency also increased to 56.4% with 72.2% removal of TN (effluent TN was 27.3 mg L−1, Fig. 3C), and the conductivity of concentrate increased to 3912.4 μS cm−1 by recovering 61.4% of TN (604.2 mg L−1) and 42.7% of NH4+-N (409.1 mg L−1). As a contrast, with an open circuit the removal efficiency of conductivity and TN were only 16.3% and 16.7% respectively, and the concentrate recovered negligible salinity and nitrogen, indicating efficient current generation, desalination, and salinity and nitrogen recovery in the M-MDC with AACC operation mode.However, despite the enhanced current generation, desalination efficiency and salt/nitrogen recovery, the removal efficiency of TN in AACC mode was lower than that in ACAC mode, due to that nitrogen was only removed by electric migration of NH4+, NO3−, and NO2−, but not through biological denitrification (Fig. 3B). The desalination efficiency (56.4%) was also lower than the previous MDC of >80%.27 One reason was the non-stacked configuration causing a low current efficiency, another reason was that the low COD of wastewater could not provide sufficient electrons to drive desalination at the present level of coulombic efficiency. In this study, the coulombic efficiency was 8.4%, the influent COD of 397.8 ± 10.2 mg L−1 could theoretically realize only 28.4% desalination of the wastewater (1673.3 ± 39.2 μS cm−1). To explore the maximum desalination performance of the M-MDC, the COD of the wastewater was increased to ∼1900 mg L−1 by adding 1.4 g L−1 glucose, and the HRT was increased to 64 h to improve COD utilization in the anode chambers. Results showed that current generation increased slightly to 17.8 mA (7.9 mA for MDC-1, 4.1 mA for MDC-2, 5.8 mA for MDC-3), desalination efficiency reached 75.7%, and COD removal efficiency was 96.2% (Fig. 3D and S7‡). The pH in anode-1 and anode-2 decreased to 4.84 ± 0.08 and 4.47 ± 0.05 respectively, while the pH values in cathode-1 and cathode-2 were much higher (7.85 ± 0.11 and 8.31 ± 0.14 respectively), indicating that pH had little impact on current production and the M-MDC was efficient in removal of high strength organics and salinity.
Advantages and challenges
The main innovation and contribution of this study was the fabrication of a multistage MDC which realized efficient removal of COD (>90.0%), nitrogen (87.0%), and partial desalination (43.4–75.7%) of real domestic wastewater, without pre-treatment for organics or suspended solids. Energy for desalination was provided by the wastewater itself, and the M-MDC had several advantages compared to T-MDCs: (i) simultaneous organics removal and self-driven desalination for the same wastewater; (ii) enhanced nitrogen removal due to cooperative biological nitrification/denitrification and electrical migration of NH4+, NO3−, and NO2−; (iii) enhanced organics removal due to multistage anaerobic/oxic conditions of the anode and cathode.28,29 In addition, there was no blocking in the desalination chamber (electrode chamber) of the M-MDC, and the two types of operational mode provided solutions for various situations in wastewater treatment. The ACAC mode had a great capability in organics/nitrogen removal but was less efficient in recovery of energy and nutrients/salts from wastewater, thus it might be utilized for (pre)treatment of high strength wastewater from pharmaceutical, food processing, and other sources that have high salinity, nitrogen, and organic concentration. On the contrary, the AACC mode had a higher coulombic efficiency for utilizing organics to produce electricity, but it was less efficient in removing nitrogen from wastewater. Therefore, the AACC mode can be utilized to produce power, desalinate salt water, and recover nutrients/chemicals using low strength wastewater.The M-MDC also had some limitations. The main challenge was that the M-MDC constructed in the study was essentially a non-stacked one, resulting in relatively low current efficiency and desalination efficiency. The biocathode also needed aeration, which was energy consuming and increased operation costs. In addition, compared to the ACAC mode removing nitrogen by converting to N2, nitrogen in the AACC mode was migrated in the concentrate in the form of NO2− and NO3− with a mixture of other salts, bringing difficulty to its post-treatment. Some measures, such as applying an external voltage and increasing the number of stages can enhance the M-MDC performance in terms of desalination and removal of organics and nutrients. Using electrodialysis and reverse osmosis to treat the produced concentrate can further decrease its volume, which can be utilized as a kind of liquid fertilizer. Future studies can also include microbial community analysis, membrane fouling etc., making the M-MDC more cost-efficient in practical application.
Acknowledgements
This research was financially supported by the Key Program of the National Natural Science Foundation of China (No. 51238004) and the Shanghai Tongji Gao Tingyao Environmental Science & Technology Development Foundation (STGEF).References
- P. L. McCarty, J. Bae and J. Kim, Environ. Sci. Technol., 2011, 45, 7100–7106 CrossRef CAS PubMed.
- K. Xiao, Y. Xu, S. Liang, T. Lei, J. Sun, X. Wen, H. Zhang, C. Chen and X. Huang, Front. Environ. Sci. Eng., 2014, 8, 805–819 CrossRef CAS.
- H. Liu, S. A. Cheng and B. E. Logan, Environ. Sci. Technol., 2005, 39, 658–662 CrossRef CAS PubMed.
- K. C. Zuo, H. Liu, Q. Y. Zhang, P. Liang, X. Huang and C. D. Vecitis, ChemSusChem, 2015, 8, 2035–2040 CrossRef CAS PubMed.
- Y. Feng, W. He, J. Liu, X. Wang, Y. Qu and N. Ren, Bioresour. Technol., 2014, 156, 132–138 CrossRef CAS PubMed.
- G. G. Kumar, Z. Awan, K. S. Nahm and J. S. Xavier, Biosens. Bioelectron., 2014, 53, 528–534 CrossRef PubMed.
- K. Zuo, S. Liang, P. Liang, X. Zhou, D. Sun, X. Zhang and X. Huang, Bioresour. Technol., 2015, 185, 426–430 CrossRef CAS PubMed.
- X. X. Cao, X. Huang, P. Liang, K. Xiao, Y. J. Zhou, X. Y. Zhang and B. E. Logan, Environ. Sci. Technol., 2009, 43, 7148–7152 CrossRef CAS PubMed.
- X. Chen, X. Xia, P. Liang, X. Cao, H. Sun and X. Huang, Environ. Sci. Technol., 2011, 45, 2465–2470 CrossRef CAS PubMed.
- N. A. Shehab, G. L. Amy, B. E. Logan and P. E. Saikaly, J. Membr. Sci., 2014, 469, 364–370 CrossRef CAS.
- A. Morel, K. Zuo, X. Xia, J. Wei, X. Luo, P. Liang and X. Huang, Bioresour. Technol., 2012, 118, 43–48 CrossRef CAS PubMed.
- F. Zhang, M. Chen, Y. Zhang and R. J. Zeng, J. Membr. Sci., 2012, 417, 28–33 CrossRef.
- K. Zuo, J. Cai, S. Liang, S. Wu, C. Zhang, P. Liang and X. Huang, Environ. Sci. Technol., 2014, 48, 9917–9924 CrossRef CAS PubMed.
- K. Zuo, L. Yuan, J. Wei, P. Liang and X. Huang, Bioresour. Technol., 2013, 146, 637–642 CrossRef CAS PubMed.
- X. Chen, D. Y. Sun, X. Y. Zhang, P. Liang and X. Huang, Sci. Rep., 2015, 5, 15744 CrossRef CAS PubMed.
- Q. Y. Ping, I. M. Abu-Reesh and Z. He, Desalination, 2015, 376, 55–61 CrossRef CAS.
- H. Y. Yuan, I. M. Abu-Reesh and Z. He, Chem. Eng. J., 2015, 270, 437–443 CrossRef CAS.
- Y. B. Lu and Z. He, Environ. Sci. Technol., 2015, 49, 10529–10535 CrossRef CAS PubMed.
- H. P. Luo, P. Xu, T. M. Roane, P. E. Jenkins and Z. Y. Ren, Bioresour. Technol., 2012, 105, 60–66 CrossRef CAS PubMed.
- Q. Y. Ping, Z. Y. Huang, C. Dosoretz and Z. He, Water Res., 2015, 77, 13–23 CrossRef CAS PubMed.
- F. Zhang and Z. He, Desalination, 2015, 360, 28–34 CrossRef CAS.
- Z. A. Stoll, C. Forrestal, Z. J. Ren and P. Xu, J. Hazard. Mater., 2015, 283, 847–855 CrossRef CAS PubMed.
- X. Chen, P. Liang, Z. Wei, X. Zhang and X. Huang, Bioresour. Technol., 2012, 119, 88–93 CrossRef CAS PubMed.
- M. Mehanna, T. Saito, J. L. Yan, M. Hickner, X. X. Cao, X. Huang and B. E. Logan, Energy Environ. Sci., 2010, 3, 1114–1120 CAS.
- V. Lindstrand, G. Sundstrom and A. S. Jonsson, Desalination, 2000, 128, 91–102 CrossRef CAS.
- J. Sun, K. Xiao, X. Yan, P. Liang, Y.-X. Shen, N. Zhu and X. Huang, Process Biochem., 2015, 50, 2224–2233 CrossRef CAS.
- K. Zuo, Z. Wang, X. Chen, X. Zhang, J. Zuo, P. Liang and X. Huang, Environ. Sci. Technol., 2016, 50, 7254–7262 CrossRef CAS PubMed.
- S. Ghaniyari-Benis, R. Borja, S. A. Monemian and V. Goodarzi, Bioresour. Technol., 2009, 100, 1740–1745 CrossRef CAS PubMed.
- O. Reyes, E. Sanchez, N. Rovirosa, R. Borja, M. Cruz, M. F. Colmenarejo, R. Escobedo, M. Ruiz, X. Rodriguez and O. Correa, Bioresour. Technol., 1999, 70, 55–60 CrossRef CAS.
Footnotes |
| † The authors declare no competing financial interest. |
| ‡ Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ew00196c |
| This journal is © The Royal Society of Chemistry 2016 |