Research highlights: applications of atomic force microscopy in natural and engineered water systems
Ruochen
Zhu
a,
David T.
Tan
b and
Danmeng
Shuai
*a
aDepartment of Civil and Environmental Engineering, The George Washington University, 800 22nd St NW Suite 3530, Science and Engineering Hall, Washington, DC 20052, USA. E-mail: danmengshuai@gwu.edu
bMalaysia Innovation Hub, Level 19, Wisma R & D Universiti Malaya, Jalan Pantai Baharu, 59990 Kuala Lumpur, Malaysia
First published on 3rd May 2016
Abstract
The mechanistic understanding of the fate, transport, and transformation of contaminants in natural and engineered water systems is of great importance for environmental remediation. Recently, atomic force microscopy (AFM) has emerged as a promising tool to provide insights on the properties and interactions of materials, chemicals, and microorganisms. This article highlights three studies using AFM to understand the molecular mechanism of membrane fouling for water and wastewater treatment, to characterize biofilm properties that influence the accumulation and release of pathogens in drinking water distribution systems, and to evaluate the nucleation and growth of manganese (Mn) (hydr)oxide for remediating Mn-contaminated environmental systems.
The environmental behavior of contaminants, minerals, biomolecules, and pathogens in natural waters and water/wastewater treatment systems is of significance for environmental engineering. A fundamental, mechanistic understanding of the behavior allows us to elucidate the fate, transport, and transformation of contaminants in water, thus shaping the design of water purification systems to improve the quality of produced water and the cost-effectiveness of the systems. To date, a variety of characterization tools have been developed to explore natural and engineered water systems at a micro- or even nano-scale, among which, atomic force microscopy (AFM) stands out not only for the high atomic resolution (angstrom scale) but also for its versatile capability in characterizing surface properties and electrostatic properties either in an atmosphere or a liquid environment (DOI: 10.1021/acs.accounts.5b00017, DOI: 10.1021/cr960068q). AFM is a scanning probe microscopic (SPM) technique that is widely used to investigate morphological, structural, and functional characteristics of materials, e.g., size, shape, friction, adhesion forces, cohesiveness, Young's modulus, and even chemical bond breaking/formation based on the interaction between the AFM probe and the sample surface. However, the application of AFM, especially characterization beyond imaging, in natural and engineered water systems is still at its nascent stage. This article highlights recent applications of AFM in the characterization of membrane fouling by organic polymers, biofilms in drinking water distribution systems, and nucleation and growth processes of manganese (Mn) (hydr)oxide nanoparticles.
Molecular mechanisms of membrane fouling
Membrane fouling, a process in which particles, colloids, or microorganisms accumulate onto the surface or into the pores of membranes, compromises the performance of membrane filtration by decreasing membrane flux and selectivity for the removal of target contaminants while increasing the operational cost and maintenance for water and wastewater treatment. Therefore, the study of membrane fouling mechanisms attracts great attention to guide the design of an improved membrane system for water purification.Membrane–foulant and foulant–foulant intermolecular interactions play critical roles in membrane fouling. However, these interactions over the concentration polarization layer (CPL) and the gel layer (GL, or ‘cake layer’) in membrane filtration have been largely overlooked. The CPL is a loose layer in the vicinity of the membrane surface with the foulant concentration higher than that in the bulk, due to the rejection and back diffusion of foulants from the membrane surface; the GL is an inter-linked structured layer of foulants next to the membrane surface due to a high foulant concentration. Both the CPL and GL contribute to membrane fouling, but GL resistance and stability in filtration is believed to be closely related with the irreversible membrane fouling. To evaluate the relationship between the membrane fouling and intermolecular interactions over the CPL and GL, Liu et al. (DOI: 10.1021/acs.est.5b04098) used an amino-functionalized AFM probe covalently bound with anionic polyacrylamide (APAM), the probe membrane foulant, to predict the polymer fouling behavior on a polyvinylidene fluoride (PVDF) ultrafiltration membrane. The atomic force microscope used a probe attached to a micro-cantilever to approach target surfaces (Fig. 1). The probe passed the surface in a raster fashion and meanwhile laser light was reflected from the cantilever to a photodiode sensor. Based on the deflection of the cantilever, the atomic force microscope detected the interaction forces acting between the sample surface and the probe attached to the cantilever.
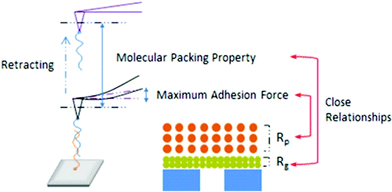 | ||
| Fig. 1 Schematic of intermolecular interactions measured by AFM on an APAM fouled PVDF ultrafiltration membrane. Reprinted with permission from DOI: 10.1021/acs.est.5b04098. Copyright 2016 American Chemical Society. | ||
A critical finding by Liu et al. (DOI: 10.1021/acs.est.5b04098) was that the CPL had a greater impact on the membrane flux loss than the GL. Specifically, the intermolecular interactions between foulant–foulant, rather than foulant–membrane, were closely related to membrane fouling. The CPL resistance (Rp) was strongly correlated with the maximum adhesion force between foulant and foulant, and the GL resistance (Rg) was closely related to the GL structure that corresponded to the molecular packing property of the foulant. Furthermore, considering the effects of Ca2+ and Na+ that are ubiquitously present in real water (AFM force characterizations with the presence of Ca2+ and Na+ are shown in Fig. 2), in the presence of Ca2+ a larger APAM–APAM maximum adhesion force resulting in a higher membrane flux loss was observed. Even though the introduction of Ca2+ did not increase the Rg significantly, nevertheless the recovery of membrane flux after backwashing was significantly decreased, indicating that Ca2+ causes more severe fouling in the GL. As to Na+, the GL structures with Na+ likely corresponded to the proposed molecular packing among APAM chains, which could be used to predict the irreversible fouling potential and guide GL cleaning. These results shed light on a promising control of membrane fouling by organic polymers, and provide a fundamental basis for cleaning the fouled membranes.
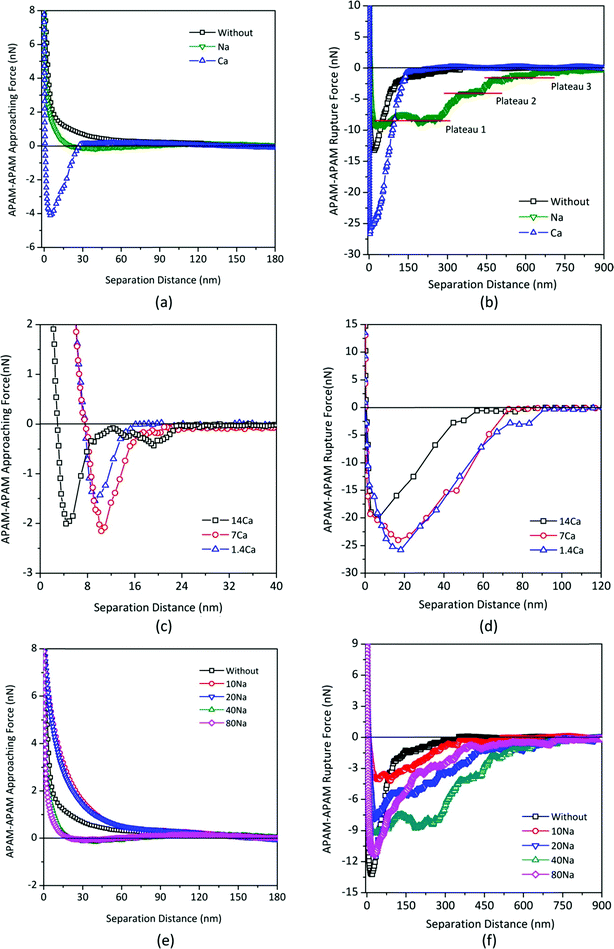 | ||
| Fig. 2 Impacts of Na+ and Ca2+ on the APAM–APAM intermolecular interactions: (a) and (b): comparison of the influence of Na+ and Ca2+ (with identical ionic strength) on approaching force curves and rupture force curves, respectively; (c) and (d): impacts of the concentration of Ca2+ on approaching force curves and rupture force curves, respectively; (e) and (f): impacts of the concentration of Na+ on approaching force curves and rupture force curves, respectively. The force curves in (e) and (f) were measured in pure water, 10 mM, 20 mM, 40 mM, and 80 mM of Na+, sequentially, using the same tip and substrate both of which were covalently grafted by the APAM molecule. Herein, the tested solutions were the inorganic salt solutions (without any addition of APAM). Reprinted with permission from DOI: 10.1021/acs.est.5b04098. Copyright 2016 American Chemical Society. | ||
Mechanical and structural properties of drinking water biofilms – implications for pathogen control in drinking-water distribution systems (DWDS)
DWDS biofilms can facilitate pathogen persistence and transmission, and the mechanical and structural properties of biofilms are two major factors influencing the accumulation and release of pathogens. A disinfectant residual, required in most drinking waters by the U.S. Environmental Protection Agency, may impact the biofilm mechanical and structural properties because of biomass loss, and chemical and biological composition changes in the biofilms. Therefore, it is of great interest to understand the mechanical and structural properties of biofilms under the long-term exposure of disinfectants, as this will shed light on determining the methods of predicting, assessing, and controlling the risk of pathogens in DWDS (Fig. 3). According to Shen et al. (DOI: 10.1021/acs.est.5b04653), AFM plays a substantial role in the characterization of biofilm structural and mechanical properties: thickness and stiffness.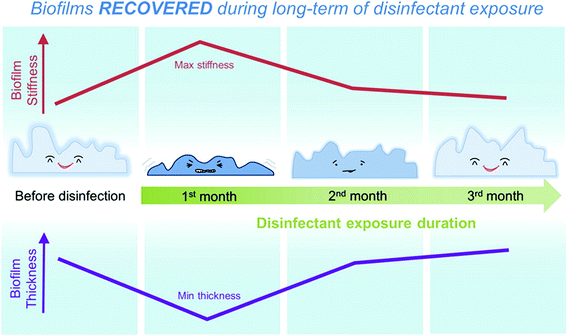 | ||
| Fig. 3 Schematic of biofilm stiffness and thickness changes over three months of disinfectant exposure. Reprinted with permission from DOI: 10.1021/acs.est.5b04653. Copyright 2016 American Chemical Society. | ||
The change in biofilm stiffness, represented by the biofilm elastic modulus (Young's modulus), was estimated by AFM indentation tests with a probe approaching to the biofilm surface at a downward velocity of 2 μm s−1. Spherical beads with a diameter of 20 μm were selected as the AFM probe to minimize contact pressures between the probe and the biofilms and to characterize the elastic modulus of the biofilms in a non-destructive way. The indentation force was measured as a function of indentation depth and the indentation curve for the biofilm outer layer had a lower slope compared to the biofilm inner layer (Fig. 4), suggesting that the Young's modulus of the biofilm outer layer was smaller than that of the biofilm inner layer. The outer-layer rather than inner-layer Young's modulus was selected to characterize the biofilm stiffness because the outer layer with direct exposure to drinking water is expected to be more important for determining pathogen accumulation and release. The outer-layer thickness was also characterized by the AFM, but the biofilm thickness was determined by optical coherence tomography (OCT) because the biofilms were relatively thick (tens or hundreds of μm) and the AFM was not able to characterize the full thickness.
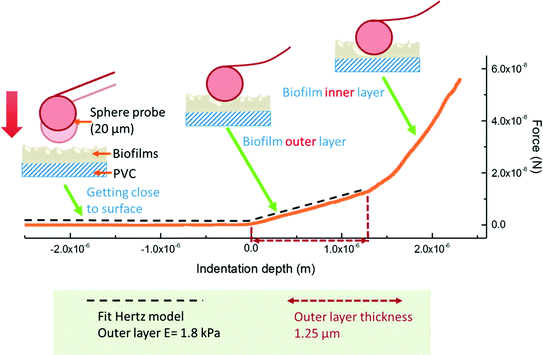 | ||
| Fig. 4 Indentation test principle. A sphere probe with the diameter of 20 μm was used in the indentation measurement. When the probe was indented into the biofilm surface, the deflection of the probe cantilever was monitored, and the applied force was calculated with the measured vertical deflection and the spring constant of the cantilever. The force as a function of indentation depth was then plotted, and the biofilm outer layer Young's modulus and thickness were determined accordingly. Reprinted with permission from DOI: 10.1021/acs.est.5b04653. Copyright 2016 American Chemical Society. | ||
The most important finding of Shen et al. (DOI: 10.1021/acs.est.5b04653) was that biofilm thickness and stiffness recovered in the long-term disinfectant exposure (Fig. 5). After the first month of the exposure to monochloramine and free-chlorine, the mean stiffness of biofilms was increased by 4 to 8 times of that before disinfectant treatment. The biofilm thickness was also observed to decrease from 120 ± 8 μm to 93 ± 6–107 ± 11 μm at the same time, probably due to biomass consumption. However, with longer disinfectant exposure up to three months, the biofilm mean stiffness showed a 2- to 5-fold decrease, along with an increasing biofilm thickness to 110 ± 7–129 ± 8 μm. It suggested that the biofilms adapted to long-term disinfectant exposure.
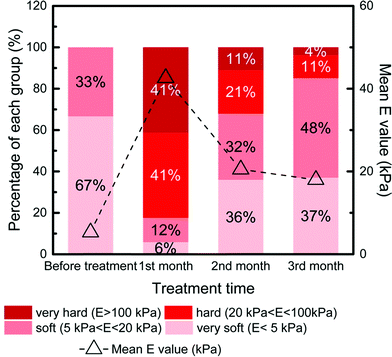 | ||
| Fig. 5 The percentage stacked bar for the Young's modulus (E) of biofilms during the three months of monochloramine treatment under stirring conditions. The dotted line shows the mean value of E at each time point. Reprinted and modified with permission from DOI: 10.1021/acs.est.5b04653. Copyright 2016 American Chemical Society. | ||
In this study, AFM characterizations indicated that one month of disinfectant exposure had the highest efficacy in controlling biofilms with the highest biofilm stiffness and the lowest biofilm thickness. The increased stiffness of the biofilms corresponded to the enhanced resistance to detachment, which would be more effective to prevent the release of biofilm associated pathogens. However, biofilm stiffness and thickness recovered following long-term exposure to disinfectants. Moreover, this study also illustrated that shear stress in DWDS could help reduce pathogen accumulation. AFM analyses provide insights in the transmission prediction and control of biofilm-associated pathogens in DWDS.
Nucleation and growth of Mn (hydr)oxide nanoparticles in natural water
Mn is one of the most abundant metals on earth, and Mn (hydr)oxide minerals are ubiquitously present in aquatic and terrestrial environments. Due to the large surface area and high reactivity of the minerals, Mn (hydr)oxide plays an important role in determining the fate, transport, and transformation of redox species and heavy metals. However, the early stage of nucleation and growth of Mn (hydr)oxide itself was largely overlooked. In a recent study, Jung et al. (DOI: 10.1021/acs.est.5b02819) explored the heterogeneous nucleation and growth of Mn (hydr)oxide nanoparticles at mineral–water interfaces by AFM and other microscopic/spectroscopic tools.By using the tapping mode of the AFM, the particle size, height, and morphology of Mn (hydr)oxide on the quartz substrates under varied ionic strengths (IS) were analyzed (Fig. 6). In the 1 mM IS system, Mn (hydr)oxide nanoparticles were formed in a relatively round shape with lateral dimensions larger than 100 nm and heights around 30 nm. However, in the 100 mM IS system, a unique rod-shaped Mn (hydr)oxide was observed. Moreover, the rod-shaped nanoparticles showed an arch-structured height profile along the rod axis, indicating that these nanoparticles were formed by oriented aggregation. The observed rod-shaped Mn (hydr)oxide nanoparticles were next characterized by high resolution X-ray diffraction and X-ray photoelectron spectroscopy, and Mn(OH)2 (s) and Mn3O4 (s) were found.
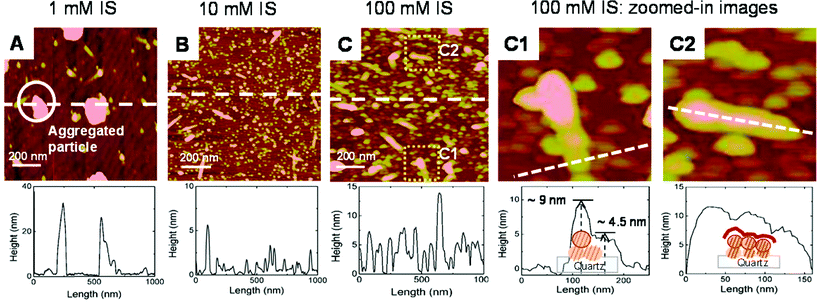 | ||
| Fig. 6 AFM height images show that the 1 mM ionic strength (IS) (A) system forms many big aggregated particles, the 10 mM IS (B) system has properties intermediate between the 1 mM and 100 mM IS systems, and the 100 mM IS (C) system contains many small particles which have formed both on quartz and on Mn oxides. The AFM height images from zoomed-in images (marked in C) for the 100 mM IS system show the pseudomonodispersed size distribution (C1) and oriented aggregation of nucleated particles (C2). The AFM image sizes (A to C) are 1 × 1 μm. Reprinted with permission from DOI: 10.1021/acs.est.5b02819. Copyright 2016 American Chemical Society. | ||
The formation process of Mn (hydr)oxide nanoparticles on the quartz substrate was also analyzed by comparing the AFM images at different elapsed times. From the AFM images in Fig. 7, it is observed that particle aggregation occurred as early as the first 5 minutes of the reaction, in which small individual particles started to cover the quartz surface. After the formation of sufficient Mn (hydr)oxide nanoparticles on the quartz surface, nucleation started to form on the top of pre-formed Mn (hydr)oxide nanoparticles and generated rod-shaped nanoparticles (>15 minutes).
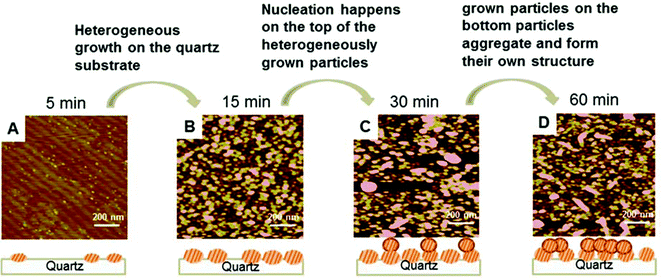 | ||
| Fig. 7 AFM height images from the 100 mM IS system at different elapsed times: 5 min (A), 15 min (B), 30 min (C), and 60 min (D). The schematic drawings explain the heterogeneous nucleation of Mn (hydr)oxide on the quartz substrate, followed by the nucleation and growth of Mn (hydr)oxide on top of the heterogeneously nucleated particles. The nucleated particles form both rod-shaped and round particles through oriented aggregation. The AFM image sizes are 1 × 1 μm. Reprinted with permission from DOI: 10.1021/acs.est.5b02819. Copyright 2016 American Chemical Society. | ||
In this study, the AFM not only precisely characterized the structural properties and morphologies of Mn (hydr)oxide nanoparticles, but also analyzed the nucleation process over a specific duration of reaction time. The mechanistic study sheds light on the nucleation and growth of Mn (hydr)oxide for remediating Mn-contaminated environmental systems (e.g., acid mine drainage).
Outlook
The selected studies highlight the significance and advantages of AFM characterization for membrane fouling, biofilm properties, and mineral nucleation in natural and engineered water systems. AFM outperforms many other microscopic or spectroscopic tools because it measures samples non-destructively under environmental conditions (e.g., in the air or water rather than vacuum). It overcomes the challenges of complicated sample preparation, and even enables the characterization of living microorganisms and cells.AFM holds great promise for understanding material properties and surface interactions in water systems. On the other hand, its limitations should also be considered for environmental sample analyses. For example, the AFM scans samples in a raster fashion and it is suitable for analyzing samples with a small size (e.g., hundreds of μm2). Other microscopic or spectroscopic tools should also be used to provide complementary characterization. In addition to AFM, other SPM (e.g., scanning tunneling microscopy, scanning electrochemical microscopy) analyses may also be beneficial to provide insights into chemical properties and reactions in natural and engineered water systems.
| This journal is © The Royal Society of Chemistry 2016 |
