Green tea beverages enriched with catechins with a galloyl moiety reduce body fat in moderately obese adults: a randomized double-blind placebo-controlled trial
Makoto
Kobayashi
*a,
Takanori
Kawano
a,
Yuuichi
Ukawa
a,
Yuko M.
Sagesaka
a and
Ikuo
Fukuhara
b
aCentral Research Institute, ITO EN, Ltd, Shizuoka, 421-0516, Japan. E-mail: m-kobayasi@itoen.co.jp; Tel: +81 548541247
bFukuhara Hospital, Hokkaido, 061-1351, Japan
First published on 5th November 2015
Abstract
Objective To determine whether ingesting a green tea beverage enriched with catechins with a galloyl moiety during a meal reduces body fat in moderately obese adults. Design Randomized double-blind placebo-controlled study. Subjects A total of 126 obese subjects (25 ≤ body mass index < 30 kg m−2) were randomly assigned to a group receiving green tea beverages without catechins (placebo), or a group receiving green tea beverages with a low or high content of catechins with a galloyl moiety. Each subject ingested 500 mL bottled green tea beverages containing 25, 180, or 279.5 mg green tea catechins (0, 149.5, or 246.5 mg catechins with a galloyl moiety, respectively), at mealtimes for 12 weeks; the subjects were instructed to ingest the beverage during the meal that had the highest fat content on that day. Methods Anthropometric measurements and blood chemistry analysis were performed during the run-in period; at weeks 0, 4, 8, and 12 of the intake period; and at the end of the withdrawal period. Abdominal fat area was measured by computed tomography at weeks 0, 8, and 12 of the intake period and at the end of the withdrawal period. Results Both the low- and high-dose groups exhibited significant reductions in visceral and subcutaneous fat areas compared to the control group at 12 weeks post-intervention. Conclusion Ingestion of a green tea beverage enriched with catechins with a galloyl moiety during a high-fat meal reduces body fat in moderately obese adults.
1. Introduction
Green tea, which is produced from the non-fermented leaves of Camellia sinensis, is a popular beverage worldwide. Green tea is rich in catechins, which is the major polyphenolic compound in green tea. Green tea catechins constitute approximately 10–18% of the total dry weight of green tea leaves. A 200 mL serving of green tea contains approximately 130 mg polyphenols, including approximately 48–72 mg catechins.1 The catechins extracted from green tea leaves mainly include (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epicatechin gallate (ECG), and (−)-epigallocatechin gallate (EGCG). Moreover, catechins can be categorized into 2 classes: free catechins such as EC and EGC, and catechins with a galloyl moiety (CGM) such as ECG and EGCG. The consumption of canned and bottled tea beverages is increasing in industrialized countries, particularly in Japan. The sterilization of these products epimerizes approximately 50% of catechins at the C2-position, forming (−)-catechin (C), (−)-gallocatechin (GC), (−)-catechin gallate (CG), and (−)-gallocatechin gallate (GCG).2,3 Therefore, canned and bottled tea beverages mainly contain 8 types of catechins.Green tea catechins have various physiological functions, including antiatherogenic,4 hypocholesterolemic,5,6 antioxidative,7,8 anticarcinogenic,9,10 and hypotriacylglycerolemic11–13 activities. Additional studies have investigated the effects of green tea and green tea catechins on body fat reduction in experimental animals14,15 and humans, but the results in humans are controversial.16–23 The reasons for the discrepancy among human studies may be the differences in caffeine intake and catechin composition. The anti-obesity effects of green tea catechins depend on the increase in energy expenditure by fat oxidation,14,24–28 and on the inhibition of fat absorption in the intestine.11,28,29 However, a meta-analysis suggests that green tea catechins synergistically stimulate daily energy expenditure with caffeine.30 The predominant hypothesis is that green tea catechins and caffeine synergistically enhance sympathetic nervous system activity, subsequently increasing energy expenditure by fat oxidation.28 Furthermore, green tea catechins, particularly CGM, are not readily absorbed in humans.31 However, the simultaneous ingestion of caffeine enhances the bioavailability of EGCG, i.e., the predominant green tea catechin.32 Gutiérrez-Salmeán et al.25 reported that supplementation with EC (a free catechin) at just 1 mg kg−1 body weight stimulates postprandial fat and carbohydrate metabolism in normal and overweight subjects. However, it is not known whether ingesting such a small amount of green tea CGM increases energy expenditure by fat oxidation in humans and whether the simultaneous ingestion of caffeine enhances the bioavailability of free catechins.
On the other hand, Juhel et al.33 reported that green tea extracts inhibit gastric and pancreatic lipases in vitro. Our group has shown that green tea CGM inhibits pancreatic lipase activity in vitro in a dose-dependent manner; moreover, we found that administration of THEA-FLAN 90S, a decaffeinated mixture of CGM, delays the lymphatic recovery of 14C-trioleoylglycerol in rats with thoracic duct cannulation.11 Meanwhile, free catechins, i.e., green tea catechins without a galloyl moiety, did not inhibit pancreatic lipase activity in that study. Furthermore, we recently reported that the ingestion of tea beverages enriched with green tea CGM increases lipid excretion into human feces.29 These findings collectively suggest that green tea CGM and free catechins exert body fat-lowering effects via different mechanisms: green tea CGM mainly inhibits fat absorption in the intestine, whereas free catechins, which have higher bioavailability than green tea CGM, mainly lead to increased energy expenditure. Therefore, it is important to consider the individual characteristics by which green tea catechins exert their beneficial effects.
Our previous placebo-controlled double-blind study showed that consumption of a beverage containing tea catechins (250 mL with 215.3 mg green tea catechins containing 211.0 mg green tea CGM) twice or thrice daily during mealtimes for 12 weeks significantly reduces abdominal fat, particularly visceral fat, compared to a control group of healthy Japanese subjects (22.5 < body mass index [BMI] ≤ 30 kg m−2).17 However, the subjects appeared to have difficultly in ingesting 250 mL beverages twice or thrice daily. The present study investigated the effects of ingesting a green tea beverage supplemented with THEA-FLAN 90S, a decaffeinated mixture of CGM, on body fat reduction in moderately obese adults. The test beverage was ingested daily at mealtime, because we hypothesized that green tea CGM would inhibit intestinal fat absorption.
2. Results
2.1 Baseline subject characteristics
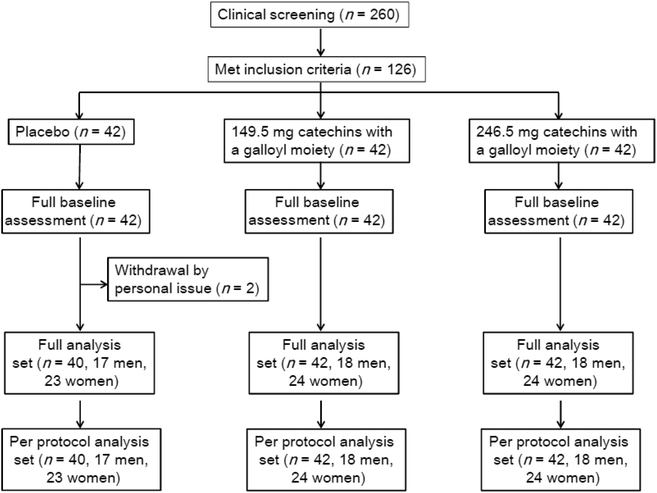
Among 126 subjects randomized to treatment, 2 dropped out for personal reasons (i.e., job relocation) unrelated to the trial; thus, 124 subjects completed the trial (Fig. 1). The percentage of individuals in the placebo, low-dose, and high-dose groups who ingested the test beverage was 99.7 ± 0.2%, 99.4 ± 0.2%, and 99.8 ± 0.1%, respectively. There were no changes to the trial outcomes after the trial commenced. The per protocol analysis set was identical to the full analysis set. Therefore, the remaining 124 subjects were analyzed. Baseline subject characteristics are shown in Table 1. There were no significant differences among the groups.| Placebo | Low | High | |
|---|---|---|---|
| Data are expressed as mean ± SE. There were no significant differences among groups.BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein. | |||
| n | 40 | 42 | 42 |
| Male/female | 17/23 | 18/24 | 18/24 |
| Age (years) | 44.7 ± 1.5 | 43.7 ± 1.4 | 44.7 ± 1.4 |
| Height (m) | 162.9 ± 1.3 | 162.7 ± 1.4 | 162.6 ± 1.5 |
| Body weight (kg) | 72.9 ± 1.3 | 72.8 ± 1.3 | 72.6 ± 1.4 |
| BMI (kg m−2) | 27.4 ± 0.2 | 27.4 ± 0.1 | 27.4 ± 0.2 |
| Body fat ratio (%) | 31.7 ± 0.9 | 32.2 ± 1.0 | 31.4 ± 0.9 |
| Waist circumference (cm) | 93.4 ± 0.8 | 93.7 ± 0.7 | 93.8 ± 0.8 |
| Total cholesterol (mg dL−1) | 220.9 ± 4.4 | 216.3 ± 5.7 | 215.9 ± 5.0 |
| HDL-cholesterol (mg) | 58.8 ± 2.4 | 56.6 ± 2.3 | 56.8 ± 2.0 |
| LDL-cholesterol (mg dL−1) | 142.2 ± 4.6 | 143.3 ± 5.4 | 143.4 ± 4.7 |
| Triacylglycerol (mg dL−1) | 125.4 ± 9.5 | 122.1 ± 10.9 | 103.5 ± 7.1 |
| Glucose (mg dL−1) | 84.6 ± 1.0 | 84.2 ± 1.0 | 83.8 ± 1.0 |
2.2 Nutrition survey and physical activity
The results of the nutrition survey and physical activity are shown in Table 2. There were no significant differences among the groups.| Group | Intake period | |||
|---|---|---|---|---|
| 0 weeks | 4 weeks | 8 weeks | ||
| Energy (kcal) | Placebo | 1803.0 ± 69.4 | 1878.1 ± 65.1 | 1812.3 ± 59.5 |
| Low | 1762.0 ± 46.1 | 1777.9 ± 52.6 | 1741.5 ± 49.6 | |
| High | 1824.6 ± 66.9 | 1784.3 ± 62.4 | 1723.7 ± 66.9 | |
| Protein (g) | Placebo | 63.4 ± 3.2 | 66.8 ± 2.4 | 64.7 ± 2.6 |
| Low | 61.7 ± 1.9 | 65.7 ± 2.4 | 62.4 ± 2.1 | |
| High | 66.5 ± 3.1 | 65.5 ± 2.3 | 61.8 ± 2.3 | |
| Fat (g) | Placebo | 57.7 ± 3.0 | 59.8 ± 3.3 | 57.2 ± 2.3 |
| Low | 57.5 ± 2.4 | 61.2 ± 2.9 | 59.5 ± 2.5 | |
| High | 61.7 ± 3.4 | 59.3 ± 2.5 | 59.4 ± 3.9 | |
| Carbohydrate (g) | Placebo | 247.0 ± 9.7 | 262.1 ± 10.8 | 252.5 ± 10.2 |
| Low | 236.9 ± 7.8 | 232.3 ± 7.6 | 230.5 ± 7.7 | |
| High | 236.7 ± 9.2 | 235.8 ± 10.6 | 223.4 ± 10.2 | |
| Physical activity (steps per day) | Placebo | 6354.4 ± 445.8 | 6341.7 ± 431.1 | 6498.0 ± 451.2 |
| Low | 6035.9 ± 456.9 | 5682.0 ± 451.0 | 5559.7 ± 423.4 | |
| High | 5880.9 ± 429.1 | 5922.8 ± 438.8 | 6197.9 ± 463.5 | |
| Group | Intake period | Withdrawal | |
|---|---|---|---|
| 12 weeks | 17 weeks | ||
| Data are expressed as mean ± SE. n = 40, 42, and 42 in the placebo, low-dose, and high-dose groups, respectively. There were no significant differences among groups and trial periods. | |||
| Energy (kcal) | Placebo | 1866.5 ± 69.5 | 1790.5 ± 68.5 |
| Low | 1732.7 ± 47.3 | 1675.5 ± 46.3 | |
| High | 1805.4 ± 60.8 | 1715.9 ± 70.6 | |
| Protein (g) | Placebo | 67.0 ± 2.7 | 65.2 ± 2.7 |
| Low | 60.2 ± 1.5 | 62.0 ± 1.9 | |
| High | 65.2 ± 2.1 | 63.0 ± 2.7 | |
| Fat (g) | Placebo | 59.3 ± 3.2 | 58.5 ± 3.1 |
| Low | 57.9 ± 2.7 | 57.3 ± 3.1 | |
| High | 62.2 ± 3.0 | 59.8 ± 3.6 | |
| Carbohydrate (g) | Placebo | 257.4 ± 9.9 | 238.0 ± 9.9 |
| Low | 232.4 ± 7.4 | 224.0 ± 8.6 | |
| High | 243.2 ± 11.0 | 227.8 ± 10.7 | |
| Physical activity (steps per day) | Placebo | 6789.1 ± 633.7 | 6693.9 ± 705.1 |
| Low | 6482.8 ± 498.5 | 6143.7 ± 428.4 | |
| High | 5885.0 ± 432.5 | 5878.0 ± 452.9 | |
2.3 Anthropometric values in the trial period
Anthropometric data are shown in Table 3. Body weight had increased significantly in the placebo group at 12 and 17 weeks compared to that at 0 weeks (P < 0.001 and P = 0.0013, respectively). Meanwhile, body weight had decreased significantly in the low-dose group at 12 weeks (P = 0.0249), and in the high-dose group at 8 and 12 weeks (P = 0.0281 and P = 0.0074, respectively), compared to that at 0 weeks. The body fat ratio had increased significantly in the placebo group at 8, 12, and 17 weeks compared to that at 0 weeks (P < 0.001, P = 0.0192, and P = 0.0123, respectively). Meanwhile, the fat ratio in the low- and high-dose groups did not change throughout the intake period. BMI in the placebo group was significantly higher at 12 and 17 weeks than that at 0 weeks (P < 0.001 and P = 0.0012, respectively). Meanwhile, BMI in the low-dose group was significantly lower at 12 weeks (P = 0.02639) and in the high-dose group at 8 and 12 weeks (P = 0.0235 and P = 0.0094, respectively) than that at 0 weeks. Waist circumference was significantly higher in the placebo group at 12 and 17 weeks than that at 0 weeks (P = 0.0085 and P = 0.0066, respectively). Meanwhile, waist circumference in the low- and high-dose groups was significantly lower at 12 weeks than that at 0 weeks (P = 0.0166 and P < 0.001, respectively). There were no significant differences in any parameter among groups. Hip circumference did not change significantly in any group.| Group | Intake period | |||
|---|---|---|---|---|
| 0 weeks | 4 weeks | 8 weeks | ||
| Body weight (kg) | Placebo | 72.9 ± 1.3a | 73.1 ± 1.3a | 73.3 ± 1.3ab |
| Low | 72.7 ± 1.3a | 72.6 ± 1.3ab | 72.5 ± 1.3ab | |
| High | 72.5 ± 1.4a | 72.3 ± 1.4ab | 72.0 ± 1.4b | |
| Body fat ratio (%) | Placebo | 32.8 ± 0.9a | 33.3 ± 0.9ab | 34.5 ± 0.9c |
| Low | 34.1 ± 1.0ab | 34.1 ± 1.1ab | 33.8 ± 0.9a | |
| High | 32.6 ± 1.0a | 32.7 ± 1.0a | 32.8 ± 1.0a | |
| BMI (kg cm−2) | Placebo | 27.4 ± 0.2a | 27.5 ± 0.2a | 27.5 ± 0.2ab |
| Low | 27.4 ± 0.2a | 27.3 ± 0.2ab | 27.3 ± 0.2ab | |
| High | 27.3 ± 0.2a | 27.3 ± 0.2ab | 27.2 ± 0.2b | |
| Waist circumference (cm) | Placebo | 93.4 ± 0.7a | 93.6 ± 0.8ab | 93.7 ± 0.7ab |
| Low | 93.8 ± 0.7a | 93.7 ± 0.7a | 93.6 ± 0.7a | |
| High | 93.5 ± 0.8a | 93.3 ± 0.8a | 93.1 ± 0.8ab | |
| Hip circumference (cm) | Placebo | 98.9 ± 0.6 | 98.8 ± 0.6 | 98.8 ± 0.6 |
| Low | 98.5 ± 0.5 | 98.7 ± 0.5 | 98.7 ± 0.5 | |
| High | 98.9 ± 0.5 | 99.0 ± 0.5 | 98.9 ± 0.6 | |
| Group | Intake period | Withdrawal | |
|---|---|---|---|
| 12 weeks | 17 weeks | ||
| Data are expressed as mean ± SE. n = 40, 42, and 42 in the placebo, low-dose, and high-dose groups, respectively. Means not sharing a common letter differ significantly between the trial periods (P < 0.05). There were no significant differences among groups.BMI, body mass index. | |||
| Body weight (kg) | Placebo | 73.6 ± 1.3b | 73.5 ± 1.3b |
| Low | 72.3 ± 1.3b | 73.2 ± 1.3c | |
| High | 71.9 ± 1.4b | 72.6 ± 1.4a | |
| Body fat ratio (%) | Placebo | 33.8 ± 0.9bc | 33.9 ± 0.8bc |
| Low | 33.8 ± 1.0a | 34.7 ± 1.0b | |
| High | 32.8 ± 1.0a | 34.0 ± 1.0b | |
| BMI (kg cm−2) | Placebo | 27.7 ± 0.2b | 27.6 ± 0.2b |
| Low | 27.2 ± 0.2b | 27.6 ± 0.2c | |
| High | 27.1 ± 0.2b | 27.4 ± 0.2a | |
| Waist circumference (cm) | Placebo | 94.0 ± 0.7b | 94.0 ± 0.7b |
| Low | 93.3 ± 0.7b | 93.9 ± 0.7b | |
| High | 92.8 ± 0.8b | 93.3 ± 0.8a | |
| Hip circumference (cm) | Placebo | 99.0 ± 0.6 | 99.2 ± 0.6 |
| Low | 98.7 ± 0.5 | 99.0 ± 0.5 | |
| High | 98.7 ± 0.6 | 99.1 ± 0.5 | |
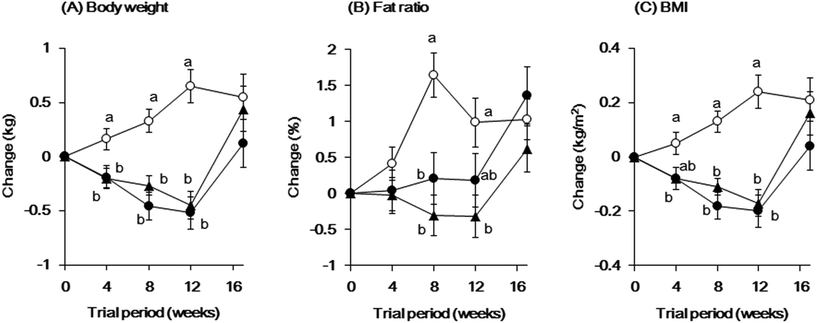
The changes in body weight, fat ratio, and BMI are shown in Fig. 2. The change in body weight at 4, 8, and 12 weeks was significantly smaller in the low- and high-dose groups than that in the placebo group (low-dose group: P = 0.0429, P < 0.001, and P < 0.001; high-dose group: P = 0.0465, P < 0.001, and P < 0.001). The change in fat ratio at 8 and 12 weeks in the low-dose group was significantly smaller than that in the placebo group (P < 0.001 and P = 0.0061, respectively); similarly, this change in fat ratio at 8 weeks in the high-dose group was significantly smaller than that in the placebo group (P = 0.0191). The change in BMI was significantly smaller in the low-dose group than that in the placebo group at 4, 8, and 12 weeks (P = 0.0485, P < 0.001, and P < 0.001, respectively), whereas the change in BMI was significantly smaller in the high-dose group than that in the placebo group at 8 and 12 weeks (P < 0.001 and P < 0.001, respectively). There were no significant differences in these parameters between the low- and high-dose groups.
2.4 Abdominal fat area
Abdominal fat area data are shown in Table 4. Visceral fat area (VFA), subcutaneous fat area (SFA), and total fat area (TFA) did not change significantly in the placebo group throughout the trial period. Meanwhile, all of these values in the low- and high-dose groups were significantly lower at 8 and 12 weeks, respectively, than those at 0 weeks (VFA: low-dose group, P = 0.0207 and P = 0.0078, high-dose group, P = 0.0048 and P < 0.001, SFA: low-dose group, P < 0.001 and P < 0.001, high-dose group, P < 0.001 and P < 0.001, TFA: low-dose group, P < 0.001 and P < 0.001, high-dose group, P < 0.001 and P < 0.001). However, during the withdrawal period, the significant reductions in abdominal fat areas due to the continuous ingestion of green tea CGM were attenuated. There were no significant differences among groups.| Group | Intake period | Withdrawal | |||
|---|---|---|---|---|---|
| 0 weeks | 8 weeks | 12 weeks | 17 weeks | ||
| Data are expressed as mean ± SE. n = 40, 42, and 42 in the placebo, low-dose, and high-dose groups, respectively. Means not sharing a common letter differ significantly between the trial periods (P < 0.05). There were no significant differences among groups.VFA, visceral fat area; SFA, subcutaneous fat area; TFA, total fat area. | |||||
| VFA (cm2) | Placebo | 96.0 ± 6.1 | 97.0 ± 5.9 | 98.8 ± 5.9 | 96.7 ± 6.0 |
| Low | 93.8 ± 5.8a | 88.9 ± 5.4b | 88.3 ± 5.1b | 92.0 ± 5.3ab | |
| High | 92.2 ± 6.7a | 85.8 ± 6.1bc | 84.8 ± 5.7c | 90.5 ± 5.8ab | |
| SFA (cm2) | Placebo | 226.9 ± 8.3 | 227.1 ± 8.7 | 221.7 ± 8.4 | 223.4 ± 8.4 |
| Low | 239.2 ± 8.9a | 228.9 ± 8.9b | 221.0 ± 9.0c | 229.2 ± 8.9b | |
| High | 232.1 ± 7.9a | 222.0 ± 8.7b | 215.4 ± 8.5c | 224.5 ± 8.3b | |
| TFA (cm2) | Placebo | 322.9 ± 8.8 | 324.2 ± 9.0 | 320.5 ± 8.9 | 320.1 ± 8.8 |
| Low | 333.0 ± 7.7a | 317.8 ± 7.9b | 309.4 ± 7.5c | 321.2 ± 8.2b | |
| High | 324.3 ± 8.2a | 307.8 ± 8.4b | 299.7 ± 8.8c | 315.0 ± 9.0b | |
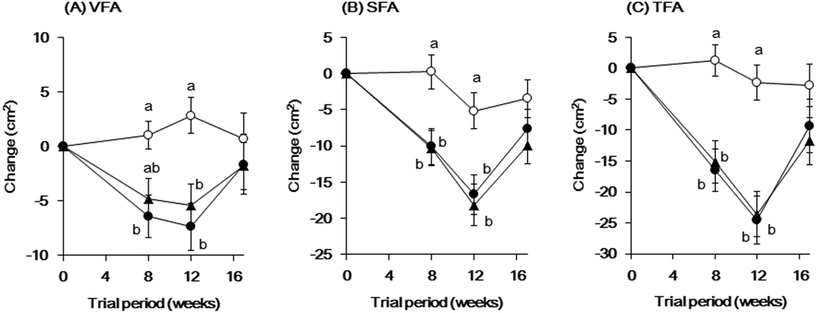
The changes in VFA, SFA, and TFA are shown in Fig. 3. The change in VFA was significantly smaller in the low-dose groups at 12 weeks (P = 0.01) and in the high-dose groups at 8 and 12 weeks (P = 0.0162 and P < 0.001, respectively), compared to that in the placebo group. The changes in SFA and TFA at 8 and 12 weeks were significantly smaller in the low- and high-dose groups than those in the placebo group (SFA: low-dose group, P = 0.0082 and P = 0.0026, high-dose group, P = 0.0096 and P = 0.0085; TFA: low-dose group, P = 0.0012 and P < 0.001, high-dose group, P < 0.001 and P < 0.001). There were no differences in these parameters between the low- and high-dose groups. However, during the withdrawal period, the significant reductions in these abdominal fat areas due to the continuous ingestion of green tea CGM disappeared.
2.5 Blood and urine
Baseline levels of total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triacylglycerol, and glucose are shown in Table 1. There were no significant differences among groups with respect to any of these parameters or other blood or urine parameters. The blood total cholesterol level in the placebo group was significantly higher at 8 weeks than that at 0 weeks (P = 0.0128). Although there were significant changes in several blood parameters during the trial period, they were within their respective normal ranges (data not shown). Furthermore, there were no significant differences in blood or urine parameters among groups (data not shown).2.6 Adverse events
No subjects withdrew from the trial because of adverse effects or discomfort due to the beverage intervention. During the trial period, 14 cases of abdominal pain (6, 3, and 5 in the placebo, low-dose group, and high-dose groups, respectively) and 4 cases of diarrhea (2, 1, and 1 in the placebo, low-dose group, and high-dose groups, respectively) were reported. In addition, numerous subjective symptoms related to body conditions were reported (i.e., nasal congestion); however, recovery from all of these symptoms was spontaneous. Furthermore, no major adverse effects were noted. Therefore, the physician responsible concluded that no adverse events related to the trial had occurred.3. Discussion
In the present study, the subjects were instructed not to ingest green tea except for the test beverages throughout the trial period. Furthermore, free catechins were removed as much as possible from the test beverages, and the caffeine contents were minimized and equalized as precisely as possible; this was done in order to clarify the effect of green tea CGM, because green tea CGM and free catechins may exert their body fat-lowering effects via different mechanisms.The accumulation of body fat, especially visceral fat, leads to metabolic syndrome, which is a risk factor for cardiovascular disease.34 The Japan Society for the Study of Obesity defines obesity as BMI ≥ 25 kg m−2. The present study investigated the effects of ingesting a green tea beverage supplemented with THEA-FLAN 90S, a decaffeinated mixture of CGM, on body fat reduction in moderately obese adults who ingested a test beverage daily with a meal for 12 weeks. The results revealed significant decreases in VFA, SFA, and TFA at both doses of green tea CGM. Furthermore, body weight and BMI decreased significantly in both the low- and high-dose groups. However, there were no significant differences in the changes in VFA, SFA, TFA, body weight, fat ratio, or BMI between the low- and high-dose groups. Nevertheless, significant decreases in body weight and BMI occurred earlier in the high-dose group than in the low-dose group.
Nakagawa et al.32 reported that the bioavailability of EGCG is enhanced by the simultaneous ingestion of caffeine; they found a larger area under the curve of EGCG when subjects ingested a beverage containing 95 mg EGCG and 40 mg caffeine than when subjects ingested a beverage containing 95 mg EGCG alone or 95 mg EGCG and 180 mg caffeine. In the present study, all test beverages contained caffeine. There is no evidence indicating that the EGCG/caffeine ratio enhances the bioavailability of EGCG. Furthermore, Chow et al.35 reported that the oral bioavailability of green tea catechins is dramatically lower in a sated state than in a fasting state. Because the subjects in the present study ingested the test beverages at mealtimes, the oral bioavailability of green tea CGM might have been very low. Therefore, it is unlikely that absorbed green tea CGM leads to increased energy expenditure, followed by reduced abdominal body fat area.
We recently showed that the ingestion of tea beverages enriched with green tea CGM increases lipid excretion into human feces.29 Inhibition of dietary fat absorption decreases the deposition of visceral fat. Meanwhile, it is possible that slower absorption of dietary fat also decreases the deposition of visceral fat. After a meal, the increase in blood glucose stimulates the secretion of insulin. Insulin activates peripheral lipoprotein lipase and then hydrolysis of chylomicron triacylglycerols is stimulated. Fatty acids originating from chylomicron triacylglycerols are mainly incorporated into adipose tissue and deposited as triacylglycerols. If the concentration of chylomicron triacylglycerols is higher, it is possible that the deposition of fatty acids originating from chylomicron triacylglycerols may be higher in adipose tissues. Therefore, the suppression of postprandial hypertriacylglycerolemia may result in the reduction of fat deposition. It has been reported that green tea catechins, particularly CGM,suppressed postprandial hypertriacylglycerolemia in experimental animals11,12 and in humans.36,37 In this trial, subjects ingested the test beverage during the mealtime. Therefore, although the present study did not examine whether the ingestion of the test beverages increased lipid excretion or suppressed postprandial hypertriacylglycerolemia, it is reasonable to consider that green tea CGM exerts its abdominal body fat-lowering effects by inhibiting or slowing intestinal fat absorption.
In the placebo group, body weight increased during the trial period. Gordon et al.38 reported that body weight increases from summer to winter. Therefore, the increase in body weight in the placebo group may be attributable to seasonal factors, because the present trial was conducted during the period from summer to winter.
Green tea catechins have hypocholesterolemic activity.5,6 Our previous placebo-controlled double-blinded study showed that consumption of beverages containing tea catechins (250 mL containing 215.3 mg green tea catechins and 211.0 mg green tea CGM) twice or thrice daily at mealtimes for 12 weeks significantly reduced serum total and low-density lipoprotein cholesterol concentrations compared to that in a control group of healthy Japanese subjects (22.5 < BMI ≤ 30 kg m−2).17 However, in the present study, green tea CGM did not exert any hypocholesterolemic activity. The reasons for the discrepancy between our studies may be the different dosages of green tea CGM. Moreover, we recently showed that EGCG, a green tea CGM, decreases the micellar solubility of cholesterol via a specific interaction with phosphatidylcholine (a phospholipid), but not via a direct interaction with cholesterol.39 Thus, the restricted cholesterol solubility in bile salt micelles due to green tea CGM may be a major cause of the observed inhibition of cholesterol absorption.40,41 In humans, the amounts of cholesterol and phospholipids delivered to the intestinal lumen via the biliary pathway are higher than those due to dietary intake.42 Phosphatidylcholine is the predominant phospholipid in the intestinal lumen. Bile containing endogenous phosphatidylcholine is generally secreted postprandially. Therefore, it may be important to consider the amounts and ingestion state (sated/fasted) of green tea CGM in order to determine how it exerts its hypocholesterolemic activity in humans.
4. Limitations
One limitation of this study is that we did not evaluate whether the ingestion of the test beverages enriched with green tea CGM increased lipid excretion. Therefore, we could not confirm that green tea CGM exerts its abdominal body fat-lowering effect by inhibiting intestinal fat absorption. Furthermore, additional research is required to confirm that beverages rich in green tea CGM exert their abdominal body fat-lowering effect not only in Japanese but also in Caucasian populations; this is because Hursel et al.43 reported that the body weight-reduction effect of green tea catechins in Caucasian subjects is weaker than that in Asian subjects.5. Conclusion
The results of this study demonstrate that the ingestion of a green tea beverage enriched with green tea CGM reduces abdominal body fat in moderately obese adults who ingest such beverages with a high-fat meal. Green tea CGM may exert its abdominal body fat-lowering effects by inhibiting or slowing intestinal fat absorption. These results suggest that the ingestion of green tea beverages enriched with CGM together with high-fat meals may be an effective strategy for reducing body fat in moderately obese adults.6. Experiments
6.1 Subjects
A 12 week randomized placebo-controlled double-blind trial with a parallel design was performed (Fig. 1). This study was approved by the Ethics Committee of Fukuhara Hospital (Eniwa, Japan) in accordance with the principles of the Declaration of Helsinki. The subjects were recruited by a clinical trials coordinator, New Drug Research Center, Inc. (Hokkaido, Japan), and were fully informed regarding the content and methods of this trial. Written informed consent was obtained prior to participation. Four weeks prior to the trial, a preliminary screening of 260 subjects aged 20–65 years was performed, and 126 subjects with 25 ≤ BMI < 30 kg m−2 were selected. The Japan Society for the Study of Obesity defines obesity as BMI ≥ 25 kg m−2. The exclusion criteria were as follows: a history of drug or food allergy, chronic disease or frequent medicine use, blood donation (i.e., of 400 mL within 12 weeks or 200 mL within 4 weeks, or the donation of blood components within 2 weeks), current use of medications, and current use of functional or health foods that may affect the trial outcome. Subjects judged to be inappropriate by the trial investigator were also excluded. Two subjects in the placebo group dropped out at 0 and 3 weeks for personal reasons.6.2 Test beverages
Three types of test beverages were manufactured industrially. Tea was extracted from 90 kg green tea leaves (ITO EN, Ltd, Shizuoka, Japan) by 11250 L hot water. Green tea catechins were removed from the green tea infusion by the addition of polyvinylpolypyrrolidone.17,44 Cyclodextrin and ascorbic acid were added to the resultant infusion in order to adjust its taste as much as possible. Finally, the tea was packed in plastic bottles by using an ultra-high temperature heating method for sterilization, and this was used as the placebo beverage. Meanwhile, 2 other infusions were prepared by adding cyclodextrin and ascorbic acid and these were supplemented with 5.3 or 8.7 kg THEA-FLAN 90S (ITO EN, Ltd) and packed in plastic bottles using the same method as that used for the placebo beverage. These preparations were designated as low- and high-CGM beverages, and they contained 149.5 and 246.5 mg CGM per 500 mL respectively. Prior to the initiation of the trial, we confirmed that the flavor, taste, and packaging of these 3 types of beverages were difficult to distinguish. The catechin and caffeine contents in these beverages were analyzed by high-performance liquid chromatography with UV detection (Table 5).45 Approximately 50% of the CGM present in the beverages were heat-epimerized tea catechins (CG and GCG), and the remaining were ECG and EGCG (Table 5).| Placebo | Low | High | |
|---|---|---|---|
| (mg per 500 mL bottle) | |||
| EGCG, (−)-epigallocatechin gallate; ECG, (−)-epicatechin gallate; GCG, (−)-gallocatechin gallate; CG, (−)-catechin gallate; EGC, (−)-epigallocatechin; EC, (−)-epicatechin; GC, (−)-gallocatechin; C, (−)-catechin; CGM, catechins with a galloyl moiety; N.D., not detected. | |||
| EGCG | N.D. | 50.5 | 84.5 |
| ECG | N.D. | 17.5 | 29.5 |
| GCG | N.D. | 61.5 | 102.0 |
| CG | N.D. | 20.0 | 30.5 |
| EGC | 5.5 | 6.5 | 7.0 |
| EC | 2.5 | 3.5 | 4.0 |
| GC | 13.0 | 16.0 | 17.0 |
| C | 4.0 | 4.5 | 5.0 |
| Total catechins | 25.0 | 180.0 | 279.5 |
| CGM | 0.0 | 149.5 | 246.5 |
| Caffeine | 32.5 | 34.0 | 35.0 |
6.3 Design
We conducted a placebo-controlled double-blind trial between August 2014 and January 2015. The trial period consisted of a 4 week run-in period, a 12 week intake period, and a 5 week withdrawal period. After stratification by age and sex during preliminary screening, 126 subjects were divided into 3 groups by stratified randomization, which was performed by a statistician working with New Drug Research Center, Inc. (Hokkaido, Japan), independent of the trial investigators in order to ensure that the mean BMI and waist circumference in each group were similar. The 3 groups were randomly allocated to receive 1 of the 3 test beverages by a hospital director in Hokkaido, independent of the trial investigators. The test beverages were delivered to the subjects by a person in charge of allocation who was working with the site management organization, Medifform Inc. (Hokkaido, Japan), independent of the trial investigators. During the intake period, the subjects were instructed to ingest the beverage during the meal with the highest fat content on that day. The subjects were instructed to decide which meal was the fattest meal on that day by their subjective judgment. The subjects were instructed to visit Fukuhara Hospital 6 times: at the beginning of the run-in period; at weeks 0, 4, 8, and 12 of the intake period; and at the end of the withdrawal period. The subjects were instructed not to change their daily activities including eating habits and exercise, and not to ingest green tea except for the test beverages.6.4 Nutrition survey and determination of physical activity
The subjects were instructed to record and photograph the contents of daily meals, snacks, and beverages in a dietary diary for 3 consecutive days prior to weeks 0, 4, 8, and 12 of the intake period, and at the end of the withdrawal period. The intakes of energy, protein, fat, and carbohydrates were calculated from the diary records and photographs by a nutritionist using Excel Eiyo-Kun version 6.0 (Kenpakusha Co., Ltd, Tokyo, Japan). The dietary records and photographs were submitted at the beginning of the run-in period; at weeks 0, 4, 8, and 12 of the intake period; and at the end of the withdrawal period. Physical activity was measured by the number of steps taken using a pedometer (HJ-005, OMRON Co., Kyoto, Japan).6.5 Anthropometric measurements
All anthropometric measurements were made by well-trained investigators. Height was measured at the beginning of the run-in period. During each visit, the body weight, body fat ratio, waist circumference, hip circumference, systolic blood pressure, and diastolic blood pressure were measured; blood and urine samples were also collected. Body weight and body fat ratio were simultaneously measured by using a body fat scale (TBF-310, TANITA Co., Tokyo, Japan). BMI was calculated as height (in m) divided by body weight (in kg) squared.6.6 Abdominal fat measurement
The primary outcome variable in this trial was abdominal fat area. All subjects underwent abdominal fat analysis by computed tomography (CT-W450, Hitachi Medical Co., Tokyo, Japan). The VFA and SFA were estimated using FatScan version 3.0 (N2 System Co., Osaka, Japan) according to the method of Tokunaga et al.46 These areas were added to obtain the TFA. Computed tomography was performed at weeks 0, 8, and 12 of the intake period, as well as at the end of the withdrawal period. On examination days, the subjects fasted for at least 5.5 hours.6.7 Blood and urine sampling and clinical analysis
After 5.5 hours of fasting, blood and urine were collected for hematological and biochemical analyses at the beginning of the run-in period; at weeks 0, 4, 8, and 12 of the intake period; and at the end of the withdrawal period. All blood and urine analyses were performed by SRL, Inc., Ltd (Hokkaido, Japan). The following blood parameters were evaluated: white blood cells, red blood cells, hemoglobin, hematocrit, platelets, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, total protein, albumin, total bilirubin, aspartate aminotransferase, alanine aminotransferase, lactase dehydrogenase, alkaline phosphatase, γ-glutamyl transpeptidase, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triacylglycerol, glucose, hemoglobin A1c, uric acid, blood urea nitrogen, creatinine, sodium, chloride, and potassium. The following urine parameters were evaluated: protein, glucose, urobilinogen, occult blood, bilirubin, and ketone body.6.8 Adverse events
Subjects were instructed to record any subjective symptoms regarding body conditions in their diary, which were submitted to the responsible physician at the beginning of the run-in period; at weeks 0, 4, 8, and 12 of the intake period; and at the end of the withdrawal period.6.9 Statistical analysis
Statistical analysis was performed with the per protocol analysis set. Data are expressed as mean ± standard error (SE). The significance of differences at different time points was evaluated by using the Šidák method.47 Differences between intervention groups were evaluated by the Bartlett method followed by the Tukey–Kramer test or the Steel–Dwass test where appropriate. All statistical analyses were performed with SAS R9.3 (SAS Institute, Tokyo, Japan). Differences were considered significant at P < 0.05.Acknowledgements
We are grateful to Mr Hidenori Takahashi from the Production Department, Research and Development Division, ITO EN Ltd for the industrial-manufacture of the placebo and test beverages. We also thank all subjects who participated in this study and the team of medical scientists from the New Drug Research Center, Inc.References
- C. D. Wu and G. X. Wei, Tea as a functional food for oral health, Nutrition, 2002, 18, 443–444 CrossRef CAS PubMed.
- Z. Y. Chen, Q. Y. Xhu, D. Tsang and Y. Huang, Degradation of green tea catechins in tea drinks, J. Agric. Food Chem., 2001, 49, 477–482 CrossRef CAS PubMed.
- R. Seto, H. Nakamura, F. Nanjyo and Y. Hara, Preparation of epimers of tea catechins by heat treatment, Biosci., Biotechnol., Biochem., 1997, 61, 1434–1439 CrossRef CAS.
- Y. Miura, T. Chiba, I. Tomita, H. Koizumi, S. Miura, K. Umegaki, Y. Hara, M. Ikeda and T. Tomita, Tea catechins prevent the development of atherosclerosis in apoprotein E-deficient mice, J. Nutr., 2001, 131, 27–32 CAS.
- O. Kajimoto, Y. Kajimoto, M. Yabune, A. Nozawa, K. Nagata and T. Kakuda, Tea catechins reduce serum cholesterol levels in mild and borderline hypercholesterolemia patients, J. Clin. Biochem. Nutr., 2003, 33, 101–111 CrossRef CAS.
- O. Kajimoto, Y. Kajimoto, M. Takeda, A. Nozawa, Y. Suzuki and T. Kakuda, A beverage containing tea catechins with a galloyl moiety reduce serum cholesterol level in hypercholesterolemic women, Health Sci., 2006, 22, 60–71 Search PubMed . (In Japanese with English summary).
- T. Okuda, Y. Kimura, T. Yoshida, T. Hatano, H. Okuda and S. Arichi, Studies on the activities of tannins and related compounds from medicinal plants and drugs. I. Inhibitory effects on lipid peroxidation in mitochondria and microsomes of liver, Chem. Pharm. Bull., 1983, 31, 1625–1631 CrossRef CAS PubMed.
- K. Yoshino, I. Tomita, M. Sano, I. Oguni, Y. Hara and M. Nakano, Effects of long term dietary supplement of tea polyphenols on lipid peroxide levels in rats, Age, 1994, 17, 79–85 CrossRef CAS.
- T. Kada, K. Kaneko, S. Matsuzaki, T. Matsuzaki and Y. Hara, Detection and chemical identification of natural bioantimutagens. A case of the green tea factor, Mutat. Res., 1985, 150, 127–132 CrossRef CAS PubMed.
- H. Fujiki, M. Suganuma, S. Okabe, N. Sueoka, A. Komori, E. Sueoka, T. Kozu, Y. Tada, K. Suga, K. Imai and K. Nakachi, Cancer inhibition by green tea, Mutat. Res., 1998, 402, 307–310 CrossRef CAS PubMed.
- I. Ikeda, K. Tsuda, Y. Suzuki, M. Kobayashi, T. Unno, H. Tomoyori, H. Goto, Y. Kawata, K. Imaizumi, A. Nozawa and K. Kakuda, Tea catechins with a galloyl moiety suppress postprandial hypertriacylglycerolemia by delaying lymphatic transport of dietary fat in rats, J. Nutr., 2005, 135, 155–159 CAS.
- P. T. Chan, W. P. Fong, Y. L. Cheung, Y. Huang, W. K. K. Ho and Z. Y. Chen, Jasmine green tea epicatechins are hypolipidemic in hamsters (Mesocricetus auratus) fed a high fat diet, J. Nutr., 1999, 129, 1094–1101 CAS.
- S. I. Koo and S. K. Noh, Green tea as inhibitor of the intestinal absorption of lipids: potential mechanism for its lipid-lowering effect, J. Nutr. Biochem., 2007, 8, 179–183 CrossRef PubMed.
- T. Murase, A. Nagasawa, J. Suzuki, T. Hase and I. Tokimitsu, Beneficial effects of tea catechins on diet-induced obesity: stimulation of lipid catabolism in the liver, Int. J. Obes., 2002, 26, 1459–1464 CrossRef CAS PubMed.
- I. Ikeda, R. Hamamoto, K. Uzu, K. Imaizumi, K. Nagao, T. Yanagita, Y. Suzuki, M. Kobayashi and T. Kakuda, Dietary gallate esters of tea catechins reduce deposition of visceral fat, hepatic triacylglycerol, and activities of hepatic enzymes related to fatty acid synthesis in rats, Biosci., Biotechnol., Biochem., 2005, 69, 1049–1053 CrossRef CAS PubMed.
- T. Nagao, Y. Komine, S. Soga, S. Meruro, T. Hase, Y. Tanaka and I. Tokimitsu, Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men, Am. J. Clin. Nutr., 2005, 81, 122–129 CAS.
- O. Kajimoto, Y. Kajimoto, M. Yabune, T. Nakamura, K. Kotani, Y. Suzuki, A. Nozawa, K. Nagata, T. Unno, Y. M. Sagesaka, T. Kakuda and T. Yoshikawa, Tea catechins with a galloyl moiety reduce body weight and fat, J. Health Sci., 2005, 51, 161–171 CrossRef CAS.
- H. Wang, Y. Wen, Y. Du, X. Yan, H. Guo, J. A. Rycroft, N. Boon, E. M. Kovacs and D. J. Mela, Effects of catechin enriched green tea on body composition, Obesity, 2010, 18, 773–779 CrossRef CAS PubMed.
- T. Nagao, T. Hase and I. Tokimitsu, A green tea extract high in catechins reduces body fat and cardiovascular risks in human, Obesity, 2007, 15, 1473–1483 CrossRef CAS PubMed.
- K. C. Maki, M. S. Reeves, M. Farmer, K. Yasunaga, N. Matsuo, Y. Katsuragi, I. Tokimitsu, D. Wilder, F. Jones, J. F. Blumberg and Y. Cartwright, Green tea catechin consumption enhances exercise-induced abdominal fat loss in overweight and obese adults, J. Nutr., 2009, 139, 264–270 CrossRef CAS PubMed.
- A. Basu, K. Sanchez, M. J. Leyva, M. Wu, N. M. Betts, C. E. Aston and T. J. Lyons, Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome, J. Am. Coll. Nutr., 2010, 29, 31–41 CrossRef CAS PubMed.
- A. M. Hill, A. M. Coates, J. D. Buckley, R. Ross, F. Thielecke and P. R. C. Howe, Can EGCG reduce abdominal fat in obese subject?, J. Am. Coll. Nutr., 2007, 26, 396S–402S CrossRef CAS PubMed.
- P. Auvichayapat, M. Orapochanung, O. Tunkamnerdthai, B. O. Sripanidkulchai, N. Auvichayapat, B. Thinkhamrop, S. Kunhasura, S. Wongpratoom, S. Sinawat and P. Hongprapas, Effectiveness of green tea on weight reduction in obese Thais: A randomized, controlled trial, Physiol. Behav., 2008, 93, 486–491 CrossRef CAS PubMed.
- U. Harada, A. Chikama, S. Saito, H. Takase, T. Nagao, T. Hase and I. Tokimitsu, Effects of the long-term ingestion of tea catechins on energy expenditure and dietary fat oxidation in healthy subjects, J. Health Sci., 2005, 51, 248–252 CrossRef CAS.
- G. Gutiérrez-Salmeán, P. Ortiz-Vilchis, C. M. Vacaseydel, I. Rubio-Gayosso, E. Meaney, F. Villarreal, I. Ramírez-Sánchez and G. Ceballos, Acute effects of an oral supplement of (−)epicatechin on postprandial fat and carbohydrate metabolism in normal and overweight subjects, Food Funct., 2014, 5, 521–527 Search PubMed.
- F. Thielecke, G. Rahn, J. Böhnke, F. Adams, A. L. Birkenfeld, J. Jordan and M. Boschmann, Epigallocatechin-3-gallate and postprandial fat oxidation in overweight/obese male volunteers: a pilot study, Eur. J. Clin. Nutr., 2010, 64, 704–713 CrossRef CAS PubMed.
- A. G. Dulloo, C. Duret, D. Rohrer, L. Girardier, N. Mensi, M. Fathi, P. Chantre and J. Vandermander, Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans, Am. J. Clin. Nutr., 1999, 70, 1040–1045 CAS.
- T. M. Rains, S. Agarwal and K. C. Maki, Antiobesity effects of green tea catechins: a mechanistric review, J. Nutr. Biochem., 2011, 22, 1 CrossRef CAS PubMed.
- Y. Ukawa, Y. Hatakeyama, A. Noro, I. Fukuhara and Y. M. Sagesaka, Effect of consumption of tea beverage containing catechins with a galloyl moiety on lipid excretion into feces, Jpn. Pharmacol. Ther., 2013, 41, 919–927 CAS . (In Japanese with English summary).
- R. Hursel, W. Viechtbauer, A. G. Dulloo, A. Tremblay, L. Tappy, W. Rumpler and M. S. Westertero-Plantenga, The effects of catechin rich teas and caffeine on energy expenditure and fat oxidation: a meta-analysis, Obes. Rev., 2011, 12, e573–e581 CrossRef CAS PubMed.
- K. Nakagawa, S. Okuda and T. Miyazawa, Dose-dependent incorporation of tea catechins, epigallocatechin-3-gallate and epigallocatechin, into human plasma, Biosci., Biotechnol., Biochem., 1997, 61, 1981–1985 CrossRef CAS PubMed.
- K. Nakagawa, K. Nakayama, M. Nakamura, P. Sookwong, T. Tsuduki, H. Niino, F. Kimura and T. Miyazawa, Effects of co-administration of tea epigallocatechin-3-gallate (EGCG) and caffeine on absorption and metabolism of EGCG in humans, Biosci., Biotechnol., Biochem., 2009, 73, 2014–2017 CrossRef CAS PubMed.
- C. Juhel, M. Armand, Y. Pafumi, C. Rosier, J. Vandermander and D. Lairon, Green tea extract (AR25) inhibits lipolysis of triglycerides in gastric and duodenal medium in vitro, J. Nutr. Biochem., 2000, 11, 45–51 CrossRef CAS PubMed.
- J. P. Després, Body fat distribution and risk of cardiovascular disease: an update, Circulation, 2012, 126, 1301–1313 CrossRef PubMed.
- H. H. S. Chow, Y. I. A. Hakim, D. R. Vining, J. A. Crowell, J. Ranger-Moore, W. M. Chew, C. A. Celaya, S. R. Rodney, Y. Hara and D. S. Alberts, Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals, Clin. Cancer Res., 2005, 11, 4627–4633 CrossRef CAS PubMed.
- T. Unno, M. Tago, Y. Suzuki, A. Nozawa, Y. M. Sagesaka, T. Kakuda, K. Egawa and K. Kondo, Effect of tea catechins on postprandial plasma lipid responses in human subjects, Br. J. Nutr., 2005, 93, 543–547 CrossRef CAS PubMed.
- T. Unno, Y. Suzuki, A. Nozawa, Y. Sagesaka, T. Kakuda, K. Egawa and K. Kondo, Suppressive effect of tea catechins on elevation of postprandial serum triglycerides, Jpn. J. Nutr. Assess., 2005, 22, 207–212 CAS . (in Japanese).
- D. J. Gordon, J. Hyde, D. C. Trost, F. S. Whaley, P. J. Hannan, D. R. Jacobs and L. G. Ekelund, Cyclic seasonal variation in plasma lipid and lipoprotein levels. The Lipid Research Clinics Coronary Primary Prevention Trial Placebo Group, J. Clin. Epidemiol., 1988, 41, 679–689 CrossRef CAS PubMed.
- M. Kobayashi, M. Nishizawa, N. Inoue, T. Hosoya, M. Yoshida, Y. Ukawa, Y. M. Sagesaka, T. Doi, T. Nakayama, S. Kumazawa and I. Ikeda, Epigallocatechin gallate decreases the micellar solubility of cholesterol via specific interaction with phosphatidylcholine, J. Agric. Food Chem., 2014, 62, 2881–2890 CrossRef CAS PubMed.
- I. Ikeda, Y. Imasato, E. Sasaki, M. Nakayama, H. Nagao, T. Takeo, F. Yayabe and M. Sugano, Tea catechins decrease micellar solubility and intestinal absorption of cholesterol in rats, Biochim. Biophys. Acta, 1992, 1127, 141–146 CrossRef CAS.
- I. Ikeda, M. Kobayashi, T. Hamada, T. Tsuda, H. Goto, K. Imaizumi, A. Nozawa, A. Sugimoto and T. Kakuda, Heat-epimerized tea catechins rich in gallocatechin gallate and catechin gallate are more effective to inhibit cholesterol absorption than tea catechins rich in epigallocatechin gallate and epicatechin gallate, J. Agric. Food Chem., 2003, 51, 7303–7307 CrossRef CAS PubMed.
- J. S. Cohn, A. Kamili, E. Wat, R. W. Chung and S. Tandy, Dietary phospholipids and intestinal cholesterol absorption, Nutrients, 2010, 2, 116–127 CrossRef CAS PubMed.
- R. Hursel, W. Viechtbauer and M. S. Westertero-Plantenga, The effects of green tea on weight loss and weight maintenance: a meta-analysis, Int. J. Obes., 2009, 33, 956–961 CrossRef CAS PubMed.
- M. Asaka, K. Murai, R. Nakanishi and Y. Aoyama, An increase in antibacterial activity of green tea infusion by heat-treatment, Nippon Shokuhin Kagaku Kogaku Kaishi., 2000, 47, 708–715 CrossRef CAS . (In Japanese with English summary).
- T. Goto, Y. Yoshida, M. Kiso and H. Nagashima, Simultaneous analysis of individual catechins and caffeine in green tea, J. Chromatogr. A, 1996, 749, 295–299 CrossRef CAS.
- K. Tokunaga, Y. Matsuzawa, K. Ishikawa and S. Tarui, A novel technique for the determination of body fat by computed tomography, Int. J. Obes., 1983, 7, 473–445 Search PubMed.
- Z. Šidàk, Rectangular confidence region for the means of multivariate normal distributions, J. Am. Stat. Assoc., 1967, 62, 626–633 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |