Phenolic-rich lychee (Litchi chinensis Sonn.) pulp extracts offer hepatoprotection against restraint stress-induced liver injury in mice by modulating mitochondrial dysfunction
Dongxiao
Su†
ab,
Ruifen
Zhang†
a,
Cuilan
Zhang
a,
Fei
Huang
a,
Juan
Xiao
a,
Yuanyuan
Deng
a,
Zhencheng
Wei
a,
Yan
Zhang
a,
Jianwei
Chi
a and
Mingwei
Zhang
*ab
aSericultural & Agri-Food Research Institute, Guangdong Academy of Agricultural Sciences/Key Laboratory of Functional Foods, Ministry of Agriculture/Guangdong Key Laboratory of Agricultural Products Processing, Guangzhou 510610, China. E-mail: mwzhh@vip.tom.com; Fax: +86-20-8723 6354; Tel: +86-20-8723 7865
bDepartment of Food Science and Engineering, College of Life Science, Yangtze University, Jingzhou, Hubei 434025, P. R. China
First published on 5th November 2015
Abstract
The pulp from lychee, a tropical to subtropical fruit, contains large quantities of phenolic compounds and exhibits antioxidant activities both in vitro and in vivo. In the present study, we investigated the mechanisms underlying the hepatoprotective effects of lychee pulp phenolics (LPPs) against restraint stress-induced liver injury in mice. After 18 h of restraint stress, increased levels of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were observed. High levels of thiobarbituric acid reactive substances (TBARS) were also found. Restraint stress causes liver damage, which was protected against by LPP pretreatment at a dosage of 200 mg (kg d)−1 for 21 consecutive days. This treatment remarkably decreased the serum ALT, AST and TBARS levels, elevated the liver glutathione (GSH) content, and the activities of glutathione peroxidase (GPx), superoxide dismutase (SOD) and catalase (CAT). Furthermore, respiratory chain complex and Na+–K+–ATPase activities were enhanced in liver mitochondria, while mitochondrial membrane potential levels and reactive oxygen species (ROS) production decreased. Thus, treatment with LPPs ameliorated restraint stress-induced liver mitochondrial dysfunction. These results suggest that LPPs protect the liver against restraint stress-induced damage by scavenging free radicals and modulating mitochondrial dysfunction. Thus, lychee pulp may be a functional biofactor to mitigate oxidative stress.
1. Introduction
Previous studies have demonstrated that long-term exposure to stress triggers numerous health problems and lifestyle diseases, including cardiovascular injury1 and Alzheimer's disease.2 Increased oxidative stress and diminished antioxidant protection are the primary contributors to the development of stress-induced diseases. Phenolics in fruits and vegetables have potent antioxidant properties, which may help combat oxidative stress and improve the pro/antioxidant balance within the body.3Lychee, a tropical to subtropical fruit, has become increasingly popular throughout the world.4 Recent work has suggested that lychee pulp, which is the most commonly consumed part of the fruit, contains large quantities of phenolic compounds. Several phenolics, including quercetin, kaempferol, trans-cinnamic acid, gallic acid, chlorogenic acid, (+)-catechin, caffeic acid, (−)-epicatechin and rutin, have been detected in lychee pulp extracts via high-performance liquid chromatography (HPLC) in tandem with mass spectrometry.5–7 Our group previously isolated and purified major antioxidant compounds from lychee pulp. These included quercetin 3-o-rut-7-o-α-L-rha, rutin and (−)-epicatechin, which were subjected to cellular antioxidant activity and oxygen radical absorbance capacity assays. We also identified the structural formulae of these compounds using nuclear magnetic resonance and electrospray ionisation mass spectrometry.8 Previous studies reported that fruit extracts rich in quercetin 3-rut-7-rha could reduce serum cholesterol and triglycerides in diabetic rats fed with high cholesterol diet.9 Rutin exerts hepatoprotective effects10 and antioxidant properties.11 (−)-Epicatechin also provides cardiovascular protection12,13 as well as anti-inflammatory14 and antioxidant effects.
Lychee pulp phenolic (LPP) compounds exhibit excellent antioxidant activities, including ferric reducing antioxidant power as well as 2,2-diphenyl-1-picrylhydrazyl and oxygen radical absorbance capacity, as demonstrated by cellular antioxidant activity assays.5,7,15 However, whether in vitro methods can predict in vivo antioxidant activity is a matter of debate; as such, the in vivo data are more robust. It has been reported that lychee pulp extracts can decrease alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels following CCl4-induced liver injury.16 The hepatoprotective effects of lychee pulp extracts on CCl4-induced hepatotoxicity are believed to be related to lychee pulp's antioxidant properties.
Oxidative damage causes mitochondrial dysfunction and thus has a critical role in the development of human diseases.17,18 Restraint stress can induce serious liver injury manifested as increased serum ALT and malondialdehyde (MDA) levels19 and mitochondrial dysfunction in the liver.20,21 Mitochondria are important for energy production and play pivotal roles in basic cellular processes, such as pyruvate oxidation, free radical generation and fatty acid metabolism.17,22 Mitochondrial membrane potential (MMP) and ATP synthase (ATPase) activity are the key parameters used in the assessment of cellular energy metabolism. ATPase dysfunction has been associated with increased oxidative stress.18,23 LPP compounds exhibit good antioxidant activities and hepatoprotective effects on chemical-induced liver injury. However, the possible protection of LPP against the restraint stress-induced liver injury in mice and the mechanisms underlying the hepatoprotective effects of LPPs remain unknown.
This report extends the previous work regarding the structures, potential hepatoprotective effects and antioxidant activities of LPPs in mice subjected to restraint-induced stress. The mechanisms underlying LPP activity were further determined by evaluating the mitochondrial function, which appeared to be improved. The findings of the present study provide evidence to promote the use of lychee pulp as a functional biofactor to mitigate oxidative stress.
2. Materials and methods
2.1. Plant materials
Lychee (cv. Feizixiao), which is one of the main cultivars in South China, was purchased at commercial maturity from a local fruit market in Guangzhou, China. Uniformly mature fresh fruits were selected and washed with tap water. The pericarp and seed were then manually removed. The fresh lychee pulp was weighed and immersed in chilled acetone/water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v).
20, v/v).
2.2. Chemicals
Rutin and (−)-epicatechin were purchased from Sigma-Aldrich (St Louis, MO, USA). HPLC-grade acetic acid and acetonitrile were obtained from Thermo Fisher Scientific (Waltham, MA, USA). Deionised water was prepared using a Milli-Q water purification system (Billerica, MA, USA). ALT, AST, thiobarbituric acid reactive substances (TBARS), superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), xanthine oxidase (XOD) and Coomassie brilliant blue kits were all obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).2.3. Preparation and analysis of LPPs
LPPs were prepared as previously described.8 In brief, samples (10 kg) were extracted twice with 20 L of chilled acetone/water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v). The supernatants were combined and concentrated at 45 °C. The concentrated supernatant (1 L) was then fractionated on a HPD826 resin (Cangzhou Bonchem Co., Ltd, Cangzhou, China) column (∅ 10 cm, length 150 cm) to remove most of the non-phenolic compounds. Elution was performed using 10 L of deionised water and 95% aqueous ethanol (v/v). The organic phase fraction was collected, rotary evaporated and freeze-dried to produce LPP powder, which was then analysed using HPLC.
20, v/v). The supernatants were combined and concentrated at 45 °C. The concentrated supernatant (1 L) was then fractionated on a HPD826 resin (Cangzhou Bonchem Co., Ltd, Cangzhou, China) column (∅ 10 cm, length 150 cm) to remove most of the non-phenolic compounds. Elution was performed using 10 L of deionised water and 95% aqueous ethanol (v/v). The organic phase fraction was collected, rotary evaporated and freeze-dried to produce LPP powder, which was then analysed using HPLC.
The phenolic composition and contents of the extract were determined using the HPLC-DAD method, which has been described previously.15 Briefly, the extract was filtered before being applied to an Agilent Zorbox SB-C18 column (250 × 4.6 mm, 5 μm, Palo Alto, CA, USA) and eluted at a flow rate of 1.0 mL min−1 using a binary gradient that consisted of solution A (water/acetic acid 996![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v) and solution B (acetonitrile) as the mobile phase. Elution was monitored based on spectrophotometric absorption at 280 nm. The gradient elution programme was as follows: 0–40 min, solution A 95–75%; 40–45 min, solution B 75–65%; and 45–50 min, solution B 65–50%, followed by a 5 min equilibration period with 95% solution A. Peak identities were confirmed based on retention times determined for standard compounds. The total phenolic content was determined according to a previously described method,24 the moisture content was evaluated based on the methodology of Varith et al.,25 total sugar was measured spectrophotometrically according to the colorimetric method26 and protein was assayed using a modified Kjeldahl method.27
4 v/v) and solution B (acetonitrile) as the mobile phase. Elution was monitored based on spectrophotometric absorption at 280 nm. The gradient elution programme was as follows: 0–40 min, solution A 95–75%; 40–45 min, solution B 75–65%; and 45–50 min, solution B 65–50%, followed by a 5 min equilibration period with 95% solution A. Peak identities were confirmed based on retention times determined for standard compounds. The total phenolic content was determined according to a previously described method,24 the moisture content was evaluated based on the methodology of Varith et al.,25 total sugar was measured spectrophotometrically according to the colorimetric method26 and protein was assayed using a modified Kjeldahl method.27
The three major phenolics identified in the lychee pulp were quercetin 3-o-rutinoside-7-o-α-L-rhamnosidase, rutin and (−)-epicatechin. These components constituted 230.03 ± 15.14, 37.10 ± 3.11 and 25.11 ± 1.43 mg g−1 of the LPP freeze-dried powder, respectively. The total phenolic content accounted for up to 53.40 ± 2.37% of the total weight of the LPP freeze-dried powder; other components included moisture (10.00 ± 1.63%), total sugar (15.01 ± 0.42%) and protein (3.51 ± 0.14%) as well as the unknown ones.
2.4. Animals and experimental design
The experiment was approved by the Ethics Committee on Animals Experiment of Guangdong Academy of Agricultural Sciences. The animal care and treatment protocols complied with the national guidelines for the care and use of laboratory animals. Seven-week-old pathogen-free male Kunming (KM) mice were purchased from the Center of Laboratory Animal Science Research of Southern Medical University (Guangzhou, China) and acclimated for 1 week before the experiment. All animals were housed in a specific pathogen-free and environmentally controlled room under controlled temperature (23 ± 2 °C) and humidity (60 ± 5%) conditions with a 12 h light/dark cycle. The mice were fed a standard laboratory diet and provided with tap water ad libitum in accordance with the national standards outlined in “Laboratory Animal Requirements of Environment and Housing Facilities” (GB 14925–2010).The mice were randomly divided into five groups of 10 animals each. These groups were designated as normal control, model (restraint stress), LPP-L, LPP-M, and LPP-H treatment groups. LPP was dissolved in distilled water and the mice were orally administered 50, 100 and 200 mg per kg body weight LPP in the afternoon per day for 3 weeks. The animals of the normal control and model groups were given distilled water instead. The body weight and food intake were recorded twice a week. Thirty minutes after the final oral gavage, all animals except those in the normal control group were physically restrained in 50 mL polypropylene tubes with holes for 18 h before being sacrificed for serum and liver collection.
Serum was collected by centrifuging the blood samples at 3000g for 10 min at 4 °C and stored at −20 °C for later biochemical analysis. Liver samples were immediately excised, washed with chilled normal saline, blotted dry and weighed. The liver was cut into 2 portions, one of which was used for mitochondria isolation. The other portion was stored at −80 °C for later biochemical determination.
2.5. Measurement of ALT and AST activities in serum
The serum levels of ALT and AST were measured using an automatic biochemical analyser (7600 Series, Hitachi, Tokyo, Japan) and commercial kits. Enzyme activities are expressed in units per litre (UL−1).2.6. Measurement of SOD, T-AOC, GSH, GPx, CAT and XOD activities in serum and liver
The frozen liver samples were homogenized in chilled normal saline in an ice bath to obtain a 10% (w/v) liver homogenate. The supernatant was collected by centrifuging the homogenate at 4000g for 10 min at 4 °C. Then the homogenate supernatant was aliquoted and stored at −80 °C for biochemical analysis. The Bradford method28 was used to determine the protein concentrations of the liver homogenate with bovine serum albumin as a standard. SOD, T-AOC, GSH, GPx, CAT and XOD activities were quantified using a commercial kit according to the manufacturer's protocol. SOD activity was determined according to the xanthine and xanthine oxidase method. Briefly, 20 mL of the sample and 20 mL of the enzyme working solution were mixed thoroughly with 200 mL of WST-1 working solution (from the kit) in each well of a 96-well microplate. The plate was incubated at 37 °C for 20 min, and its absorbance at 450 nm was measured using an Infinite M200 PRO plate reader (Tecan Austria GmbH, Grodig, Austria). The total antioxidant capacity (T-AOC) of the liver was assessed using a colorimetric method. Briefly, the samples prepared as above and a working solution were added to test tubes containing phenanthroline substances and then incubated at 37 °C for 30 min. Fe3+ was reduced to Fe2+, which then formed complexes with phenanthroline that could be measured at 520 nm. GPx activity was measured using a spectrophotometric assay that involved calculating the catalysis rate of the oxidation of GSH to GSSG. The GSH content was determined using a DTNB-GSH reductase-recycling assay. The CAT levels in the liver were determined based upon the decomposition of H2O2, which can be measured based on absorbance at 415 nm. XOD catalysed the oxidation of hypoxanthine to xanthine to produce superoxide anion radicals, which eventually resulted in a fuchsia adduct measurable at 530 nm using an Infinite M200 PRO plate reader.2.7. Measurement of TBARS content in serum and liver
MDA is a product of lipid peroxidation and is therefore an indicator of this process. MDA reacts with thiobarbituric acid (TBA) to generate pink MDA-TBA adducts with measurable absorbance at 532 nm. The MDA contents in the serum and liver were determined by performing a TBARS assay using a commercially available kit according to the supplier's instructions and expressed as nmol MDA equivalents per mL or per mg of protein.2.8. Isolation of liver mitochondria
The mitochondria were isolated from liver tissue by using a commercial tissue mitochondria isolation kit (Beyotime Institute of Biotechnology, Guangzhou, China) according to the manufacturer's instructions. Briefly, 100 mg fresh mice liver tissues were cut into small pieces and homogenized with a Dounce tissue grinder in a 1 ml isolation reagent (supplied by the commercial kit) in an ice bath. The homogenate was centrifuged at 600g for 5 min at 4 °C. Then, the supernatant was centrifuged again at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min at 4 °C to obtain mitochondrial pellets. The mitochondrial pellets were then resuspended in 40 μL ice-cold preserving solution (supplied by the commercial kit). Three hundred milligrams of liver tissue of each animal were used to isolate mitochondria by repeating the above procedure 3 times and combining the suspension. In order to obtain enough mitochondria for later analysis, mitochondrial suspensions from two mice in the same group were mixed together. The activity of mitochondria was determined by Janus green B staining and observed with a microscope. Freshly isolated mitochondria were used to measure ROS generation and membrane potential, and the remaining mitochondrial suspension was aliquoted and stored at −80 °C for enzyme activity determination. The protein concentration in the mitochondrial suspension was determined with the Bradford method.28
000g for 10 min at 4 °C to obtain mitochondrial pellets. The mitochondrial pellets were then resuspended in 40 μL ice-cold preserving solution (supplied by the commercial kit). Three hundred milligrams of liver tissue of each animal were used to isolate mitochondria by repeating the above procedure 3 times and combining the suspension. In order to obtain enough mitochondria for later analysis, mitochondrial suspensions from two mice in the same group were mixed together. The activity of mitochondria was determined by Janus green B staining and observed with a microscope. Freshly isolated mitochondria were used to measure ROS generation and membrane potential, and the remaining mitochondrial suspension was aliquoted and stored at −80 °C for enzyme activity determination. The protein concentration in the mitochondrial suspension was determined with the Bradford method.28
2.9. Measurement of ROS generation in liver mitochondria
ROS were assayed using 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA).29 DCFH was oxidised in the presence of ROS into highly fluorescent 2′,7′-dichlorofluorescein (DCF). Briefly, 2 μL of DCFH-DA was added to 50 μL of the mitochondrial suspension and incubated in the dark at 37 °C for 15 min. The resulting fluorescence was measured using an Infinite M200 PRO plate reader with an excitation wavelength of 490 nm and an emission wavelength of 530 nm.2.10. Measurement of liver mitochondrial membrane potential (ΔΨm)
Mitochondrial membrane potentials (ΔΨm) were assayed using the fluorescent probe Rhodamine 123. The mitochondrial suspension samples (50 μL) prepared as described above were mixed with membrane potential reaction buffer (150 μL) and 1 μL Rhodamine 123 (1 mmol L−1) and accessed via flow cytometry at an excitation wavelength of 503 nm and an emission wavelength of 527 nm.2.11. Measurement of liver mitochondrial complexes
The activities of the mitochondrial complex I (NADH coenzyme Q10 oxidoreductase) and complex II (succinate coenzyme Q10 oxidoreductase) were measured spectrophotometrically according to ref. 30. Complex I activity was measured by recording the decreases in absorbance caused by the oxidation of NADH at 340 nm for 3 min. Complex II activity was measured by monitoring the reduction of 2,6-dichlorophenolindophenol at 600 nm for 3 min.2.12. Measurement of liver mitochondrial ATPase
The ATPase activity was determined at 37 °C by measuring the initial rate of inorganic phosphate release following ATP hydrolysis according to a previously described method.31 Na+–K+–ATPase activity was calculated by determining the difference between total ATPase activity (Na+–K+–Mg2+–ATPase) and Mg2+ ATPase activity via a colorimetric assay. One unit of ATPase activity is defined as the amount of enzyme required to produce 1 μmol Pi per mg protein per h via ATP hydrolysis.2.13. Statistical analysis
All data are presented as the mean ± standard deviation (SD). Biochemical indicators of the serum and liver were analysed in 10 animals from each group. ROS levels, mitochondrial membrane potential, mitochondrial complex activities and ATPase activity were determined for 5 replicates in each group due to the combination of mitochondrial suspensions from two mice. One-way analysis of variance (ANOVA) was used to assess inter-group differences. SPSS 13.0 was used for statistical analysis. In the cases of statistically significant differences, Dunnett's post hoc test was employed for multiple pairwise comparisons. Differences at p < 0.05 were considered statistically significant.3. Results
3.1. General conditions of mice
No animal died during the whole experimental period. The body weights and food intake of each group of animals were equivalent (data not shown). Abnormal signs related to toxicity were not observed in LPP treated animals including rough hair coats, diarrhea, ataxia, hunched posture, and hypoactivity.3.2. Effects of LPP on ALT and AST levels in the serum of restraint-stressed mice
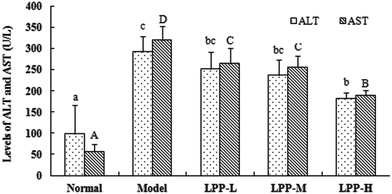
The mean serum ALT and AST levels in the normal control mice were 97.53 ± 67.87 U L−1 and 55.73 ± 18.93 U L−1, respectively. These values significantly increased to 292.60 U L−1 and 320.47 U L−1, corresponding to 1.98- and 4.75-fold increases over the baseline, respectively, after the mice were subjected to restraint-induced stress for 18 h (Fig. 1). All evaluated LPP doses decreased the serum ALT and AST levels to varying extents in the restraint-stressed mice. The highest dose of LPP significantly decreased the serum ALT and AST levels (p < 0.05).3.3. Effects of LPP on biochemical indicators in the serum and liver of restraint-stressed mice
Compared with the normal control group, serum SOD activity in the restraint-stressed mice dramatically decreased (p < 0.05), as indicated in Table 1. Conversely, in the restraint-stressed mice, the TBARS levels were markedly higher than in the normal control group (p < 0.05). LPP treatment increased the SOD activity of the restraint-stressed mice and the middle and high doses of LPP significantly increased serum SOD activity (p < 0.05). In contrast, the TBARS content decreased in all treatment groups; in the high-dose group, the TBARS content was reduced to the level of the normal control group (p > 0.05).| Normal | Model | LPP-L | LPP-M | LPP-H | |
|---|---|---|---|---|---|
| Normal: normal control group, administered distilled water by oral gavage for 3 consecutive weeks; Model: restraint stress group, administered distilled water by oral gavage for 3 consecutive weeks and then subjected to restraint stress starting 30 min after the final oral gavage and lasting for 18 h before being sacrificed; LPP groups: administered 50 (LPP-L), 100 (LPP-M), or 200 (LPP-H) mg LPP per kg body weight per day by oral gavage for 3 consecutive weeks and then subjected to restraint stress starting 30 min after the final oral gavage and lasting for 18 h before being sacrificed. Biochemical indicators were quantified using commercial kits. Values are reported as the mean ± SD (n = 10). Values within each column without a common letter are significantly different (p < 0.05). | |||||
| Serum | |||||
| SOD (U mL−1) | 230.31 ± 5.98d | 157.42 ± 8.24a | 163.56 ± 8.33a | 180.76 ± 7.32b | 203.77 ± 8.51c |
| TBARs (nmol MDA equivalent per mL) | 1.12 ± 0.44a | 3.04 ± 0.78b | 2.61 ± 0.98ab | 2.13 ± 0.55ab | 1.34 ± 0.33a |
| Liver tissue | |||||
| GSH (mg per g prot.) | 8.56 ± 1.39c | 3.06 ± 0.67a | 4.49 ± 0.85ab | 5.77 ± 1.01b | 6.34 ± 1.43b |
| TBARs (nmol MDA equivalent per mg prot.) | 6.57 ± 1.12a | 17.41 ± 1.61c | 13.07 ± 1.80b | 9.65 ± 2.11a | 8.04 ± 1.39a |
| GPx (U per mg prot.) | 43.76 ± 3.23c | 34.20 ± 1.98a | 36.77 ± 2.69ab | 39.60 ± 2.31abc | 40.53 ± 1.87bc |
| SOD (U per mg prot.) | 125.31 ± 7.30c | 99.75 ± 6.32a | 101.32 ± 6.31a | 109.73 ± 7.13ab | 118.75 ± 5.41bc |
| XOD (U per mg prot.) | 36.37 ± 1.38a | 48.70 ± 1.90c | 46.39 ± 3.46bc | 45.59 ± 3.82bc | 41.14 ± 2.03ab |
| CAT (U per mg prot.) | 325.49 ± 7.49c | 300.61 ± 5.79a | 302.45 ± 6.92a | 307.06 ± 9.13ab | 318.78 ± 5.55bc |
| T-AOC (U per mg prot.) | 0.58 ± 0.08c | 0.18 ± 0.10a | 0.24 ± 0.07a | 0.39 ± 0.06b | 0.43 ± 0.07b |
The phenolics in lychee pulp affected the biochemical indicators in the livers of the restraint-stressed mice. Compared with the normal control group, the restraint-stressed mice had a lower GSH content (p < 0.05) and T-AOC capacity and higher TBARS levels (p < 0.05), as indicated in Table 1. Meanwhile, SOD, GPx and CAT activities decreased, while XOD activity increased in the restraint-stressed group. LPP administration reduced the TBARS content from 17.41 to 8.04 nmol per mg protein, which was not significantly different from the value measured for the normal group (p > 0.05). The XOD content also decreased. Meanwhile, GPx activity increased in a dose-dependent manner in the restraint-stressed mice treated with lychee pulp extract. The high-dose group exhibited significantly higher GPx activity than the model group (p < 0.05) and did not significantly differ from the normal group (p > 0.05). Similar results were found for SOD and CAT. The GSH content and T-AOC capacity were accordingly elevated as SOD, GPx and CAT increased. Both the high-dose and middle-dose groups exhibited an increased GSH content and T-AOC capacity compared with the restraint-stressed group (p < 0.05).
3.4. Effects of LPPs on the mitochondrial function in the livers of restraint-stressed mice
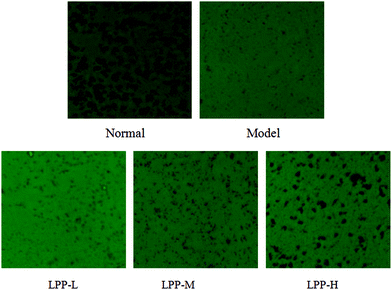
Nearly all the mitochondria became blue-green in colour in the normal control mice as visualized under a light microscope with a green filter (Fig. 2). There were markedly fewer blue-green mitochondria in the restraint-stressed group versus the normal control group. The number of blue-green mitochondria decreased in response to restraint stress but was partially and dose-dependently restored following pretreatment with lychee pulp extract.LPPs altered ROS generation and membrane potential in the liver mitochondria of the restraint-stressed mice. The mice subjected to restraint stress exhibited approximately 2-fold higher ROS production than the normal control group (p < 0.05) (Table 2). However, pretreatment with LPP significantly attenuated the elevation in the ROS level in a dose-dependent manner. The high-dose group had an approximately 40% lower ROS level than that in the restraint-stressed group (p < 0.05).
| Normal | Model | LPP-L | LPP-M | LPP-H | |
|---|---|---|---|---|---|
| The normal control group was administered distilled water by oral gavage for 3 consecutive weeks; the restraint stress model group was administered distilled water by oral gavage for 3 consecutive weeks and then subjected to restraint stress starting 30 min after the final oral gavage and lasting for 18 h before being sacrificed; LPP groups were administered 50 (LPP-L), 100 (LPP-M), or 200 (LPP-H) mg LPP per kg body weight per day by oral gavage for 3 consecutive weeks and then subjected to restraint stress starting 30 min after the final oral gavage and lasting for 18 h before being sacrificed. ROS were assayed using 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA). Mitochondrial membrane potentials were assayed using the fluorescent probe Rhodamine 123. Values are reported as the mean ± SD (n = 5). Values within each column without a common letter are significantly different (p < 0.05). | |||||
| ROS (RFU) | 452.62 ± 4.58a | 891.26 ± 32.13e | 791.96 ± 13.63d | 594.33 ± 13.58c | 553.73 ± 3.90b |
| Membrane potential (FI) | 9.61 ± 0.71a | 19.92 ± 1.26c | 18.84 ± 1.83c | 15.93 ± 2.36b | 13.98 ± 1.29b |
Restraint stress increased the MMP levels, as indicated in Table 2. When the mice were subjected to restraint stress, their MMP levels increased, which was indicated by their high fluorescence intensities. Approximately 2-fold higher fluorescence intensity was observed in the model group compared with the normal control group (p < 0.05). After the addition of LPPs, the fluorescence decreased; in the high-dose group, this value was approximately 26% lower than in the model group (p < 0.05).
LPPs exerted positive effects on respiratory chain complex and ATPase activities in the liver mitochondria from the restraint-stressed mice. The restraint-stress group did not exhibit significant differences in respiratory chain complex I or Mg2+–ATPase activities compared to the normal group. Pretreatment with lychee pulp extract also had little effect on these activities, as indicated in Table 3. However, restraint stress significantly decreased the respiratory chain complex total ATPase and Na+–K+–ATPase activities (p < 0.05) by 23% and 50%, respectively, compared to the normal mice. The administration of LPP partially blocked the restraint stress-induced depletion of respiratory complex and Na+–K+–ATPase activities. At 200 mg (kg d)−1, lychee pulp extract could reverse the decreased activity of liver Na+–K+–ATPase to a level comparable to the normal control group.
| Normal | Model | LPP-L | LPP-M | LPP-H | |
|---|---|---|---|---|---|
| The normal control group was administered distilled water by oral gavage for 3 consecutive weeks; the restraint stress model group was administered distilled water by oral gavage for 3 consecutive weeks and then subjected to restraint stress starting 30 min after the final oral gavage and lasting for 18 h before being sacrificed; LPP groups were administered 50 (LPP-L), 100 (LPP-M), or 200 (LPP-H) mg LPP per kg body weight per day by oral gavage for 3 consecutive weeks and then subjected to restraint stress starting 30 min after the final oral gavage and lasting for 18 h before being sacrificed. Mitochondrial complexes and ATPase activity were measured by spectrophotometric analysis. Values are reported as the mean ± SD (n = 5). Values within each column without a common letter are significantly different (p < 0.05). | |||||
| Complex I (nmol min−1 mg−1) | 746.25 ± 77.37a | 611.68 ± 108.54a | 656.54 ± 116.70a | 728.58 ± 76.76a | 698.67 ± 122.45a |
| Complex II (μmol min−1 mg−1) | 3.86 ± 0.56c | 0.88 ± 0.08a | 1.07 ± 0.14a | 1.16 ± 0.11a | 1.93 ± 0.26b |
| Total ATPase (μmol Pi per mg prot. per h) | 56.70 ± 8.33a | 43.32 ± 6.07b | 46.03 ± 5.86a | 49.14 ± 6.93a | 51.74 ± 6.97a |
| Mg2+–ATPase (μmol Pi per mg prot. per h) | 26.49 ± 2.50a | 26.90 ± 2.96a | 25. 94 ± 1.80a | 23.21 ± 3.31a | 20.27 ± 2.11a |
| Na+–K+–ATPase (μmol Pi per mg prot. per h) | 30.21 ± 5.83b | 16.42 ± 3.11a | 20.09 ± 4.06a | 25.93 ± 3.62ab | 31.47 ± 4.86b |
4. Discussion and conclusions
In recent years, numerous studies have demonstrated that lychee pulp contains large quantities of phenolic compounds, which scavenge superoxide anion and hydroxyl radicals both in vitro and in vivo.7,8,15,16 In accordance with our previous reports, the major phenolic compounds that we identified in lychee pulp were quercetin 3-o-rutinoside-7-o-α-L-rhamnosidase, rutin and (−)-epicatechin.8 Lv et al. determined that quercetin rhamnosyl-rutinoside accounted for the majority of the total phenolic content of a Feizixiao cultivar using HPLC–MS.32 This result is in accordance with our results and provides further support for our conclusions. Quercetin 3-rut-7-rha, rutin and (−)-epicatechin together accounted for up to 53.40% of the total weight of LPPs. All of these compounds also exhibited good antioxidant activity. Based on their predominant contents and significantly higher antioxidant activity than other phenolics in lychee pulp, quercetin 3-rut-7-rha, rutin and (−)-epicatechin would be the major contributors to lychee pulp antioxidant activity in the present study. Mosaddegh et al. found that Paliurus spina-christi fruit extracts rich in quercetin 3-rut-7-rha and rutin could reduce the levels of serum cholesterol and triglycerides in rats.9 Quercetin 3-rut-7-rha is a rutin derivative, with rhamnose substitution at the 7-hydroxyl group. A previous study showed that alkylation of the hydroxyl at position 7 enhanced the free radical scavenging activity.33 Therefore, it can be deduced that quercetin 3-rut-7-rha would exhibit good antioxidant activity in vivo. Rutin is used as a vasoprotectant34 and it exerts hepatoprotective effects10 because of its excellent antioxidant activities.10,11 Indeed, its antioxidant properties are an important component of its biological activities. (−)-Epicatechin also provides cardiovascular protection12,13 as well as exerts anti-inflammatory14 and antioxidant effects. Additionally, (−)-epicatechin and its metabolites protect against oxidative stress via their direct antioxidant effects.35It has been reported that LPPs can decrease the serum ALT and AST activities in livers that have suffered CCl4-induced damage.16 Increased serum ALT and AST activities also serve as markers of liver damage in restraint-stressed mice. In the present study, we observed that LPP pretreatment could attenuate restraint stress-induced liver damage in mice. This result arose from the antistress effects exerted by LPPs, as reflected by the recovery of ALT and AST activities in the serum. In addition, the mice subjected to restraint stress for 18 h exhibited accelerated formation of ROS.19 Imbalances between ROS scavenging and generation provoked by restraint stress can lead to excessive ROS levels. Harmful free radicals subsequently react with proteins and lipids, thereby resulting in oxidative damage. Lipid peroxidation was observed in the restraint-stressed mice. Our study suggested that the contents of TBARS, which are the end products of lipid peroxidation, were elevated in both serum and liver. Previous studies have indicated that immobilisation stress induces increased TBARS levels.36 Increases in the ALT, AST and TBARS levels in the serum and livers of the restraint-stressed mice suggest that this stress induces damage to the hepatic cell structure. However, the administration of lychee pulp extract significantly altered hepatic pathological damage, as reflected by the increased ALT, AST and TBARS levels.
There are two major intracellular antioxidant defence systems: low molecular weight antioxidants (such as GSH) and antioxidant enzymes (including GPx, SOD and CAT). GSH is an important intracellular antioxidant that utilizes a non-protein thiol to quench ROS.37 GPx, a GSH-related enzyme, can degrade lipid hydroperoxides into their corresponding alcohols. SODs catalyse the breakdown of superoxide anions into oxygen and hydrogen peroxide, which can be further catalysed by GPx and CAT enzymes into water.38 The mechanism driving XOD activity differs from those driving SOD, GPx and CAT activities. XOD overexpression can catalyse the oxidation of hypoxanthine to xanthine and generate unwanted free radicals.39 In our present study, mice subjected to restraint stress exhibited decreases in GPx, CAT, and SOD activities and GSH content and increases in the XOD activity and MDA levels in the liver. Similar changes in intracellular antioxidant defence system indicators have been reported by Li et al.40 All of the above results are associated with antioxidant capacity; this is further supported by the T-AOC data corresponding to the livers of the stressed mice. However, treatment with lychee pulp extract significantly altered the oxidative stress status and compensated for hepatocellular damage. Similar results have been previously observed in restraint-stressed mice pretreated with bilberry extracts or myelophil.36
ROS are produced during cellular respiration. Mitochondria are the most important cellular source of ROS.41 Dysfunctional mitochondria produce excessive amounts of ROS. Imbalances in the ROS levels result in damage to cellular macromolecules, such as membrane lipids.42 Because of the decreased level of GSH and reduced activities of SOD, GPx and CAT, increased ROS production was observed in the liver mitochondria of the restraint-stressed mice in the present study. Mitochondria are the specific targets of oxidative stress, which results in impaired mitochondrial function.43 The MMP level and ATPase activity are the key parameters used to assess mitochondrial functioning under physiological and pathological conditions.23,44 The observed 2-fold increase in the MMP levels in the liver mitochondria of the restraint-stressed mice clearly indicates that mitochondrial ROS generation is also activated by MMP, which was demonstrated via cytofluorometric analysis of ΔΨm. This conclusion is in accordance with the low ATPase content measured in cells from patients with mitochondrial ATPase deficiency.44 ATPase dysfunction decreases the mitochondrial synthesis of ATP. This results in elevated mitochondrial ROS production, which is associated with increased oxidative stress.44 Our results demonstrated that ATPase, Na+–K+–ATPase and mitochondria respiratory chain complex II activities decreased in restraint-stressed mice as a consequence of mitochondrial respiratory chain dysfunction. Na+–K+–ATPase acts as an energy-transducing ion pump and a signal transducer.45 When the Na+–K+–ATPase function is impaired, the fluidity of the mitochondrial membrane decreases. This change blocks electron transfer and therefore decreases respiratory chain complex activity.46,47 Pretreatment with LPPs could attenuate MMP and enhance respiratory chain complex and ATPase activities in mitochondria, thus blocking ROS generation. These observations are in agreement with Bao et al.'s report that treatment with bilberry extract enhanced mitochondrial complex II activity, Na+–K+–ATPase activity and MMP (ΔΨm) in restraint-stressed mice.20 These findings indicate that LPPs exhibit potent protective effects against restraint stress-induced liver damage by scavenging free radicals and modulating mitochondrial dysfunction.
In summary, restraint stress-induced liver damage is primarily caused by oxidative stress. Pretreatment with LPPs provides hepatoprotection, which is associated with mitochondrial protection and antioxidant activities. Lychee pulp is therefore a potential candidate functional food.
Acknowledgements
This work was supported by a Joint Fund from the NSFC and the Guangdong Provincial Government (U1301211), the National Nature Science Foundation of China (31571828), the Special Prophase Project of The National Basic Research Program of China (2012CB722904), and the National Key Technology Research and Development Program for the 12th Five-year Plan (2012BAD31B03).References
- A. Steptoe and M. Kivimaki, Nat. Rev. Cardiol., 2012, 9, 360–370 CrossRef CAS PubMed.
- R. Sultana and D. A. Butterfield, J. Alzheimer's Dis., 2010, 19, 341–353 Search PubMed.
- K. L. Wolfe and R. H. Liu, J. Agric. Food Chem., 2007, 55, 8896–8907 CrossRef CAS PubMed.
- Y. M. Jiang, X. W. Duan, D. Joyce, Z. Q. Zhang and J. R. Li, Food Chem., 2004, 88, 443–446 CrossRef CAS.
- K. Mahattanatawee, J. A. Manthey, G. Luzio, S. T. Talcott, K. Goodner and E. A. Baldwin, J. Agric. Food Chem., 2006, 54, 7355–7363 CrossRef CAS PubMed.
- S. Saxena, S. N. Hajare, V. More, S. Kumar, S. Wadhawan, B. B. Mishra, M. N. Parte, S. Gautam and A. Sharma, Food Chem., 2011, 126, 39–45 CrossRef CAS.
- R. Zhang, Q. Zeng, Y. Deng, M. Zhang, Z. Wei, Y. Zhang and X. Tang, Food Chem., 2013, 136, 1169–1176 CrossRef CAS PubMed.
- D. Su, H. Ti, R. Zhang, M. Zhang, Z. Wei, Y. Deng and J. Guo, Food Chem., 2014, 158, 385–391 CrossRef CAS PubMed.
- M. Mosaddegh, M. Khoshnood, M. Kamalinejad and E. Alizadeh, Iran. J. Pharm. Res., 2010, 3, 51–54 Search PubMed.
- K. H. Janbaz, S. A. Saeed and A. H. Gilani, Fitoterapia, 2002, 73, 557–563 CrossRef CAS PubMed.
- J. Yang, J. Guo and J. Yuan, LWT-Food Sci. Technol., 2008, 41, 1060–1066 CrossRef CAS.
- M. Morrison, R. van der Heijden, P. Heeringa, E. Kaijzel, L. Verschuren, R. Blomhoff, T. Kooistra and R. Kleemann, Atherosclerosis, 2014, 233, 149–156 CrossRef CAS PubMed.
- G. Gutiérrez-Salmeán, P. Ortiz-Vilchis, C. M. Vacaseydel, L. Garduño-Siciliano, G. Chamorro-Cevallos, E. Meaney, S. Villafaña, F. Villarreal, G. Ceballos and I. Ramírez-Sánchez, Eur. J. Pharmacol., 2014, 728, 24–30 CrossRef PubMed.
- E. J. B. Ruijters, G. R. M. M. Haenen, A. R. Weseler and A. Bast, PharmaNutrition, 2014, 2, 47–52 CrossRef CAS.
- D. Su, R. Zhang, F. Hou, M. Zhang, J. Guo, F. Huang, Y. Deng and Z. Wei, BMC Complementary Altern. Med., 2014, 14, 9 CrossRef PubMed.
- L. Bhoopat, S. Srichairatanakool, D. Kanjanapothi, T. Taesotikul, H. Thananchai and T. Bhoopat, J. Ethnopharmacol., 2011, 136, 55–66 CrossRef CAS PubMed.
- M. T. Lin and M. F. Beal, Nature, 2006, 443, 787–795 CrossRef CAS PubMed.
- M. K. Shigenaga, T. M. Hagen and B. N. Ames, Proc. Natl. Acad. Sci. U. S. A., 1994, 91, 10771–10778 CrossRef CAS.
- L. Bao, X.-S. Yao, C.-C. Yau, D. Tsi, C.-S. Chia, H. Nagai and H. Kurihara, J. Agric. Food Chem., 2008, 56, 7803–7807 CrossRef CAS PubMed.
- L. Bao, K. Abe, P. Tsang, J.-K. Xu, X.-S. Yao, H.-W. Liu and H. Kurihara, Fitoterapia, 2010, 81, 1094–1101 CrossRef PubMed.
- J. L. Madrigal, R. Olivenza, M. A. Moro, I. Lizasoain, P. Lorenzo, J. Rodrigo and J. C. Leza, Neuropsychopharmacology, 2001, 24, 420–429 CrossRef CAS PubMed.
- S. Chaiyarit and V. Thongboonkerd, Anal. Biochem., 2009, 394, 249–258 CrossRef CAS PubMed.
- G. Juan, M. Cavazzoni, G. T. Saez and J. E. O'Connor, Cytometry, 1994, 15, 335–342 CrossRef CAS PubMed.
- V. Dewanto, X. Wu, K. K. Adom and R. H. Liu, J. Agric. Food Chem., 2002, 50, 3010–3014 CrossRef CAS PubMed.
- J. Varith, P. Dijkanarukkul, A. Achariyaviriya and S. Achariyaviriya, J. Food Eng., 2007, 81, 459–468 CrossRef.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. A. Rebers and F. Smith, Anal. Chem., 1956, 28, 350–356 CrossRef CAS.
- A. Barker and R. Volk, Anal. Chem., 1964, 36, 439–441 CrossRef CAS.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS PubMed.
- D. HaMai, A. Campbell and S. C. Bondy, Free Radical Biol. Med., 2001, 31, 763–768 CrossRef CAS PubMed.
- M. Spinazzi, A. Casarin, V. Pertegato, M. Ermani, L. Salviati and C. Angelini, Mitochondrion, 2011, 11, 893–904 CrossRef CAS PubMed.
- M. Candeias, P. Abreu, A. Pereira and J. Cruz-Morais, J. Ethnopharmacol., 2009, 121, 117–122 CrossRef CAS PubMed.
- Q. Lv, M. Si, Y. Yan, F. Luo, G. Hu, H. Wu, C. Sun, X. Li and K. Chen, J. Funct. Foods, 2014, 7, 621–629 CrossRef CAS.
- M. Kessler, G. Ubeaud and L. Jung, J. Pharm. Pharmacol., 2003, 55, 131–142 CrossRef CAS PubMed.
- I. Erlund, T. Kosonen, G. Alfthan, J. Mäenpää, K. Perttunen, J. Kenraali, J. Parantainen and A. Aro, Eur. J. Clin. Pharmacol., 2000, 56, 545–553 CrossRef CAS PubMed.
- E. J. B. Ruijters, A. R. Weseler, C. Kicken, G. R. M. M. Haenen and A. Bast, Eur. J. Pharmacol., 2013, 715, 147–153 CrossRef CAS PubMed.
- H. G. Kim, J. S. Lee, J. S. Lee, J. M. Han and C. G. Son, J. Ethnopharmacol., 2012, 142, 113–120 CrossRef PubMed.
- M. Venukumar and M. Latha, Indian J. Physiol. Pharmacol., 2002, 46, 223–228 CAS.
- G. N. Landis and J. Tower, Mech. Ageing Dev., 2005, 126, 365–379 CrossRef CAS PubMed.
- H. Chung, S. Song, H. J. Kim, Y. Ikeno and B. Yu, J. Nutr., Health Aging, 1998, 3, 19–23 Search PubMed.
- W.-X. Li, Y.-F. Li, Y.-J. Zhai, W.-M. Chen, H. Kurihara and R.-R. He, J. Agric. Food Chem., 2013, 61, 6328–6335 CrossRef CAS PubMed.
- J.-J. Kuo, H.-H. Chang, T.-H. Tsai and T.-Y. Lee, Int. J. Mol. Med., 2012, 30, 673–679 CAS.
- K. K. Griendling, D. Sorescu, B. Lassègue and M. Ushio-Fukai, Arterioscler., Thromb., Vasc. Biol., 2000, 20, 2175–2183 CrossRef CAS.
- A. M. Schmeichel, J. D. Schmelzer and P. A. Low, Diabetes, 2003, 52, 165–171 CrossRef CAS PubMed.
- T. Mráček, P. Pecina, A. Vojtíšková, M. Kalous, O. Šebesta and J. Houštěk, Exp. Gerontol., 2006, 41, 683–687 CrossRef PubMed.
- Z. Xie and A. Askari, Eur. J. Biochem., 2002, 269, 2434–2439 CrossRef CAS PubMed.
- C. R. Hackenbrock, Trends Biochem. Sci., 1981, 6, 151–154 CrossRef CAS.
- E. Slater, J. Berden and M. Herweijer, Biochim. Biophys. Acta, Rev. Bioenerg., 1985, 811, 217–231 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |