Modulation of hepatocarcinogenesis in N-methyl-N-nitrosourea treated Balb/c mice by mushroom extracts
Srishti
Ramsaha
ag,
Vidushi S.
Neergheen-Bhujun
*ag,
Shalini
Verma
b,
Ashok
Kumar
c,
Rahul Kumar
Bharty
b,
Amit Kumar
Chaudhary
b,
Poornima
Sharma
d,
Ranjan Kumar
Singh
d,
Priya
Huzar Futty Beejan
e,
Kang
Kyung-Sun
f and
Theeshan
Bahorun
*g
aDepartment of Health Sciences, Faculty of Science, University of Mauritius, Réduit, Republic of Mauritius. E-mail: v.neergheen@uom.ac.mu; Fax: +230 465 6928; Tel: +230 4037412
bInstitute of Biosciences and Biotechnology, Chhatrapati Shahuji Maharaj University, Kanpur, Uttar Pradesh, India
cDeen Dayal Upadhyay Gorakhpur University, Gorakhpur, Uttar Pradesh, India
dDepartment of Physics, Banaras Hindu University, Varanasi, Uttar Pradesh, India
eMushroom Unit, Food and Agricultural Research and Extension Institute, La Brasserie, Republic of Mauritius
fDepartment of Veterinary Public Health, College of Veterinary Medicine, Seoul National University, Seoul, South Korea
gANDI Centre of Excellence for Biomedical and Biomaterials Research, CBBR, Building, MSIRI, University of Mauritius, Réduit, Republic of Mauritius. E-mail: tbahorun@uom.ac.mu; Tel: +230 4675582
First published on 3rd November 2015
Abstract
The hepatoprotective potential of edible mushrooms from Mauritius, namely Pleurotus sajor-caju and Agaricus bisporus was evaluated using an N-methyl-N-nitrosourea (MNU)-induced hepatocarcinogenesis Balb/c mice model. Mushroom extracts restored normal weight in MNU treated mice over a 3 month supplementation period. Blood parameter analyses indicated a clear modulation of hemoglobin concentration, leukocyte, platelet, lymphocyte, neutrophil, monocyte and eosinophil counts in MNU-induced mice (p < 0.05). Mushroom extract supplementation effectively reduced oxidative damage in MNU-primed mice, which was marked by a significant decrease in the extent of lipid peroxidation (p < 0.05) and a concomitant increase in the enzymatic antioxidant levels, primarily catalase, superoxide dismutase, glutathione reductase and peroxidase, and FRAP values (p < 0.05). DNA protective effects of the extracts were confirmed by Raman spectroscopy, where, the MNU–DNA interaction, as evidenced by an intense peak at 1254 cm−1, was normalized. The findings demonstrate hepatoprotective, immunomodulatory and anti-carcinogenic effects and suggest the use of mushrooms as potential dietary prophylactics in cancer chemoprevention.
1. Introduction
Cancer in all its guises continues to undermine global health, and is among the foremost causes of death worldwide, accounting for 8.2 million deaths in 2012, with a predicted rise of 13 million in the coming decades.1 Epidemiological studies have convincingly shown that lifestyle plays a major role in the aetiology of human cancers with approximately 90% of all cancers being linked to adverse lifestyle, and 30–40% associated with unhealthy diet.2 Numerous strategies leading to effective anticancer therapies have been adopted for several decades with, however, mitigated success as the morbidity and mortality from this disease have been unexpectedly high over the past few years.3The prevention of cancer through dietary intervention has thus gained considerable interest, thereby fueling the need for comprehensive data on natural biofactors with potential therapeutic and chemopreventive properties.4 Evidence from biochemical and molecular studies has revealed that phytophenolics, in view of their pluripharmacological properties, can exert modulatory actions in cancer cells by interacting with a wide range of cellular and molecular targets that are vital to the cell signaling machinery.5
There is currently an upsurge of interest in macrofungi (mushrooms) which have been part of the human diet and a source of both healthy food and antioxidants with potential chemopreventive properties. A number of clinical trials have assessed the benefits of medicinal mushrooms, in particular, Ganoderma lucidum, whereby their potential uses, individually and as adjuncts to cancer therapy were revealed.6 As natural immune-enhancers, mushroom extracts were reported to complement chemotherapy and radiation therapy by countering the side-effects of cancer such as nausea, bone marrow suppression, anemia, and lowered resistance.7
Pharmacological activities of mushrooms involve their antioxidant, antidiabetic, hypocholesterolemic, anti-tumor, anti-cancer, immunomodulatory, anti-allergic, nephroprotective, and anti-microbial properties.6,8,9 Mushrooms contain, in addition to antioxidant phytophenolics, polysaccharides that have been shown to exert antitumor effects against HeLa tumour cells.10 Sarangi et al.11 and Shah et al.12 also reported the immunomodulatory and antitumor properties of proteoglycans derived from the fruiting body and mycelia of Pleurotus ostreatus. In vitro and in vivo anticancer activities of white button mushrooms (Agaricus bisporous) have indicated their possible use in breast cancer treatment as they suppress aromatase activity and estrogen biosynthesis.13
In the light of rising cancer incidence and growing interest and demand for effective functional foods, the phenolic composition of mushroom extracts and their modulatory effects on hepatocarcinogenic Balb/c mice were studied. The immune-modulating effects of P. sajor-caju and A. bisporus extracts were examined for a number of haematological and biochemical parameters and the alteration of DNA bases and the backbone structure was studied by Laser Raman Spectroscopy.
2. Materials and methods
2.1. Chemicals and reagents
MNU was purchased from Sigma-Aldrich (Steinheim, Germany) and superoxide dismutase (from bovine liver, Lot #091M7019 V) was from Sigma-Aldrich Co. (St Louis, USA). Epinephrine bitartrate, oxidised glutathione, reduced glutathione, nicotinamide adenine dinucleotide phosphate, reduced tetrasodium salt (NADPH), isoamyl alcohol, 5,5-dithiobis (2-nitrobenzoic acid) (DTNB), ethylenediaminetetraacetic acid (EDTA), 2,4,6-tripyridyl-s-triazine (TPTZ), bovine liver catalase enzyme (Lot 000167575), reduced glutathione (Lot 0000046991) and molecular grade saturated phenol (w/10 mM Tris, 1 mM EDTA) were from Hi-Media (Mumbai, India). All the other reagents used for analysis were of analytical grade.2.2. Mushroom extracts
P. sajor-caju (ME1) was harvested from the mushroom unit of the Food and Agricultural Research and Extension Institute (FAREI) and A. bisporus (ME2) was purchased from S.K.C Surat & Co Ltd (Curepipe, Mauritius). The substrate used for the cultivation of P. sajor-caju consists of a mixture of sugarcane bagasse (20%), lime (10%) and crushed maize (10%). The lyophilised mushrooms were extracted as per the modified method of Barros et al.34 Lyophilised mushroom samples (500 g) were subjected to exhaustive extraction using 100% methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/v). After each extraction and overnight-maceration at 4 °C, filtration and evaporation to dryness were carried out at 37 °C. The extracts were re-dissolved in distilled water prior to lyophilisation.
2 w/v). After each extraction and overnight-maceration at 4 °C, filtration and evaporation to dryness were carried out at 37 °C. The extracts were re-dissolved in distilled water prior to lyophilisation.
2.3. Phytophenolic analyses
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water, 1
water, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (v/v), pH 2.5; solvent B: acetonitrile
9 (v/v), pH 2.5; solvent B: acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water, 1
water, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v), pH 2.5; adjusted with phosphoric acid). Gallic acid, (+)-catechin, chlorogenic acid, 3-coumaric acid, ferulic acid, gentisic acid, 3-hydroxybenzoic acid, protocatechuic acid, pyrogallol and trans-cinnamic acid and ergothioneine were identified and quantified by comparing their retention times and spectral data with those of their authentic standards at 275 nm and 320 nm. Mushroom extracts were analysed in five replicates and results were expressed as mg DW per ml of respective standards.
1 (v/v), pH 2.5; adjusted with phosphoric acid). Gallic acid, (+)-catechin, chlorogenic acid, 3-coumaric acid, ferulic acid, gentisic acid, 3-hydroxybenzoic acid, protocatechuic acid, pyrogallol and trans-cinnamic acid and ergothioneine were identified and quantified by comparing their retention times and spectral data with those of their authentic standards at 275 nm and 320 nm. Mushroom extracts were analysed in five replicates and results were expressed as mg DW per ml of respective standards.
2.4. Experimental design
Balb/c mice of either sex were housed under standard conditions (20 ± 2 °C; 65 ± 15% relative humidity). 120 animals of the same age (7 weeks) and weight (20 ± 2 g) were selected for the study. The mice were routinely examined for any symptoms of ill health and any visible morphological changes. Furthermore, the body weight was monitored throughout the treatment period and the liver weight was noted after sacrifice. The animals were studied 24 hours after the last dose. The mice were sacrificed at scheduled periods and complete autopsies were performed. Liver samples were stored and used for DNA isolation. The study protocol was in accordance with the rules and regulations of the Chhatrapati Shahuji Maharaj University and ethical clearance was obtained from the University Ethical Committee (Ethical Clearance Reference: 1589/PO/a/12/CPCSEA).2.5. Treatment groups
After an acclimatization period of 3 weeks, the animals were randomly assigned to 12 groups, each having 10 mice. Group I (control or normal mice) was given only phosphate buffer saline (PBS), while Group II mice were given the carcinogen N-methyl, N-nitrosourea (MNU) at a concentration of 50 mg per kg body weight (b.w.) (stock: 1 g per 100 ml) intraperitoneally (i.p.). Mice were induced with MNU and simultaneously given oral extract treatment in the Groups III (150 mg kg−1 ME1 + MNU, i.p.), IV (300 mg kg−1 ME1 + MNU, i.p.), V (450 mg kg−1 ME1 + MNU, i.p.), VI (600 mg kg−1 ME1 + MNU, i.p.), VIII (150 mg kg−1 ME2 + MNU, i.p.), IX (300 mg kg−1 ME2 + MNU, i.p.), X (450 mg kg−1 ME2 + MNU, i.p.) and XI (600 mg kg−1 ME2 + MNU, i.p.). Mice of Groups VII (600 mg kg−1 ME1) and XII (600 mg kg−1 ME2) were given only mushroom extract treatment. The treatment period for the twelve groups lasted 3 months (92 days).The dose selection for this study was based on the level of ergothioneine, a natural antioxidant, reported in mushroom species and on its bioavailability in the human body which is estimated within a range of 1 to 4 mg per 100 ml blood.15 The amount of ergothioneine in an average adult of 64 kg would be 44–176 mg (concentration: 0.69–2.76 mg per kg body weight). Dose selected for the mice weighing around 20 g was therefore estimated based on this amount. Considering the fact that the mushroom extracts contain a cocktail of metabolites and since the Pleurotus sp. (oyster mushrooms) have been reported to contain about 119 mg ergothioneine per kilogram of mushrooms,16 a dose of 150–600 mg per kg body weight was selected.
2.6. Biochemical analyses
Blood samples were collected via retro orbital sinus bleeding, without using any anticoagulant. Centrifugation at 4695g (5000 rpm) was carried out for 10 minutes to separate the serum. Liver tissue homogenate (10%) was prepared by homogenising 0.1 g of tissue in 1 ml of cold buffer-pH 7.5 (50 mM phosphate buffer and 1 mM EDTA), followed by centrifugation at 10, 285g (10,000 rpm) for 10 minutes at 4 °C. All the assays used for the biochemical analyses were optimised for mice serum and liver tissue homogenate.2.7. Haematological analysis
Prior to cervical dislocation of mice, blood samples were collected between 9.00 and 10.00 a.m. after overnight fasting and dispensed in EDTA-K2 containing tubes (Sarstedt, Germany) for haematological studies. The following were assessed: red blood cell (RBC) count, hemoglobin concentration, platelet count, leucocytes, lymphocytes, neutrophils, monocytes and eosinophils.2.8. DNA isolation
DNA from the liver samples of all the experimental mice was isolated using a standard phenol–chloroform protocol.232.9. Raman analysis
DNA isolated from the liver of Balb/c mice was used for Raman spectral analysis. The spectra of all four experimental DNA samples were recorded on a micro-Raman setup from Renishaw, UK, equipped with a grating of 1800 lines per mm and a peltier cooled CCD. GRAM-32 software was used for data collection. DNA (10 ml) was placed in a quartz cell on an automated X–Y stage below the Olympus long distance 50× microscope objective. The accumulation time for one window was selected as 60 s and 3 spectra were accumulated in each window. With these experimental parameters, reasonably good quality Raman spectra were obtained. In one window, approximately 800 cm−1 regions were covered. The resolution of the spectrometer was slightly better than 1 cm−1. A MX50 A/T Olympus microscope (Florida, USA) was attached to the spectrometer. It focused the laser light onto the sample and collected the scattered light at 180° scattering geometry. The 514.5 nm wavelength line of Ar+ laser was used as an excitation source for DNA samples. The recorded data were first saved in “.spc” extension files and were then converted to ASC II files. The Raman spectra were recorded repeatedly to ensure reproducibility of the results.2.10. Data analysis
Mean slope analysis was carried out to assess significant differences in weight gain for the 12 different treatment groups in the animal studies. Trend lines were generated for each replicate of the different groups and the slope analysis was carried out using statistical software STATISTICA (Release 7). The specific activity of the enzymes in the different biochemical assays, mean and standard deviation were calculated and one-way ANOVA, followed by LSD test at 5% significance level was applied to assess any significant differences between the different treatment groups, using STATISTICA. The Raman spectra recorded as ASC II files compatible with Spectra Calc and Excel software, and Origin 9.1 (Graphing and Analysis) were used for generating the spectra for the 12 different treatment groups.3. Results
3.1. Polyphenolic analysis of mushroom extracts
The total phenolic content of mushroom extracts varied widely, with the highest level recorded in the A. bisporus extract (133.7 ± 3.2 mg per g DW) and the lowest in P. sajor-caju (33.3 ± 0.9 mg per g DW). The extracts were generally poor in flavonoids (P. sajor-caju-4.6 ± 0.1 and A. bisporus-0.5 ± 0.02 mg per g DW) and negligible in proanthocyanidins. Gallic acid (P. sajor-caju-356.9 ± 38.9 μg per g DW; A. bisporus-726.2 ± 4.3 μg per g DW), pyrogallol (P. sajor-caju-2831.3 ± 105.8 μg per g DW; A. bisporus-2354.7 ± 88.6 μg per g DW) and protocatechuic acid (P. sajor-caju-630.0 ± 15.2 μg per g DW; A. bisporus-84.9 ± 16.1 μg per g DW) were the major phenolic acids detected in the mushroom extracts. L-Ergothioneine, a potent antioxidant, was identified in micro amounts in the two species (P. sajor-caju-2518.9 ± 22.2 μg per g DW; A. bisporus-2261.2 ± 14.6 μg per g DW).3.2. Effect on body and liver weight
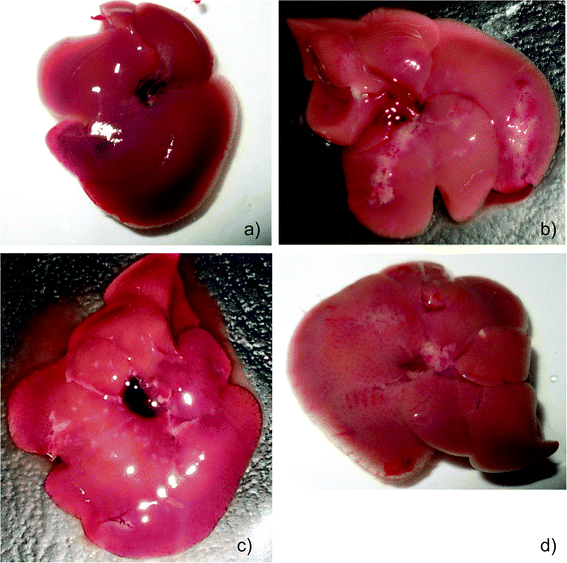
The animals in all the groups experienced an increase in weight during the first week of treatment with visible differences in growth patterns observed from day 14. MNU treated mice (group II) showed a significant decrease in body weight after 22 days compared to the PBS and extract treated groups. MNU-primed mice showed ill health symptoms like loss of weight, uneven shedding of hairs, decreased activity, hunched posture, thin appearance, labored and rapid breathing, at the end of the experimentation period, while control mice remained healthy.Moreover, the liver from MNU treated mice, showed formation of several micronodular lesions appearing as small islands or large stripes. These lesions eventually altered the normal architecture of the liver lobes with continued carcinogen exposure (Fig. 1a and b). The 56% decrease in body weight of MNU-primed mice by the end of the treatment period, was modulated by extract supplementation in groups III (48%), IV (36%), V (45%), VI (42%), VIII (46%), IX (55%), X (51%) and XII (51%).
Mice treated with ME1 and ME2 at 600 mg per kg b.w showed an increase in weight close to the control group. Treatment with ME2 (450 mg kg−1) and ME1 (300 mg kg−1) along with MNU, restored near normal growth and considerably reduced uneven shedding of hairs in Balb/c mice. Moreover, supplementation at these concentrations reduced the formation of micronodular lesions and restored the dark red colour of a normal liver (Fig. 1c and d).
The liver/body weight ratio is highly indicative of tumor presence. In MNU treated mice, the liver weight was 275 mg higher than the control mice, thus resulting in an increase of 2.8 folds in the liver/body weight ratio. Significant differences in the organ weight were observed for the liver isolated from the different treatment groups (p < 0.05). Mushroom extracts (groups VII and XII) restored the liver weight close to the control mice. Treatment in groups III, V, VIII, X and XI, showed protective effects by reducing the high liver/body weight ratio of MNU by 53% (p < 0.05). Overall, the ratios for all the treatments groups except that of Group II were within the range of 3–5% (Table 1).
| Treatment groups | Liver weight (g) | Body weight (g) | Liver/body weight ratio (%) |
|---|---|---|---|
| Data expressed as mean ± standard deviation (n = 5); ANOVA and Fisher's LSD were carried out at 5% significance; similar superscripts within the columns represent no significant differences between the treatment groups. | |||
| I-Normal (PBS only) | 1.359j ± 0.019 | 38.0a ± 2.8 | 3.6d ± 0.3 |
| II-MNU only | 1.634a ± 0.005 | 16.6d ± 2.8 | 10.1a ± 2.0 |
| III-ME1 (150 mg kg−1) + MNU | 1.544c ± 0.005 | 31.6b ± 2.7 | 4.9c ± 0.5 |
| IV-ME1 (300 mg kg−1) + MNU | 1.452g ± 0.002 | 25.8c ± 2.9 | 5.7b ± 0.6 |
| V-ME1 (450 mg kg−1) + MNU | 1.494f ± 0.005 | 29.8c ± 4.8 | 5.1c ± 0.8 |
| VI-ME1 (600 mg kg−1) + MNU | 1.553b ± 0.002 | 28.5c ± 2.9 | 5.5b ± 0.5 |
| VII-ME1 only (600 mg kg−1) | 1.323l ± 0.009 | 31.7b ± 2.8 | 4.2c ± 0.4 |
| VIII-ME2 (150 mg kg−1) + MNU | 1.533d ± 0.002 | 30.4c ± 0.9 | 5.0c ± 0.2 |
| IX-ME2 (300 mg kg−1) + MNU | 1.397h ± 0.005 | 36.8a ± 3.0 | 3.8d ± 0.3 |
| X-ME2 (450 mg kg−1) + MNU | 1.374i ± 0.003 | 33.7b ± 2.1 | 4.1c ± 0.2 |
| XI-ME2 (600 mg kg−1) + MNU | 1.511e ± 0.008 | 33.5b ± 2.7 | 4.5c ± 0.4 |
| XII-ME2 only (600 mg kg−1) | 1.340k ± 0.001 | 38.0a ± 1.1 | 3.5d ± 0.1 |
3.3. Protein concentration and biochemical analysis
Serum protein levels were lowest in mice from groups II and IX (p < 0.05). MNU treated mice, showed comparatively lower levels of protein in both serum and liver homogenate. Protein levels in mice serum from groups VII and XII, where solely extracts were given, were comparable to that of the control (Table 2). In the liver homogenate samples, the lowest protein concentration was observed in the group VIII and the highest in group V (p < 0.05).| Treatment groups (n = 5) | Serum protein concentration (μg protein per ml) | Liver tissue homogenate protein concentration (μg protein per ml) |
|---|---|---|
| Data expressed as mean ± standard deviation (error bars) (n = 5); ANOVA and Fisher's LSD were carried out at 5% significance; similar superscripts within the columns represent no significant differences between the treatment groups. | ||
| I-Normal (PBS only) | 21.5a,b ± 1.6 | 9.0c ± 0.1 |
| II-MNU only | 19.0c ± 1.1 | 5.7g ± 0.1 |
| III-MNU + ME1 (150 mg kg−1) | 20.0b ± 0.5 | 8.5d ± 0.2 |
| IV-MNU + ME1 (300 mg kg−1) | 20.9b ± 1.9 | 4.9h ± 0.2 |
| V-MNU + ME1 (450 mg kg−1) | 21.4b ± 0.9 | 10.0a ± 0.2 |
| VI-MNU + ME1 (600 mg kg−1) | 20.9b ± 2.8 | 7.6f ± 0.2 |
| VII-ME1 only (600 mg kg−1) | 19.8b ± 1.9 | 9.3b ± 0.1 |
| VIII-MNU + ME2 (150 mg kg−1) | 22.5a ± 1.1 | 3.8i ± 0.1 |
| IX-MNU + ME2 (300 mg kg−1) | 18.8c ± 1.1 | 8.1e ± 0.2 |
| X-MNU + ME2 (450 mg kg−1) | 21.0b ± 0.5 | 8.0e ± 0.1 |
| XI-MNU + ME2 (600 mg kg−1) | 20.8b ± 1.0 | 9.2b ± 0.2 |
| XII-ME2 only (600 mg kg−1) | 23.2a ± 0.4 | 9.4b ± 0.2 |
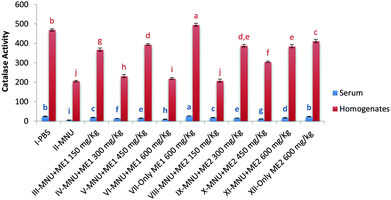
Investigation of CAT activity in both serum and liver homogenate samples showed significant differences for the different treatment groups (p < 0.05) (Fig. 2). MNU treatment reduced both serum and liver CAT activity (p < 0.05). Highest liver CAT activity was noted in Group VII (495.8 ± 7.6 mmol H2O2 per ml per mg protein), followed by control (469.4 ± 5.8 mmol H2O2 per ml per mg protein) and Group XII (412.4 ± 8. mmol H2O2 per ml per mg protein) (p < 0.05). Extract supplementation in both the groups upregulated serum and liver CAT activities in mice whereby administration of MNU decreased the enzymatic activity by 78% and 56% respectively, compared to healthy control.
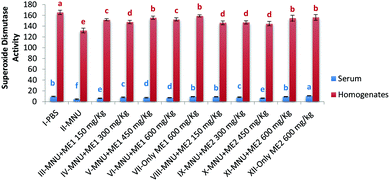
In comparison with healthy controls, mice injected with MNU showed a decline of 20% and 52% in serum and liver SOD activity, respectively. Mushroom treatment had a modulating effect with the highest superoxide dismutase (SOD50) activity being assayed in the serum of Group XII (10.8 ± 0.4 U ml−1) and Group I (165.5 ± 4.1 U per mg protein) liver homogenate (p < 0.05). Lowest activity (SOD50) occurred in MNU treated mice for both serum (4.5 ± 0.9 U ml−1) and liver homogenate samples (131.9 ± 4.4 U per mg protein) (p < 0.05). No significant differences were observed in serum SOD50 activity for Groups I, VII, VIII and IX; while the liver SOD50 activity for Groups VII, V, XI and XII were similar (p > 0.05) (Fig. 3).
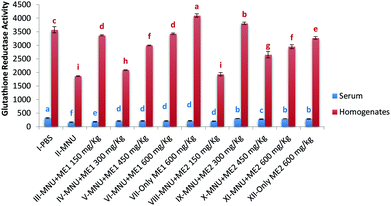
Administration of MNU decreased liver and serum GR activity (serum-166.0 ± 14.4 nmol NADPH oxidized per min per ml); liver-1865.8 ± 14.0 nmol NADPH oxidized per min per mg protein) by 48–49% compared to healthy mice, while extract treatment upregulated GR activity. The highest serum GR activity was noted in Group I (322.4 ± 17.0 nmol NADPH oxidized per min per ml), while in the liver, highest GR activity was obtained for Group VII (4099.6 ± 62.8 nmol NADPH oxidized per min per mg protein) (p < 0.05). Lowest GR activity was measured in MNU treated mice (Fig. 4). No significant differences in serum GR activity of Groups IX, XI and XII were observed, while for liver GR activity, groups VI and III; V and XI were similar (p > 0.05).
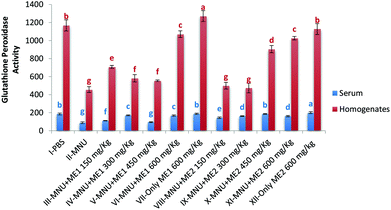
Extract supplementation also modulated liver GSH-Px activity in MNU induced mice with a fall of 50% and 45% in serum and liver respectively. Serum GSH-Px activity was similar in groups VII, X and I; while liver GSH-Px activity was comparable for control and Group XII (p > 0.05) (Fig. 5). Significant levels of GSH-Px were noted in Group XII serum (200.6 ± 9.8 μmol per min per ml) and Group VII liver (1271.9 ± 60.7 μmol per min per mg protein) samples (p < 0.05). GSH-Px activity was lowest in serum (88.5 ± 10.0 μmol per min per ml) and liver homogenate (456.9 ± 32.1 μmol per min per mg protein) of MNU treated mice (p < 0.05).
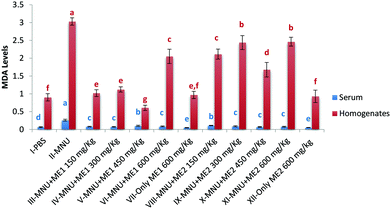
Lipid peroxidation, as measured by TBARS levels, was important in MNU-treated mice serum (0.3 ± 0.0 nmol TBARS per ml) and liver homogenate (3.0 ± 0.1 nmol TBARS per mg protein), while its occurrence was the lowest in the serum of Group VII (0.04 ± 0.0 nmol TBARS per ml) and XII (0.5 ± 0.1 nmol TBARS per mg protein), and the liver sample of Group V (0.6 ± 0.1 nmoles TBARS per mg protein) (p < 0.05). Lower levels of liver TBARS were measured for control, and extract treated groups (XII and VII) with no statistical difference amongst them (p > 0.05) (Fig. 6). Mushroom treatment reduced lipid peroxidation in MNU induced mice, whereby a rise in serum (75%) and liver (70%) MDA levels, was apparent compared to normal mice.
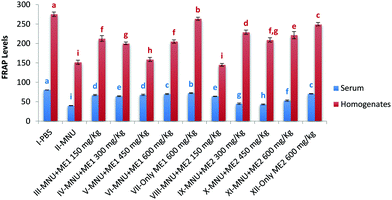
Extract treatment without carcinogen more effectively reduced circulating levels of TBARS, compared to PBS-treated mice, thus indicating an improved antioxidant status. The highest ferric reducing potential however was recorded in serum and liver homogenate samples of Groups I (serum: 80.4 ± 0.2 mmol Fe(II) per ml; liver: 275.4 ± 5.7 mmol Fe(II) per mg protein), VII (serum: 72.5 ± 1.0 mmol Fe(II) per ml; liver: 263.8 ± 3.8 mmol Fe(II) per mg protein) and XII (serum: 70.5 ± 0.6 mmoles Fe(II) per ml; liver: 249.6 ± 4.4 mmol Fe(II) per mg protein) (p < 0.05). FRAP levels in serum (39.9 ± 0.6 mmoles Fe(II) per ml) and liver (151.9 ± 5.2 mmol Fe(II) per mg protein) of MNU treated mice were very low (p < 0.05) (Fig. 7). Extract supplementation upregulated serum and liver FRAP levels in mice whereby administration of MNU decreased the FRAP value by 50% and 45% respectively compared to the healthy mice.
3.4. Haematological analyses
Analysis of blood parameters showed that the haemoglobin concentration of MNU treated mice was 31% lower than the control group and was well below the normal range of 12–17 g dL−1. The highest haemoglobin level was measured in the control mice and groups IV, VI, VII and XII (12.5–13.3 g dL−1). The leucocyte counts for all the groups were within the normal range of (4–10) × 103 cell per cumm, except for MNU (39% higher than control). Furthermore, significantly high levels of eosinophils (82%), monocytes (76%), and red blood cell count (27%) were observed in the MNU treated mice in comparison with control (p < 0.05) (Table 3).| Treatment groups (n = 10) | Hb (g dL−1) | TLC × 103 (cells per cumm) | Polymorphs (%) | Lymphocytes (%) | Eosinophils (%) | Monocytes (%) | Basophils (%) | Platelets (lacs per mm) | RBC (million cells per cumm) |
|---|---|---|---|---|---|---|---|---|---|
| Data expressed as mean ± standard deviation (error bars) (n = 10); ANOVA and Fisher's LSD were carried out at 5% significance; similar superscripts within the columns represent no significant differences between the treatment groups. | |||||||||
| I-Normal (PBS only) | 13.0b ± 0.8 | 8.6d ± 0.7 | 67.6b ± 3.3 | 41.6a ± 3.0 | 2.4c ± 1.2 | 2.9b ± 1.1 | 0 ± 0.00 | 5.8c ± 0.6 | 7.9b ± 1.0 |
| II-MNU only | 9.0d ± 0.7 | 13.2a ± 0.6 | 73.5a ± 4.3 | 20.8e ± 2.6 | 13.4a ± 1.3 | 12.2a ± 1.1 | 0 ± 0.00 | 9.6a ± 0.8 | 10.7a ± 1.4 |
| III-MNU + ME1(150 mg kg−1) | 12.0b ± 0.8 | 9.0d ± 0.8 | 60.6c ± 7.4 | 29.0c ± 2.4 | 3.4b ± 1.7 | 3.5b ± 1.7 | 0 ± 0.00 | 6.3c ± 1.3 | 6.5c ± 1.5 |
| IV-MNU + ME1(300 mg kg−1) | 12.5b ± 1.1 | 9.5c ± 0.6 | 66.4b ± 7.7 | 21.8e ± 1.9 | 3.3b ± 1.5 | 3.0b ± 1.9 | 0 ± 0.00 | 6.7b ± 1.1 | 5.5d ± 1.1 |
| V-MNU + ME1(450 mg kg−1) | 12.1b ± 1.6 | 8.8d ± 0.8 | 62.0c ± 8.9 | 21.5e ± 2.3 | 3.5b ± 1.8 | 3.2b ± 1.3 | 0 ± 0.00 | 6.6b ± 1.0 | 5.0d ± 0.8 |
| VI-MNU + ME1(600 mg kg−1) | 12.5b ± 1.6 | 9.7c ± 1.0 | 67.8b ± 8.5 | 22.1e ± 2.3 | 3.4b ± 1.6 | 2.5b ± 1.3 | 0 ± 0.00 | 7.0b ± 0.8 | 5.0d ± 1.2 |
| VII-ME1 only (600 mg kg−1) | 13.3a ± 1.4 | 8.8d ± 0.5 | 66.6b ± 7.5 | 24.2e ± 1.6 | 3.7b ± 1.8 | 2.7b ± 1.4 | 0 ± 0.00 | 6.4c ± 0.9 | 5.8d ± 1.1 |
| VIII-MNU + ME2(150 mg kg−1) | 11.3c ± 1.0 | 10.6b ± 1.0 | 66.0b ± 1.4 | 26.8d ± 3.0 | 2.8b ± 1.3 | 2.3b ± 1.1 | 0 ± 0.00 | 6.7b ± 0.8 | 6.8c ± 0.8 |
| IX-MNU + ME2(300 mg kg−1) | 10.9b ± 1.0 | 10.4b ± 1.0 | 72.2a ± 5.1 | 26.2d ± 6.9 | 3.1b ± 1.7 | 2.9b ± 1.0 | 0 ± 0.00 | 7.6b ± 1.4 | 6.1c ± 1.0 |
| X-MNU + ME2(450 mg kg−1) | 11.7c ± 1.6 | 10.9b ± 0.7 | 63.1c ± 3.3 | 30.0c ± 4.5 | 3.6b ± 1.5 | 2.4b ± 1.0 | 0 ± 0.00 | 7.4b ± 0.6 | 7.2b ± 0.7 |
| XI-MNU + ME2(600 mg kg−1) | 12.2b ± 1.4 | 10.5b ± 1.0 | 67.7b ± 4.2 | 30.9c ± 4.4 | 3.4b ± 1.7 | 3.5b ± 1.8 | 0 ± 0.00 | 7.6b ± 1.1 | 7.8b ± 1.0 |
| XII-ME2 only (600 mg kg−1) | 14.1a ± 0.5 | 8.9d ± 0.5 | 68.3a ± 3.2 | 38.1b ± 4.3 | 3.9b ± 1.7 | 3.3b ± 1.3 | 0 ± 0.00 | 7.0b ± 1.0 | 6.1c ± 0.7 |
Extract treatments in groups VII and XII (without carcinogen), gave a blood parameter profile close to that of normal mice. When these mushroom extracts were administered along with MNU, protective effects were observed at all concentrations tested (p < 0.05). The decline in haemoglobin concentration was prevented in extract treated groups: III (25%), IV (28%), V (26%), VI (28%), VIII (20%), IX (18%), X (23%) and XII (26%) and lymphocyte levels were also reduced in these groups: III (28%), IV (5%), V (3%), VI (6%), VIII (22%), IX (21%), X (31%) and XII (33%).
Moreover, mushroom supplementation reduced the elevated levels of leukocytes in MNU-induced mice in groups: III (32%), IV (28%), V (33%), VI (26%), VIII (20%), IX (22%), X (17%) and XII (21%). Abnormally high eosinophil levels were also reduced in groups III (75%), IV (75%), V (74%), VI (75%), VIII (79%), IX (77%), X (73%) and XII (75%) groups. The high percentage of monocytes was downregulated by extract treatment: III (71%), IV (75%), V (74%), VI (80%), VIII (81%), IX (76%), X (80%) and XII (71%).
The administration of MNU led to a decline in the platelet count in group II mice, which was restored to a certain extent by mushroom treatment in groups: III (34%), IV (30%), V (31%), VI (26%), VIII (30%), IX (21%), X (22%) and XII (20%). In general, haematologcial parameters were comparable when MNU primed mice were treated with ME1 at concentrations 150 mg per kg b.w and 450 mg per kg b.w and ME2 at concentrations 300 mg per kg b.w and 600 mg per kg b.w (p > 0.05).
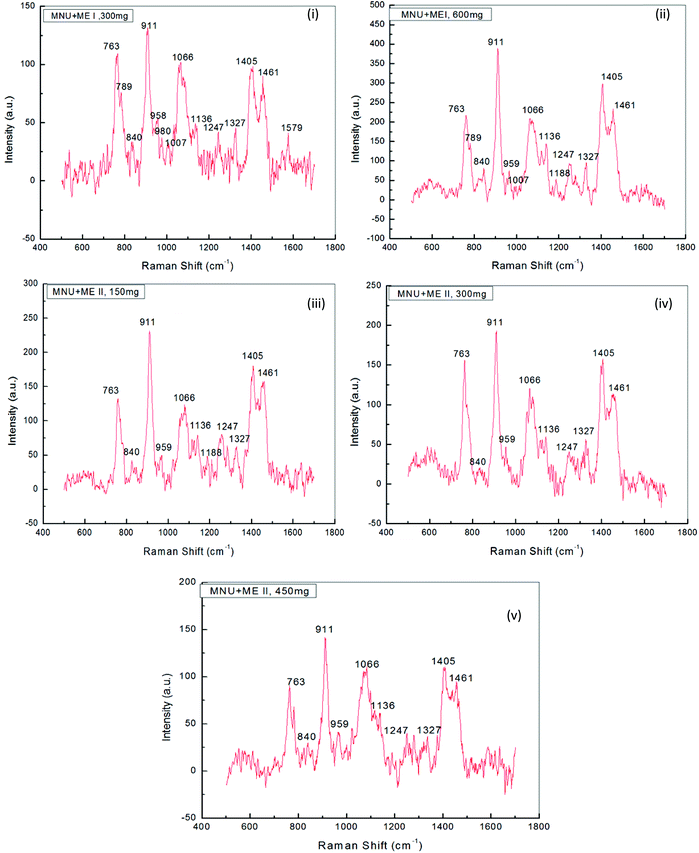
3.5. Raman analysis
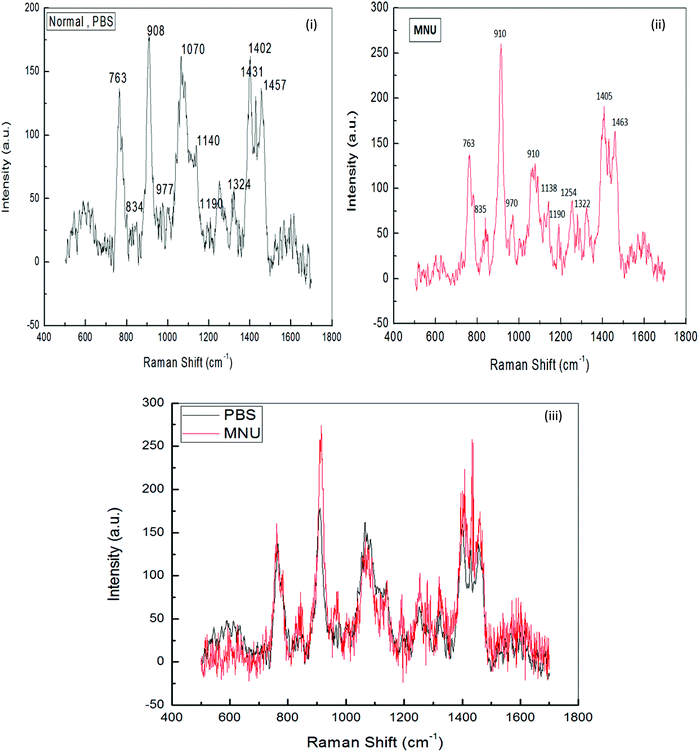
Raman Spectroscopy was performed to evaluate the effects of mushroom extracts ME1 and ME2, in reducing MNU carcinogenic damage to the DNA structure at nucleic bases and phosphate backbone levels. Fig. 8 and 9 show the spectra of liver DNA samples at the end of the 3 months treatment period. Peak intensities are studied by using the standard analysis Software Spectra Calc. Peaks appearing in each region were superimposed to the mixture of Lorenz Ian and Gaussian bands to evaluate the extent in intensity, frequency and peak area changes (Fig. 8(iii)). To assess the precise change in peak intensity and shift, an internal standard (1434 cm−1 band) was selected. This band 1434 cm−1 does not appear to change in intensity in any of the four samples. The peak normalization eliminated the effect of variation in DNA sampling. Each measurement was done separately on the same day of DNA isolation. The Raman band peaks were analysed and compared to literature data, as given in Table 4.| Raman spectra peak as per our experimental DNA sample and their assignments as per observations | Raman spectra peaks and their assignments as per earlier reports and references | |||
|---|---|---|---|---|
| Region of the peak position (cm−1) in Raman spectra. | Peak position (cm−1) in Raman spectra. | Peak assignments for Raman spectra of liver DNA. | Literature reference of peak assignments in DNA Raman spectra | Literature value for the reported peak position in DNA Raman spectra |
| Region-1 (740–900) | 763 | O–P–O stretching | Prescott et al. (1984)74 | 807 |
| Duguid et al. (1996)70 | ||||
| 840 | Phosphodiester stretching | Prescott et al. (1984)74 | 838 | |
| Duguid et al. (1996)70 | ||||
| Region-2 (900–1040) | 911 | Deoxyribose | Ke et al. (1999)75 | 920 |
| 959 | Deoxyribose | Ke et al. (1999)75 | 956 | |
| 1007 | Deoxyribose | Sipro (1987)76 | 1012 | |
| Region-3 (1080–1190) | 1066 | Deoxyribose–phosphate | Ke et al. (1999)75 | 1153 |
| 1136 | DNA backbone | Gao et al. (2005)77 | 1167 | |
| Region-4 (1190–1280) | 1247 | O–P–O stretching | Guan and Thomas (1996)78 | 1220 |
| 1254 | Adenine, cytosine | Ke et al. (1999)75; Ruiz-Chica et al. (2006)72; Verma et al. (2013)23 | 1252 | |
| 1256 | ||||
| Region-5 (1280–1400) | 1327 | Adenine, guanine | Ruiz-Chica et al. (2006)72 | 1339 |
| Region-6 (1400–1525) | 1405 | Deoxyribose | Ruiz-Chica et al. (2006)72 | 1422 |
| 1461 | Deoxyribose | Ke et al. (1999)75 | 1462 | |
The general features of the Raman spectra of liver DNA of control (PBS), MNU, ME1 (300 mg per kg b.w.) and ME1 (600 mg per kg b.w.) mice were relatively similar according to their band assignments and the regions in which the peaks were obtained. However differences in their relative intensity were apparent. For the analysis of the Raman bands, the complete spectra (600–1600 cm−1) were divided into six regions (Table 4). A total of ten peaks including 763 cm−1, 840 cm−1 and 911 cm−1 for phosphodiester stretching; 959 cm−1, 1007 cm−1, 1066 cm−1, 1136 cm−1 and 1257 cm−1 peaks for deoxyribose and 1327 cm−1, 1405 cm−1 and 1461 cm−1 for purine and pyramidine bases were characterised. These assigned peaks increased in intensity (shown at the Y-axis) by 50–70% for MNU treated (Group II) liver DNA samples (Fig. 8(ii)).
The region 1200–1600 cm−1 corresponds to nucleic bases which are prone to any type of alkylation by MNU. The mushroom protective effect was confirmed by Raman spectroscopy where, the MNU–DNA interaction as evidenced by an intense peak at 1254 cm−1 was normalised and was not apparent in any of the mushroom-treated DNA samples. The increase in the peak intensity value was within the same range of 0–200 (a.u.) in Group I (PBS treated) and MNU + ME1 (300 mg per kg b.w.) (Group IV).
Mushroom extract treatment with ME1 at a concentration of 300 mg per kg b.w. was more effective in reducing DNA damage after 92 days of supplementation compared to 600 mg per kg b.w. The combined effect of the carcinogen and ME1 extract (600 mg per kg b.w.) significantly increased the overall intensity of the Raman peaks by one fold compared to MNU treated mice (Fig. 8(ii) and 9(ii)). The major change in spectra was observed at peak 959 cm−1, 1007 cm−1 and 1188 cm−1 where the latter was more intense and prominently increased (2 folds) in ME1 (600 mg per kg b.w.) treated samples (Fig. 9(ii)). In the case of ME2 extract treatment, the Raman spectra for MNU + ME2 (450 mg per kg b.w.) (Group X) showed an increase in the peak intensity comparable to control mice (Fig. 9(v)).
Extract treatment with MNU + ME2 (300 mg per kg b.w.) showed a less prominent increase in the peak intensity compared to MNU + ME2 (150 mg per kg b.w.) (Fig. 9(iv) and (iii)). The protective effects of extract treatment in ME2 groups seemingly increased in a dose dependent manner, with a major change in spectra at peaks 911 cm−1, 959 cm−1, 1247 cm−1, 1066 cm−1 and 1188 cm−1. The intensity of these peaks was much lower in Group X, and rose sequentially in Groups IX and VIII. The rise in the intensity of the peaks in the Raman spectra of Groups X, IX, VIII and IV were much lower than that of Group VI. The biochemical scenario demonstrated that treatment by both P. sajor-caju and A. bisporus effectively reduced oxidative damage in MNU primed mice, as evidenced by a significant decrease in the extent of lipid peroxidation and a concomitant increase in the antioxidant enzyme activities notably CAT, SOD, GR and GSH-Px and FRAP levels.
4. Discussion
Mounting evidence increasingly suggests the putative role of mushrooms in the management of health and disease and supports the use of mushrooms for the development of functional food ingredients.24–26 Reports have ascribed their protective effects to the pluripharmacological effects of their bioactive constituents such as polysaccharides, amino acids, polyphenols and terpenoids,27–29 subsequently prompting a number of research initiatives into their anti-cancer properties. In this vein, mushrooms consumed in Mauritius, where cancer incidence has increased accounting for 13.4% deaths in 2013,30 were investigated for their prophylactic chemopreventive potential.Locally consumed mushrooms were rich in phenolics (p < 0.05). Smolskaitė et al.26 reported total phenol contents (TPC) of 4.2 ± 0.1 mg gallic acid equivalent (GAE) per g dry weight (DW) for A. bisporus and 5.3 ± 1.0 mg GAE per g DW for P. ostreatus. These values are much lower than those observed for P. sajor-caju (33.3 ± 0.9 mg GAE per g DW) and A. bisporus (133.7 ± 3.2 mg GAE per g DW) in this study.
HPLC analyses of the extracts confirmed the predominance of phenolic acids in the extracts. Several studies have also confirmed that major antioxidants in mushrooms, characterised in numerous species from Finland,31 India,32 Korea33 and Portugal,34 were phenolics. In our study, gallic acid, protocatechuic acid and pyrogallol were the most prominent compounds identified, in line with several other studies on P. sajor-caju, P. ostreatus and A. bisporus.31–33
The modulatory effects of these phenolics in cancer have been well expounded in the literature. Maurya et al.35 and Yumnam et al.36 reported the anti-cancer activity of gallic acid in human colon cancer (HCT15), breast cancer (MDA MB 231) and lung adenocarcinoma (A549) cell lines. Protocatechuic acid has been shown to inhibit in vitro chemical carcinogenesis and exert proapoptotic and antiproliferative effects in F344 male rats with DEN (diethylnitrosamine)-induced liver carcinogenesis.37 This phenolic is also an inhibitor of free radicals, plays a vital role in phases 1 and 2 of the metabolism of certain carcinogens and behaves like a blocking agent at the site where these carcinogens bind with DNA molecules.38
Mice liver cancer models represent a valuable practical tool to study the mechanisms involved in experimental tumour proliferation and the development of therapeutic strategies in the assessment of the bioefficacy of natural extracts. MNU used as a mutagen to induce cancer in mice, is a direct-acting alkylating agent interacting with DNA by transferring its methyl group to nucleobases in nucleic acids. This leads to the creation of mutagenic lesions and accumulation of mutations that play an important role in cancer initiation.39 The intake of this nitrostable compound develops an environment conducive to the activation of inflammatory cytokines (IL-1β, IL-6) with the increased expression of NF-kB, leading to hepatocarcinogenesis in Balb/c mice.40
The evaluation of the modulatory effects of P. sajor-caju (ME1) and A. bisporus (ME2) revealed protective effects against MNU. The morphological changes and weight records for mice in this study are consistent with those made by Verma et al.23,40 under similar conditions. MNU-treated mice experienced a decrease of 15.2% in body weight compared to the control after 4 weeks of treatment as the carcinogenic effects of MNU started to manifest. Verma et al.23 reported a decrease of 40% in body weight of Balb/c mice treated with MNU after a longer period of 28 weeks compared to control.
Mushroom treatment in MNU-induced hepatocarcinogenesis effectively restored the body weight of the mice after 22 days, and this beneficial effect can most probably be attributed to the anti-mutagenic and anti-cancer properties of the mushroom extracts as reported by Sarangi et al.11 and Shah et al.12 Data from Raman spectroscopy analysis support these observations, thereby clearly demonstrating a consequent reduction in the peak intensities of MNU liver DNA spectra as a result of extract treatment. P. sajor-caju and A. bisporus consistently reduced uneven shedding of hairs and formation of micronodular lesions in the liver of the animals by the end of the 3-months supplementation period. Moreover, a high liver/body weight ratio of MNU-treated mice (2.8 folds greater than control mice) was significantly lowered by extract treatment. Jayakumar et al.41 reported the hepatoprotective effects of P. ostreatus extracts in improving the antioxidant status and reverting hepatic damage in carbon tetrachloride (CCl4)-treated male Wistar rats. Moreover, Nada et al.42 also demonstrated that polysaccharides from P. ostreatus mycelium protected hepatocytes from CCl4-induced damage in Sprague Dawley rats. The in vitro antiproliferative effects of P. ostreatus extracts have also been reported in other cancers, e.g. breast (MCF-7), colon (COLO-205), and kidney (ACHN) human cancer cell lines.43
Mushroom treatment on serum and liver antioxidant enzymes of Balb/c mice clearly showed an upregulation of the latter and consequently a reduction in oxidative stress. MNU considerably decreased the protein level and antioxidant enzymes like CAT, SOD, GR and GPx in the serum and liver of the mice. However, the protein level was restored by P. sajor-caju at 150 mg kg−1 and A. bisporus at 300 and 450 mg kg−1 respectively. Similar observations were made by Llauradó et al.44 where the total serum protein levels of malnourished Balb/c mice supplemented with a cold-water extract from the fruiting bodies of Pleurotus spp. were higher than for those mice without supplementation.
This was substantiated by low reducing power and elevated MDA levels (as measured by the circulating levels of TBARS) in both serum and liver tissues of MNU treated mice. Mohd Ali et al.45 reported a significant decrease in the SOD activity and FRAP levels in liver tissues of male Balb/c mice with ethanol-mediated liver damage. This decrease in cellular enzyme activities was largely due to the impairment of antioxidant enzymes that protect cells against reactive oxygen species.46 Prolonged exposure to potent carcinogens has been shown to decrease enzyme activity in targeted tissues of organ-specific carcinogenesis.47,48
Nonetheless, in the initial phase of acute toxicity and carcinogenesis, a concomitant increase in enzyme activities curbing down the ROS levels was noteworthy. Lukaszewicz-Hussain & Moniuszko-Jakoniuk47 assessed CAT, GPx and GR activities, hydrogen peroxide and MDA levels in liver tissues of male Wistar rats after intoxication with chlorfenvinphos (an organophosphate insecticide). An initial increase in rat liver CAT, GPx and GR activities, followed by a considerable decrease by the end of the treatment, and a sharp rise in liver MDA levels were noted. In this study, a decrease in enzyme activity and increase in the rate of lipid peroxidation in MNU treated mice occurred by the end of the 3 months supplementation. Decline in SOD activities has been associated with an increase in circulating lipid peroxides which can result in the steady state of a superoxide anion, a highly diffusible and potent oxidizing radical capable of traversing membranes and causing deleterious effects far from the tumour sites.49
CAT and GPx detoxify a significant amount of hydrogen peroxide produced during electron transport chain and protect mitochondrial membranes from oxidation.50 The decreased activities of catalase found in the cancerous condition of MNU treated mice could be attributed to the exhaustion of these enzymes, leading to accumulation of hydrogen peroxide by cancerous cells.48,51 Decrease in GPx activities under various cancerous conditions has also been reported in the literature.52 The enormous production of free radicals, as is the case of several cancers, could have altered the antioxidant defense system in mice with MNU-induced hepatocarcinogenesis, thereby accounting for the decline observed in the serum and liver GPx activities in the present study.
Polyphenol, flavonoid and anthocyanin contents have been suggested to exert strong antioxidant activity, which contributes to the protective effect against liver injury in mice and rats.53 However, most dietary polyphenols being in their native form of esters, glycosides or polymers, cannot be absorbed directly and need to be hydrolyzed by intestinal enzymes or by colonic microflora, which reduces bioavailability.54,55 An indirect evidence of their absorption through the gut barrier is given by an increase in the antioxidant capacity of plasma after the consumption of foods rich in polyphenols (Young et al., 1999; Pandey & Rizvi, 2009).55,56
Phenolics like gallic acid and protocatechuic acid, identified in the mushrooms have been reported to have excellent antioxidant and antitumour properties,35–37 potentiating a protective effect against hepatocarcinogenesis. It is noteworthy that the biological functions of polyphenols such as anti-mutagenicity, and anti-carcinogenicity is partly a function of their antioxidant activity.57,58 Mice groups, given only extract treatment of ME1 and ME2 respectively, showed better or comparable CAT and SOD activities (p < 0.05).
Treatment with A. bisporus reduced TBARS levels in breast tissue of 7,12-dimethylbenz(a)anthracene induced mammary carcinogenesis in Sprague-Dawley rats.48 Fruiting body extracts of P. sajor-caju have also been reported to reduce the number of neoplastic cells in female Swiss mice inoculated with Ehrlich ascitic tumour.59 In the present study, extract treatment of ME1 (150 mg kg−1) in serum and ME2 (300 mg kg−1) in liver tissues showed appreciable FRAP levels. The FRAP assay can be used as a simple screen for assessing the ability to maintain the redox status in cells and tissues, as it detects redox potentials of less than 0.7 V (the redox potential of Fe3+-TPTZ).
However, since this assay does not measure the activity of thiol antioxidants, such as glutathione, it may not reflect antioxidant activity mechanistically and physiologically in complex systems.60 Measuring the in vivo antioxidant enzymes like CAT, SOD, GR and GPx, provided a better assessment of the antioxidant status in Balb/c mice induced with hepatocarcinogenesis. A more effective reduction of circulating TBARS levels was achieved by ME1 (600 mg kg−1) and ME2 (600 mg kg−1) compared to control mice and these groups showed an improved in the antioxidant status.
The hepatic antioxidant status was restored with an ethanolic extract of a wild edible mushroom (Macrocybe gigantea) treatment in carbon tetrachloride-induced hepatic damage in Swiss albino mice.61 Wu et al.62 reported the hepatoprotective effects of aqueous extracts of medicinal mushroom Ganoderma lucidum on liver injury induced by α-amanitin in mice, where a significant increase in the SOD and CAT activities, and a decreased MDA content in liver tissues were observed. In line with these observations, the protective effects of both P. sajor-caju and A. bisporus on biochemistry of Balb/c mice serum and liver tissues are clearly evident, and can be attributed to their antioxidant properties in vitro.
The immunomodulatory effects of the mushroom extracts were obvious on hemoglobin, leukocyte and platelet as well as lymphocyte, neutrophil, monocyte and eosinophil counts in MNU-treated mice. MNU-treated samples showed an abnormal elevated level of leucocytes, monocytes and neutrophils. A strong association between abnormally high neutrophil, monocyte or leucocyte counts and poor survival rates in patients with metastatic carcinoma has been reported.63,64 The sharp drop in the lymphocyte count in the MNU-treated sample compared to other extract-treated groups strongly confirms this association and suggests this parameter as a valuable marker in cancer prognosis.65,66
MNU-treated mice were found with a significantly high platelet count (Table 3). This hike in platelet counts is linked to an increased expression of inflammatory cytokine (IL-1b, IL-6) as observed in various types of cancer patients.67 The inflammatory response within the microenvironment of tumours is characterised by tumour-associated macrophages and tumour-infiltrating lymphocytes. These lead to the overproduction of several proinflammatory cytokines, in particular, the tumour necrosis factor, chemokines, transcription activators and interleukin-1β and -6.68 Mushroom extract treatment also normalised the leucocyte, neutrophil, monocyte and eosinophil counts after 3 months of treatment.
The haematological profile of groups III and IV, and IX and XI were comparable (p > 0.05), thus providing additional evidence of the beneficial effects of ME1 and ME2 mushroom extracts when administered at these concentrations. Verma et al.23 also reported beneficial properties of the natural extracts of A. marmelos which normalised leucocyte, neutrophil, monocyte and eosinophil counts and increased the lymphocyte count after 28 weeks of treatment in MNU-induced hepatocarcinogenesis in Balb/c mice. Haemoglobin concentration was best normalised in MNU + ME1 (300 mg kg−1) groups and in other ME1 treated groups. Similar observations were reported by Morris et al.69 where protective effects of the lyophilised extracts of Pleurotus spp. fruiting bodies on cyclophosphamide-treated Balb/c mice were assessed. Mice that were treated with the Pleurotus spp. extract prior to cyclophosphamide administration showed haemoglobin concentrations similar to that of the control mice at the end of the treatment period.
Raman spectroscopy analysis provided a valuable indication in determining the best performing extracts in terms of their ability to protect mice liver DNA from damage at the level of nucleic bases and the phosphate backbone. The changes observed in terms of the peak intensity increases in MNU-primed mice were clearly evident (Fig. 9). The increase in the peak intensity in Raman spectroscopy is reflective of a highly cooperative conformational change occurring within different portions of the MNU-treated DNA samples.40 Raman spectra for MNU + ME2 (450 mg per kg b.w.) showed a rise in the peak intensity comparable to control mice while ME1 at a concentration of 300 mg per kg b.w. proved to be more effective in reducing DNA damage after 92 days of supplementation. The 1252 cm−1 peak assigned for adenine and cytosine (Table 4) is prominent and intense in MNU-treated samples. The 1200–1600 cm−1 region has been assigned to purines and pyramidines, corresponding to ring electronic structures that are very sensitive to base unstacking and metal binding at these ring sites.70 Rizvi and Hadi71 and Ruiz-Chica et al.72 demonstrated that MNU had a strong affinity for guanine and adenine bases in synthesised calf DNA.
The 1252 cm−1 band might be a consequence of alkylation at adenine bases, resulting in a strong interaction between the MNU and DNA structure. Verma et al.40 reported a peak value of 1256 cm−1 that was assigned to adenine and cytosine. In this study, MNU–DNA interaction evidenced by an intense peak at 1254 cm−1 in the MNU Raman spectra, was normalised by mushroom extract supplementation. Prolonged exposure to potent hepatocarcinogens, may lead to an accumulation of interactions between MNU and DNA, and their relative adducts, thus causing mutations and eventually leading to cancer.23 This MNU–DNA interaction was markedly inhibited by mushroom extracts in the treatment groups MNU + ME2 (450 mg kg−1) and MNU + ME1 (300 mg kg−1) respectively. The normal structure and confirmation of nucleic acid backbone and sugar bases in DNA samples were maintained, thereby strongly suggesting the anti-carcinogenic properties of P. sajor-caju and A. bisporus against hepatocarcinogenesis in MNU-treated Balb/c mice.
Non-cytotoxic nutrients and/or pharmacological agents have the ability to target multiple aberrant cellular signaling circuits.73 Several polyphenols have been reported to correct DNA methylation imbalances. The results of Raman spectroscopy demonstrate that one of the possible mechanisms of action employed by the mushroom extracts consists of preventing and/or reversing the alteration in DNA caused by an interference of MNU in the structure of nucleic acid bases, reflected by a peak shift in the Raman spectra.
5. Conclusion
The rise in liver cancer will continue to undermine health due to the increased exposure to environmental pollutants, dietary sources of carcinogens and xenobiotics as indicated by health statistics. With the rising incidence of cancer, functional foods rich in specific antioxidants, showing promising results in vivo and in clinical trials may provide a practical alternative approach to disease control with reduced morbidity and mortality. The findings of this study demonstrate that Pleurotus sajor-caju and Agaricus bisporus are liable for modulating hepatocarcinogenesis in Balb/c mice. Mushroom extracts with modulatory effects on biochemical, haematological and biophysical parameters could therefore provide efficient points of intervention at early cancer stages.Acknowledgements
The authors would like to thank the Mauritius Research Council and Chhatrapati Shahu Ji Maharaj (CSJM) University (Kanpur, India) for funding this research work. Special acknowledgements to Dr Varsha Gupta (Institute of Biosciences and Biotechnology, CSJM University) for providing the necessary laboratory facilities, and to Mr Sandeep Beepat (Department of Biosciences, University of Mauritius) for his useful advice while using STATISTICA software.References
- J. Ferlay, I. Soerjomataram, M. Ervik, R. Dikshit, S. Eser, C. Mathers, M. Rebelo, D. M. Parkin, D. Forman and F. Bray, International Agency for Research on Cancer (IARC), 2013, available from: http://globocan.iarc.fr [accessed 12 March 2014] Search PubMed.
- P. Anand, A. B. Kunnumakkara, C. Sundaram, K. B. Harikumar, S. T. Tharakan, O. S. Lai, B. Sung and B. B. Aggarwal, Pharm. Res., 2008, 25, 2097–2116 CrossRef CAS PubMed.
- J. K. Kundu and Y. J. Surh, Mutat. Res., 2005, 591, 123–146 CrossRef CAS PubMed.
- S. Ramos, Mol. Nutr. Food Res., 2008, 52, 507–526 CAS.
- M. A. Soobrattee, V. S. Neergheen, A. Luximon-Ramma, O. I. Aruoma and T. Bahorun, Mutat. Res., 2005, 579, 200–213 CrossRef CAS PubMed.
- S. Patel and A. Goyal, 3 Biotech, 2012, 2, 1–15 CrossRef PubMed.
- S. K. Aung, Int. J. Med. Mushrooms, 2005, 7, 300–377 Search PubMed.
- M. Elmastas, O. Isildak, I. Turkekul and N. Temur, J. Food Compost. Anal., 2007, 20, 337–345 CrossRef CAS.
- H. A. El Enshasy and R. Hatti-Kaul, Trends Biotechnol., 2013, 31, 668–677 CrossRef CAS PubMed.
- H. Tong, F. Xia, K. Feng, G. Sun, X. Gao, L. Sun, R. Jiang, D. Tian and X. Sun, Bioresour. Technol., 2009, 100, 1682–1686 CrossRef CAS PubMed.
- I. Sarangi, D. Ghosh, S. K. Bhutia, S. K. Mallick and T. K. Maiti, Int. Immunopharmacol., 2006, 6, 1287–1297 CrossRef CAS PubMed.
- S. Shah, D. Ghosh, S. K. Mallick, I. Sarangi, S. K. Bhutia, I. Banerjee, S. Maiti and T. K. Maiti, Int. J. Med. Mushrooms, 2007, 9, 123–138 CrossRef CAS.
- S. Chen, S. R. Oh, S. Phung, G. Hur, J. Ye, S. L. Kwok, G. E. Shrode, M. Belury, L. S. Adams and D. Williams, Cancer Res., 2006, 66, 12026–12034 CrossRef CAS PubMed.
- V. S. Neergheen, T. Bahorun and O. I. Aruoma, J. Plant Physiol., 2005, 163, 787–799 CrossRef PubMed.
- J. A. Moncaster, D. T. Walsh, S. M. Gentleman, L. S. Jen and O. I. Aruoma, Neurosci. Lett., 2002, 328, 55–59 CrossRef CAS PubMed.
- B. M. Landes and M. A. Finamore, Patents, US 20120141611 A1, 2012. Available from: http://www.faqs.org/patents/app/20120141611#ixzz28OvkS0uN [accessed 4 November 2013] Search PubMed.
- A. K. Sinha, Anal. Biochem., 1972, 47, 389–394 CrossRef CAS PubMed.
- H. P. Misra and I. Fridovich, J. Biol. Chem., 1972, 247, 3170–3175 CAS.
- I. Carlberg and B. Mannervik, Methods Enzymol., 1985, 113, 484–490 CAS.
- D. G. Hafeman, R. A. Sunde and W. G. Hoekstra, J. Nutr., 1974, 104, 580–587 CAS.
- K. Satoh, Clin. Chim. Acta, 1978, 90, 37–43 CrossRef CAS.
- I. F. Benzie and J. J. Strain, Anal. Biochem., 1996, 239, 70–76 CrossRef CAS PubMed.
- S. Verma, T. Bahorun, R. K. Singh, O. I. Aruoma and A. Kumar, Pharm. Biol., 2013, 51, 1272–1281 CrossRef PubMed.
- M. Zhang, S. W. Cui, P. C. K. Cheung and Q. Wang, Trends Food Sci. Technol., 2007, 18, 4–9 CrossRef CAS.
- I. Schneider, G. Kressel, A. Meyer, U. Krings, R. G. Berger and A. Hahn, J. Funct. Foods, 2011, 3, 17–24 CrossRef CAS.
- L. Smolskaitė, P. R. Venskutonis and T. Talou, LWT – Food Sci. Technol., 2015, 60, 462–471 CrossRef.
- N. J. Dubost, B. Ou and R. B. Beelman, Food Chem., 2007, 105, 727–735 CrossRef CAS.
- P. Kalač, Food Chem., 2009, 113, 9–16 CrossRef.
- Y. Yaoita, M. Kikuchi and K. Machida, Nat. Prod. Commun., 2014, 9, 419–426 CAS.
- N. Jeeanody, Health Statistics Report 2013, Ministry of Health and Quality of Life, Central Statistics Office, Mauritius, 2014, http://health.govmu.org/English/Statistics/Health/Documents/healthreport%202013.pdf (accessed 12th November 2015).
- P. Mattila, K. Konko, M. Eurola, J. M. Pihlava, J. Astola, L. Vahteristo, V. Hietaniemi, J. Kumpulainen, M. Valtonen and V. Piironen, J. Agric. Food Chem., 2001, 49, 2343–2348 CrossRef CAS PubMed.
- N. G. Puttaraju, S. U. Venkateshaiah, S. M. Dharmesh, S. M. Urs and R. Somasundaram, J. Agric. Food Chem., 2006, 54, 9764–9772 CrossRef CAS PubMed.
- M. Y. Kim, P. Seguin, J. K. Ahn, J. J. Kim, S. C. Chun, E. H. Kim, S. H. Seo, E. Y. Kang, S. L. Kim, Y. J. Park, H. M. Ro and I. M. Chung, J. Agric. Food Chem., 2008, 56, 7265–7270 CrossRef CAS PubMed.
- L. Barros, M. Dueñas, I. C. F. R. Ferreira, P. Baptista and C. Santos-Buelga, Food Chem. Toxicol., 2009, 47, 1076–1079 CrossRef CAS PubMed.
- D. K. Maurya, N. Nandakumar and T. P. Devasagayam, J. Clin. Biochem. Nutr., 2011, 48, 85–90 CrossRef CAS PubMed.
- P. D. Yumnam, U. Addepally, L. N. Mangamoori and K. Chepuri, IJRANSS, 2014, 2, 269–272 Search PubMed.
- T. Tanaka, M. Shimizu, T. Kochi and H. Moriwaki, J. Exp. Clin. Med., 2013, 5, 203–209 CrossRef CAS.
- S. Kakkar and S. Bais, ISRN Pharmacol., 2014, 2014, 952943 Search PubMed.
- A. Tsubura, Y. C. Lai, H. Miki, T. Sasaki, N. Uehara, T. Yuri and K. Yoshizawa, In Vivo, 2011, 25, 11–22 CAS.
- S. Verma, T. Bahorun and A. Kumar, Prev. Med., 2012, 54, S130–S136 CrossRef CAS PubMed.
- T. Jayakumar, E. Ramesh and P. Geraldine, Food Chem. Toxicol., 2006, 44, 1989–1996 CrossRef CAS PubMed.
- S. A. Nada, E. A. Omara, O. M. Abdel-Salam and H. G. Zahran, Food Chem. Toxicol., 2010, 48, 3184–3188 CrossRef CAS PubMed.
- S. Arora, A. Aggarwal, P. Singla, S. Jyoti and S. Tandon, Homeopathy, 2013, 102, 274–282 CrossRef PubMed.
- G. Llauradó, H. J. Morris, Y. Lebeque, R. Fontaine, R. C. Bermudez, J. Marcos, Y. Beltran and N. Garcia, Rev. Cuba. Quim., 2005, 17, 102–107 Search PubMed.
- N. Mohd Ali, H. Mohd Yusof, K. Long, S. K. Yeap, W. Y. Ho, B. K. Beh, S. P. Koh, M. P. Abdullah and N. B. Alitheen, Biomed. Res. Int., 2013, 2013, 693613 Search PubMed.
- J. Du, D. He, L. N. Sun, T. Han, H. Zhang, L. P. Qin and K. Rahman, Pharm. Biol., 2010, 48, 953–958 CrossRef PubMed.
- A. Lukaszewicz-Hussain and J. Moniuszko-Jakoniuk, Pol J. Environ. Stud., 2003, 13, 397–401 Search PubMed.
- G. Dhamodharan and S. Mirunalini, Int. J. Pharm. Pharm. Sci., 2012, 4, 348–354 Search PubMed.
- R. Padmavathi, P. Senthilnathan, D. Chodon and D. Sakthisekaran, Life Sci., 2006, 78, 2820–2825 CrossRef CAS PubMed.
- K. Rahman, Clin. Interv. Aging, 2007, 2, 219–236 CAS.
- C. Glorieux, J. Auquier, N. Dejeans, B. Sid, J. B. Demoulin, L. Bertrand, J. Verrax and P. B. Calderon, Biochem. Pharmacol., 2014, 89, 217–223 CrossRef CAS PubMed.
- G. K. Balendiran, R. Dabur and D. Fraser, Cell Biochem. Funct., 2004, 22, 343–352 CrossRef CAS PubMed.
- H. A. El-Beshbishy, O. M. Tork, M. F. El-Bab and M. A. Autifi, Pathophysiology, 2011, 18, 125–135 CrossRef CAS PubMed.
- M. D'Archivio, C. Filesi, R. D. Benedetto, R. Gargiulo, C. Giovannini and R. Masella, Ann. Ist .Super. Sanità, 2007, 43, 348–361 Search PubMed.
- K. B. Pandey and S. I. Rizvi, Oxid. Med. Cell. Longevity, 2009, 2, 270–278 CrossRef PubMed.
- J. F. Young, S. E. Nielsen, J. Haraldsdóttir, B. Daneshvar, S. T. Lauridsen, P. Knuthsen, A. Crozier, B. Sandström and L. O. Dragsted, Am. J. Clin. Nutr., 1999, 69, 87–94 CAS.
- E. G. Yordi, E. M. Pérez, M. J. Matos and E. U. Villares, Antioxidant and pro-oxidant effects of polyphenolic compounds and structure–activity relationship evidence, in Nutrition, well-being and health, ed. J. Bouayed and T. Bohn, InTech, Croatia, 2012 Search PubMed.
- K. Sloczynska, B. Powroznik, E. Pekala and A. M. Waszkielewicz, J. Appl. Genet., 2014, 55, 273–285 CrossRef CAS PubMed.
- N. Dalonso, R. Souza, M. L. Silveira, A. A. Ruzza, T. M. Wagner, E. Wisbeck and S. A. Furlan, Appl. Biochem. Biotechnol., 2010, 160, 2265–2274 CrossRef CAS PubMed.
- R. L. Prior, X. Wu and K. Schaich, J. Agric. Food Chem., 2005, 53, 4290–4302 CrossRef CAS PubMed.
- K. Acharya, A. Chatterjee, G. Biswas, A. Chatterjee and G. K. Saha, Int. J. Pharm. Pharm. Sci., 2012, 4, 285–288 CAS.
- X. Wu, J. Zeng, J. Hu, Q. Liao, R. Zhou, P. Zhang and Z. Chen, Int. J. Med. Mushrooms, 2013, 15, 383–391 CrossRef PubMed.
- S. R. Walsh, E. J. Cook, F. Goulder, T. A. Justin and N. J. Keeling, J. Surg. Oncol., 2005, 91, 181–184 CrossRef CAS PubMed.
- F. Donskov, M. Hokland, N. Marcussen, H. H. Torp Madsen and H. Von Der Maase, Br. J. Cancer, 2006, 94, 218–226 CrossRef CAS PubMed.
- F. Guidozzi, S. Reddy and A. Wadee, Gynecol. Oncol., 1994, 53, 251–255 CrossRef CAS PubMed.
- V. Hespanhol, H. Queiroga, A. Magalhaes, A. R. Santos, M. Coelho and A. Marques, Lung Cancer, 1995, 13, 253–267 CrossRef CAS PubMed.
- T. Ishibashi, H. Kimura, Y. Shikama, T. Uchida, S. Kariyone, T. Hirano, T. Kishimoto, F. Takatsuki and Y. Akiyama, Blood, 1989, 74, 1241–1244 CAS.
- V. S. Neergheen, T. Bahorun, E. W. Taylor, L. S. Jen and O. I. Aruoma, Toxicology, 2010, 278, 229–241 CrossRef CAS PubMed.
- H. J. Morris, G. Llauradó, A. Gutiérrez, Y. Lebeque, R. Fontaine, Y. Beltrán, N. García, R. C. Bermúdez and I. Gaime-Perraud, ICMBMP, 2011, 7, 324–333 Search PubMed . Available from: http://www.wsmbmp.org/proceedings/7th%20international%20conference/1/ICMBMP7-Oral-3-21-%20Morris.pdf [accessed 10 November 2011].
- J. G. Duguid, V. A. Bloomfield, J. M. Benevides and G. J. J. R. Thomas, Biophys. J., 1996, 71, 3350–3360 CrossRef CAS PubMed.
- R. Y. Rizvi and S. M. Hadi, Biosci. Rep., 1984, 4, 729–735 CrossRef CAS PubMed.
- A. J. Ruiz-Chica, A. Soriano, I. Tuñón, F. M. Sánchez-Jiménez, E. Silla and F. J. Ramírez, J. Chem. Phys., 2006, 324, 579–590 CAS.
- A. R. Amin, O. Kucuk, F. R. Khuri and D. M. Shin, J. Clin. Oncol., 2009, 27, 2712–2725 CrossRef PubMed.
- B. Prescott, W. Steinmetz and G. J. Thomas, Biopolymers, 1984, 23, 235–256 CrossRef CAS PubMed.
- W. Ke, D. Yu and J. Wu, Spectrochim Acta, Part A, 1999, 55, 1081–1090 CrossRef.
- T. G. Sipro, Raman spectra on the conformations of biological macromolecules, in Biological application of Raman spectroscopy, ed. T. G. Spiro, John Wiley, New York, 1987 Search PubMed.
- X. Gao, I. S. Butler and R. Kremer, Spectrochim. Acta, Part A, 2005, 61, 27–35 CrossRef PubMed.
- Y. Guan and G. J. J. R. Thomas, Biopolymers, 1996, 39, 813–835 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |