Recent advances in fluorescent film sensing from the perspective of both molecular design and film engineering
Rong
Miao
,
Junxia
Peng
and
Yu
Fang
*
Key Laboratory of Applied Surface and Colloid Chemistry of Ministry of Education, School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an 710062, People's Republic of China. E-mail: yfang@snnu.edu.cn; Tel: +86 29 81530787
First published on 24th May 2016
Abstract
Fluorescence is now a dominant technology and is used extensively in many fields owing to its incomparable properties. On account of its advantages, scientists and engineers have been investigating novel and powerful fluorescent sensors and sensor arrays for real-life applications, in which the construction of high-performance fluorescent films plays a prominent role. The appropriate immobilization of suitable fluorescent sensing elements onto a given substrate is an efficient way to construct usable fluorescent films. Molecular design and film fabrication are two key issues in the development of films because they determine the sensing performance of the films. Via the careful design and selection of sensing fluorophores, the development of convenient film fabrication strategies and the study of the relevant sensing dynamics, a variety of fluorescent sensing films with desirable sensing performance have been developed over at least the last ten years. This review describes recent progress in this important area of science, which combines surface and interface chemistry, photophysics and materials science.
Design, System, ApplicationHigh-performance sensing films are the key to real-life usable fluorescent sensors. Different strategies have been developed to fabricate fluorescent sensing films during the previous decades, which mainly include physical coating, doping, single-layer chemistry and molecular gel-based fabrication. The as-developed films can be divided into disordered and generally densely packed physical films, 2-dimensional chemical films and 3-dimensional networked physical films. To enhance sensing performance, the structures of films should be optimized both at a molecular level and on a microscopic scale. Therefore, more factors are involved in the design of sensing molecules; in particular, some functional structures need to be introduced to obtain films with favorable adlayer structures. A molecular gel strategy possesses advantages in the fabrication of fluorescent sensing films: 1) increased surface density and effectiveness of sensing sites; 2) increased speed and reversibility of response; and 3) ease of scale-up. However, this strategy is still open to improvement. For example, a general method is urgently needed to increase the adhesive and mechanical strength of gel-based films. Films that were fabricated using the newly developed strategy have found important applications in the sensitive and fast detection of hidden explosives and illegal drugs. |
1. Introduction
The study of sensing plays a pivotal role in our ways of discovering and learning more about the world.1,2 In general, a sensor is a device that helps us to sense and generates a perceptible change in a signal (from the original signal to the response signal) when it interacts (via physical or chemical interaction) with an analyte.3 Based on the type of change, sensors can be divided into electrical sensors,4 force sensors,5 thermal sensors,6 mass sensors,7 and optical sensors,8etc. Among these, optical sensors, in particular fluorescent sensors, have received much attention owing to their advantages in sensing, such as high sensitivity, good selectivity, absence of a radiation source, and ease of operation.8,9Since the 1970s, different types of fluorescent sensing molecules have been developed and reported to be useful for environmental monitoring,10 biomedical analysis11 and anti-terrorism applications,12 to name but few. However, only a few of these can fulfill practical applications, and much more efforts are needed to develop sophisticated fluorescent sensors for the detection of analytes under specific circumstances.3
A fluorescent sensor is the key part of a sensing device and its development involves both the design and engineering of a sensing molecule. Fluorescent sensing films with high performance can be obtained only when well-designed sensing molecules are engineered via a rational technique. In contrast to research on fluorescence sensing in the early years, researchers now pay more attention to practical applications of fluorescent sensing molecules.13 An effective way to enable the practical applications of a sensing molecule is to immobilize it on a suitable substrate surface, which will be convenient for device manufacture and further integration of the sensor.14
Owing to the importance of research into fluorescent film sensing, several review papers have been published.15–18 In contrast to previous papers, we review recent progress in the development of organic fluorophore-based fluorescent sensing films and focus on the two key issues in the construction of fluorescent sensor devices: molecular design and film engineering. In addition, we pay much attention to real-life application-led fluorescent sensing films, where basic research and practical use reinforce each other. We first introduce a model of fluorescent sensing films, in which some specific terms are defined to facilitate understanding and their roles in sensing are illustrated as well. Next, we consider challenging issues in fluorescence sensing, where some specific solutions are raised and discussed. Then, we report on recent advances toward new smart fluorescent sensing films, which not only give us a deeper understanding of film sensing, but also contribute to the further development of applicable film-based fluorescent sensors or devices. We conclude the discussion with a perspective on the future challenges for research in this field.
2. Film design and the relevant research
2.1 Organic fluorophore-based fluorescent sensing films
In principle, as a sensor, one of the measurable properties (a signal) of a fluorophore will change when it encounters a specific analyte, and the signal is used for analysis. The fluorescence intensity and fluorescence emission wavelength are two of the most commonly used signals in fluorescence sensing. Usually, a fluorescent sensor contains at least three parts: 1) a light source, 2) a fluorescence sensing unit, and 3) a transducer. The sensing unit is the core of the sensor and determines the maximum performance of the sensor. Actually, it is the emission of the fluorophore in the sensing unit that is sensitive to the presence of the analyte. The change in the emission is monitored and converted into an electrical signal by the transducer. In this way, sensing is achieved. Of course, for practical sensing, the quality of the light source, the geometrical structure of the sensor, the sampling system, and further signal processing also play important or even crucial roles in the performance of the final device or instrument.Up to now, a variety of fluorescent sensing elements have been developed such as organic fluorescent dyes,19 fluorescent polymers,18 semiconductor quantum dots,20 fluorescent carbon nanomaterials,21 luminescent metal complexes22 and fluorescent metal nanoclusters,23etc. In comparison with the others, organic fluorescent dyes were developed first and enjoy enduring popularity.24 The attractive aspects of organic fluorescent dyes lie in their designable structure, ease of characterization and wide range of available choices. Therefore, different methods have been developed for organic fluorophore-based sensing, such as directly dispersing organic fluorophores into the analysis medium, doping them into a polymer matrix, employing them to functionalize nanoparticles, and immobilizing them on a solid surface to construct fluorescent sensing films, etc.15–18 It is notable that fluorescent sensing films have great advantages in device manufacture and this makes them more favorable for practical applications.15–18,25–27 In fact, few commercial fluorescent sensors can operate without their sensing films. Therefore, research into fluorescent sensing films is a prerequisite for the development of fluorescent sensors, and this is the reason why much attention has been paid to the creation of outstanding fluorescent sensing films.
2.2 Concepts, concerns and challenges in the research
Generally speaking, a fluorescent sensing film contains two parts: a substrate and an active fluorescent sensing layer, which bears a certain number of sensing sites (Fig. 1).16,17 The substrate is a solid support on which the organic fluorescent sensing molecules are immobilized. The substrate provides not only convenience in sensing operations but also great opportunities for further device manufacture. The fluorescent sensing layer is where the sensing activity occurs: the sensing sites within or above the sensing layer respond to the analyte and then produce a change in a signal, either in fluorescence intensity and/or the profile of the emission spectrum.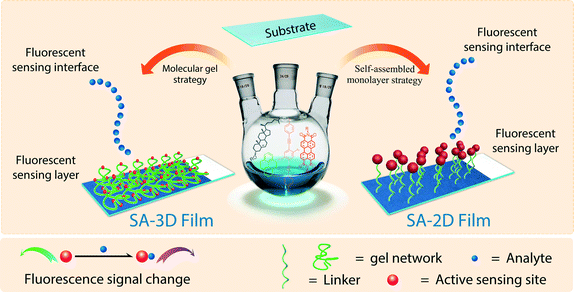 | ||
| Fig. 1 Illustration of the structures of SA-2D (right) and SA-3D (left) fluorescent sensing films and their sensing interface. | ||
Undoubtedly, research into fluorescent sensing films is a branch of surface and interface science, because their sensing activities are dependent on the structure and properties of the interface between the sensing layer and the bulk phase in which the analyte in question exists (Fig. 1). Dynamically, a sensing process includes at least three steps: 1) analyte molecules from the detection system are transferred to the fluorescent sensing layer; 2) the target molecules diffuse into or within the sensing layer; and 3) they reach and interact with the sensing sites. The second step is largely determined by the structure of the interface between the bulk phase and the fluorescent layer, as well as the internal structure of the layer. The third step, however, is mainly decided by the chemical natures of the analyte and the sensing site, as well as the microenvironment of the site, which is similar to an enzyme-catalyzed reaction in biosystems. Therefore, any factor (whether physical or chemical) that is related to the structure or properties of the surface/interface of the fluorescent layer would influence the sensing property of the film, such as the molecular structure of the sensing site, surface functionalization, interfacial reactions, and the chemical nature of the substrate, etc.
Selectivity, sensitivity, stability, response speed, reversibility and reproducibility are the six main concerns in sensor development.15–18 Accordingly, in the manufacture of fluorescent sensing films, one must realize that: 1) the sensitivity and selectivity of a fluorescent sensing film are influenced by many factors, and their optimization is the most challenging problem; 2) the stability of a sensing film mainly depends on the chemical nature of the fluorophore, the fabrication strategy and the substrate employed; 3) the response speed of a sensing film is determined by the mass transfer of the analyte across the interface between the bulk phase and the sensing layer, as well as within the sensing layer, and the kinetics of the interaction between the analyte and the sensing sites is also an important factor that affects the sensing speed; and 4) the reversibility of a sensing film relies on the reaction mechanism of the sensing process, as well as the mass transfer of the analyte within the sensing layer. Based on this understanding, to construct a fluorescent sensing film with optimized properties we need to know that: 1) the sensitivity and selectivity of a sensing film are largely dependent upon efficient and specific interactions between the analyte and the active sensing sites, and therefore the precise matching of these is a key step in the design of a sensing film; 2) a hydrophilic fluorescent film will be more accessible for hydrophilic molecules while excluding hydrophobic molecules, and this must enhance the sensitivity and selectivity of a sensing film, provided that the analyte is hydrophilic; 3) photochemical stability is another prerequisite in the selection of sensing fluorophores; 4) non-covalent interactions and some reversible chemical reactions are conducive to reversibility; and 5) reproducibility is closely related to the fabrication techniques: good reproducibility can be achieved by precisely controlling the experimental conditions, especially in the process of construction of the sensing film. However, it needs to be noted that the factors addressed above are not independent and are correlated with each other.
Although research into the development of fluorescent sensing films is a challenging task, the truth is that the molecular structure of the sensing site and the internal structure of the sensing layer determine the sensing performance of a film. Specifically, the molecular structure of the fluorophore decides the reactivity of the sensing site, and the internal structure of the film exerts a tremendous effect on the mass transfer of the analyte, which as a result affects the sensitivity, response speed and reversibility of the sensing process. In general, two things are mainly involved in the construction of a fluorescent sensing film: a well-designed or selected sensing molecule and a suitable or efficient film fabrication technology. For a given fluorophore, a variety of its derivatives could be obtained by the utilization of organic synthesis. The fluorophore or its derivatives obtained in this way can be introduced into or onto the adlayer of a film via either a chemical or physical approach. As for film fabrication, a variety of techniques can be used, whereby different techniques may result in different internal structures. In this way, fluorescent films with diverse sensing performances could be manufactured.
3. Evolution of fluorescent sensing films
The fabrication technology decides the internal structure of a fluorescent sensing film, which will greatly affect the response speed, sensitivity and reversibility of the film. Fluorescent sensing films can be divided into three main types (Fig. 2): conventional fluorescent sensing films, self-assembled two-dimensional (SA-2D) fluorescent sensing films, and self-assembled three-dimensional (SA-3D) fluorescent sensing films. Normally, films are usually obtained by two methods: one is to introduce the designed sensing fluorophore onto a chosen substrate by using a physical technique (non-covalent bonding) such as spin-coating, dip-coating, and curtain-coating, etc.; the other is to immobilize the fluorophore on a suitable substrate via a chemical reaction (covalent bonding).15–18 Thus, films can be classified as physical films and chemical films. Most SA-2D films are chemical films, whereas conventional films and SA-3D films can be either physical films or chemical films. As several reviews have focused on conventional films15,16,18 and our group reviewed progress in SA-2D films a few years ago,17 we will focus our interest on some newly developed SA-3D films in this review. Moreover, some representative films with conventional or SA-2D structures will be included as well.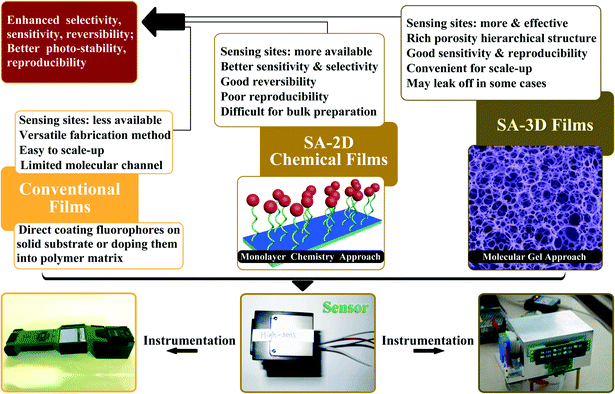 | ||
| Fig. 2 The three main types of fluorescent sensing film (top) and images of two sensor devices based on fluorescent sensing films (bottom). | ||
3.1 Conventional fluorescent sensing films
Conventional fluorescent sensing films are fabricated by the simple coating of the sensing fluorophore or one of its derivatives onto a substrate surface. For example, Yang et al. fabricated a thin solid film by using a green fluorescent pentiptycene derivative.28 The fluorescence of the film can be changed from yellow to red when different anilines are introduced, whereas the presence of benzoquinone vapor will quench the fluorescence. In addition, mechanical forces (grinding and shearing) can also cause changes in the fluorescence of the film.Among conventional films, polymer-based films are the most common type and are often obtained by chemically attaching or physically doping the sensing fluorophore onto/into a polymeric film.16 Much effort has been devoted to developing favorable polymer-based fluorescent sensing films and much progress has been achieved.15,16,29–42 Chemical bonding of the fluorophore into a polymer matrix is an efficient way to reduce leaching problems in a film sensor and helps to improve the stability of the film sensor.29 Tian et al. synthesized several types of polymerizable fluorescent monomer and prepared a series of polymer-based fluorescent sensing films.30–35 In 2010, they constructed a fluorescent sensing film by using polymerizable pH- and oxygen-sensing monomers. The film displayed good response over the pH range of 5.5–8.5 and exhibited linear Stern–Volmer quenching responses to dissolved oxygen.33 Later, another dual sensor for temperature and oxygen was also produced.34 In 2013, a polymer-based sensing film containing three different emitters (blue, green and red) was fabricated. This film generated three separate emissions and was used for the analysis of glucose and oxygen in biological conditions.35
Owing to the “molecular wire effect” and “super-quenching effect”, conjugated polymers have been used as excellent sensing materials, especially in film sensing.36–38 By introducing different fluorophores (such as quinoline, pyrene, etc.) into the backbones of conjugated polymers, a series of conjugated polymer-based fluorescent sensing films were obtained.36–39 In 2014, Swager et al. developed several conjugated cationic polymers with different accompanying counter-anions.39 The fluorescent films that were reported are sensitive to gaseous volatile amines and their detection limit (DL) could be as low as 1.4 ppm for aniline. Moreover, conjugated polymers with different counter-anions were employed to fabricate a sensor array and the resulting array was used to differentiate between amines.
Some biopolymers are also ideal candidates for fabricating fluorescent sensing films. One of the most attractive biopolymers, chitosan, possesses good film formation properties, and the resulting film often exhibits porous and networked structures.40 In other words, chitosan can not only function as a support to immobilize the sensing fluorophore but also provides extensive molecular channels for the analyte to diffuse within the layer. Based on this, several chitosan-based fluorescent sensing films were prepared.41,42 As an example, aggregates of hexaphenylsilole (HPS) were doped into chitosan (CS) film and a novel fluorescent sensing film for picric acid (PA) was obtained (Fig. 3).41 The film exhibits good selectivity in sensing PA in the solution phase and the detection limit (DL) is around 2.1 × 10−8 mol L−1. In another work, the sensing fluorophore (pyrene) was introduced by chemically reacting it with the amino group of chitosan and a sensing film for the detection of NO2− was obtained.42
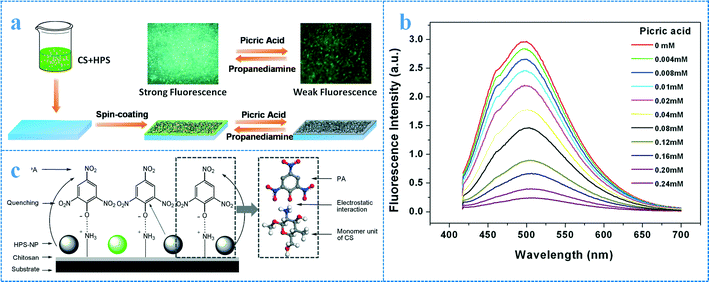 | ||
| Fig. 3 Conventional fluorescent sensing films for detecting PA: (a) illustration of film manufacture; (b) fluorescence emission spectra of the sensing film in the presence of different concentrations of PA (from top to bottom, 0, 0.004, 0.008, 0.01, 0.02, 0.04, 0.08, 0.12, 0.16, 0.2 and 0.24 mM) in an aqueous medium (λex = 397 nm); (c) schematic of selective sensing and the result of simulation of the electrostatic interaction between PA and the monomer unit of CS. Adapted from ref. 41. | ||
The matrix is crucial for the performance of conventional films; for example, in polymer-based films, the polymer composition and crosslinkers have a great influence on the sensing activity.29–35 The most outstanding advantage of conventional films is their ease of fabrication, which enables their bulk production. As discussed before, the internal structure of the sensing layer of a fluorescent film has a great impact on its sensing performance. When the sensing sites are randomly distributed within the sensing layer, a film will probably lack organized internal structures. Even when a large amount of the sensing fluorophore is loaded within the film layer, many sensing sites are liable to be submerged within the film. Therefore, many of these may have little chance of being reached by the analyte due to difficulties in mass transfer and, which is even worse, fluorescence from them will become background noise, which reduces the signal/noise ratio. Furthermore, molecules of the analyte that are bound by sensing sites located at deeper positions in the film will experience difficulties in leaving the layer again due to inconveniences in mass transfer. All of these will have negative effects on the sensing performance (such as sensitivity, response speed, and reversibility) of the film.
3.2 SA-2D fluorescent sensing films
SA-2D fluorescent sensing films are fabricated by a self-assembled monolayer (SAM) technique, in which the sensing fluorophores are covalently immobilized on a substrate surface (Fig. 1, right part). The typical feature of an SA-2D film is its monolayer structure. It is believed that most of the sensing sites are exposed in the monolayer film and are easily reached by the analyte. In other words, the utilization of the SAM technique is beneficial for improving the effectiveness of the sensing sites and this may result in an enhancement in sensitivity. Based on the SAM technique, a variety of SA-2D fluorescent sensing films were developed in the 2010s by choosing different sensing fluorophores, varying the linkers connecting the fluorophores and the substrates, and changing the substrates.17 Comprehensive studies were carried out on their constitution, structure and sensing properties. Several important concepts or models such as the spacer layer screening effect, spacer layer enrichment effect and two-dimensional solution model have been proposed, which greatly help the rational design of fluorescent sensing films with SA-2D structures. The most recent progress in this regard is the development of the concept of a ‘solvent-dependent side-chain conformation effect’, which was proposed to facilitate the development of conjugated polymer/oligomer-based fluorescent sensing films via the employment of a monolayer chemistry strategy.43–45 Unlike the construction of SA-2D films based on a low-molecular-mass fluorophore, the creation of novel SA-2D films based on a conjugated polymer or oligomer is hard to achieve via simply varying the linker structures, which is one of the most commonly adopted strategies in the creation of films with novel structures and properties. This is because varying the structure of a conjugated polymer or oligomer is much more difficult than derivatization of a low-molecular-mass fluorophore, which is partially because the reaction has to be conducted in a water- and oxygen-free environment. As is known, the conformation of an organic molecule or moiety with a relatively complex structure is usually dependent upon the solvent it encounters, and therefore the structure of the sensing layer of an SA-2D fluorescent film based on a conjugated polymer or oligomer would be altered by varying the medium (solvent in part) if the fluorescent conjugate bears suitable side chains. Based upon the considerations as described, novel SA-2D fluorescent films based on conjugated polymers or oligomers could be constructed via simply varying their side chain structures, which is the so-called ‘solvent-dependent side-chain conformation effect’. Progress in this respect will be briefly illustrated by the presentation of a few examples.In 2011, a fluorescent film was developed by the chemical immobilization of oligo(2,5-dihexadecyloxyphenyleneethynylene), which is a modified oligomer, on the surface of a glass plate via a short spacer.43 The film exhibited a reversible smart response to the presence of toxic volatile organic solvents such as benzene, toluene, chloroform, THF, and dichloromethane, etc. (Fig. 4). Fluorescence and molecular dynamics modeling studies revealed that the long, flexible alkyl chains played a crucial role in the smart sensing performance of the oligomer-based film. Moreover, the strategy that was proposed in the work provides a new way for the design of SAMs and conjugated oligomer-based fluorescent sensing films. Later, a derivative of 1,4-bis(phenylethynyl)benzene (BPEB) with cholic acid (CholA) in its side chains was synthesized and immobilized on the surface of a glass plate modified with (3-aminopropyl)trimethoxysilane to form an SA-2D fluorescent film (Fig. 5a).44 It was demonstrated that the two CholA moieties that were attached to the side chains of the fluorophore not only affected the fluorescence behavior of the film, but also favored the formation of hydrophilic pockets above the BPEB adlayer when the film was exposed to acetone. The film displayed a sensitive and selective response to the presence of HCl and some oxyacids such as HNO3, H2SO4 and H3PO4 in this solvent. Furthermore, another work was carried out in which the CholA moieties were replaced by cholesteryl (Chol) structures (Fig. 5b).45 In contrast to the film modified with CholA moieties, the as-prepared film with Chol in its side chains proved highly sensitive to the presence of PA and sensitive to 2,4,6-trinitrotoluene (TNT), 2,4-dinitrotoluene (DNT) and nitrobenzene (NB) in an aqueous phase, whereas the nitroaromatics (NACs) that were studied had little effect on the fluorescence emission of the film modified with CholA. More remarkably, the sensing process was fully reversible and free from interference by commonly found compounds, including methanol, THF, toluene, dichloromethane, ammonia, HCl, NaOH, NaCl, copper salts and seawater, etc. However, the reference films (without CholA or Chol in their side chains) in both cases mentioned above failed to sense the analyte. Therefore, it is reasonable to make the conclusion that the introduction of side units with special structures or properties into conjugated polymers/oligomers is a simple but effective way to create novel SA-2D fluorescent sensing films. This phenomenon confirmed the importance of the internal structure of a fluorescent film for its sensing performance.
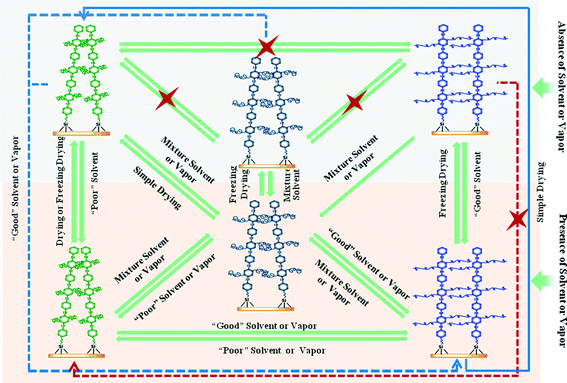 | ||
| Fig. 4 Representation of the conformations of a fluorescent film based on poly(2,5-dihexadecyloxyphenyleneethynylene) in different conditions and the relationships among them. Adapted from ref. 43. | ||
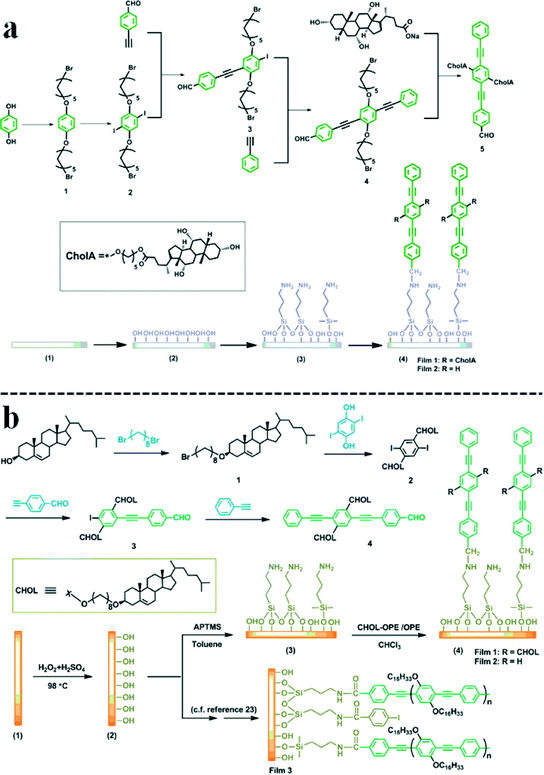 | ||
| Fig. 5 BPEB-based SA-2D fluorescent sensing film: (a) synthesis procedures of CholA-BPEB and its chemical immobilization on the surface of a glass plate; (b) preparation of OPE with cholesterol in its side chains and immobilization of BPEB and its derivatives on the surface of a glass plate modified with APTMS. Adapted from ref. 44 and 45. | ||
Although much progress was made in fluorescent film sensing when the SAM technique was employed for fabrication of SA-2D films, sensing based on these films suffers from other hindrances when they are employed in practice. Firstly, the bottleneck in the development of SA-2D films is that it is difficult to further improve the sensing sensitivity of the films. This is usually due to the low modifiable density of the substrate; that is to say, there are a limited number of reactive surface bonds on most of the substrate surface, which limits the surface density of the sensing fluorophore. As a result, only fluorophores with a high quantum yield and good photochemical stability can be used for the construction of SA-2D films. Even so, the signal/background noise ratios of these films are still low, and high-quality transducers are required when the films are used for the development of sensors. Secondly, the bulk production of SA-2D films is hard to achieve because the surface reaction encounters a number of insurmountable difficulties such as low efficiency and less uniformity, etc. These are the reasons why new strategies for the construction of high-performance fluorescent sensing films have to be developed.
3.3 SA-3D fluorescent sensing films
By learning from conventional physical films and SA-2D chemical films, it is anticipated that an efficient way to improve the sensitivity of a fluorescent sensing film is to develop a new strategy that could introduce more sensing sites into the fluorescent layer and at the same time retain most of the sites that are available for sensing. From the aspects of adjustment of the micro/nanostructure, a molecular gel strategy was involved in the fabrication of a fluorescent sensing film (Fig. 1, left part).Molecular gels are a type of attractive soft material and the most prominent characteristic of the materials is the self-assembled networked structure of the gelator within the gels.46 For a given gelator, various networked structures could be obtained when different gelation solvents were used.46–51Fig. 6 and 7 show some typical porosity networks formed within some molecular gels.48,49 It was found that tunable structures could be successfully obtained by varying the linkers in the gelators, the concentrations of the gelators or the gelling solvents. The highly ordered structures were probably formed via the self-assembly of molecules of the gelator in the solvent (Fig. 6g). In general, the amount of the gelator in a molecular gel accounts for only around 2 wt%, which means that most of the gel system is the solvent. Evaporation of the solvent produces xerogels, which are generally characterized by porous networked structures. Furthermore, the molecules of the gelator that are present within the networks are packed in a crystal-like state. In other words, they are well organized. Accordingly, porous and at least partially highly ordered fluorescent films will be produced if gel networks (formed by a fluorescent active gelator) are properly transferred onto a substrate surface.
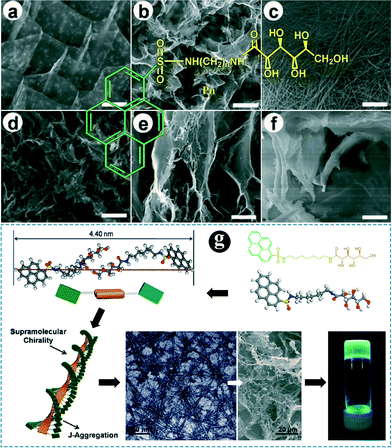 | ||
| Fig. 6 (a)–(f): SEM images of xerogels prepared from 2.0% Pn: (a) P2 in water, (b) P3 in water, (c) P4 in acetonitrile, (d) P6 in acetonitrile, (e) P7 in acetonitrile, and (f) P8 in acetonitrile. Bar distances = 10 μm. The structures of the Pn molecules with different linkers are shown in red. (g): Schematic of a possible aggregation mechanism of P7 in acetonitrile. Adapted from ref. 48. | ||
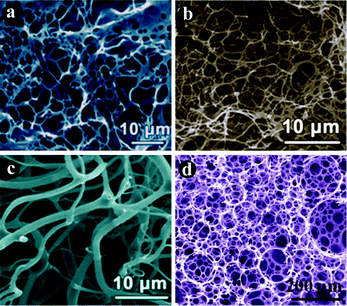 | ||
| Fig. 7 (a–c) SEM images of gel networks of several typical gelators at different concentrations. (d) A typical 3D network structure formed via a molecular gel strategy. Adapted from ref. 49. | ||
Based on the advantages of molecular gels and the requirements of fluorescent sensing films, it is rational to imagine that the introduction of a molecular gel strategy into the fabrication of fluorescent sensing films will probably result in a novel class of fluorescent films with favorable sensing performance due to ease of mass transfer and reproducibility.52,53 Hamachi et al. developed several supramolecular hydrogel hybrid systems for fluorescent sensing of polyanions or polyamines.54,55 Rissanen et al. invented a self-assembled hydrogel system and used it for the detection of pyrophosphate on a nanomolar level.56 In addition, Ajayaghosh et al. reported an SA-3D fluorescent film for the highly sensitive detection of TNT.57 In their studies, a fluorescent gelator was synthesized and used for the detection of TNT in different states. It was found that the fluorescence emission of the gelator was not sensitive to the presence of TNT in the solution state, but was highly sensitive to the analyte in the xerogel state. This finding highlights the unique capability of the SA fibrous structures as new materials in contrast to the individual molecules in specific sensing applications.
In general, an SA-3D film could be obtained by first spin-coating or dip-coating a fluorescent molecular gel onto a suitable substrate surface at temperatures above its Tgel, which is its specific phase transition (gel to solution) temperature, or at a concentration below its critical gelation concentration (CGC), and then evaporating the solvent inside.55 The films produced in this way have at least three advantages: 1) they possess more sensing sites in the fluorescent layer than those of SA-2D films, and this partially overcomes the shortcomings of SA-2D films; 2) they provide a much larger surface area and higher porosity, which are beneficial for mass transfer, resulting in a faster response speed and better reversibility, and, in addition, most of the sensing sites become active and are accessible for analyte recognition; and 3) the integration of different non-covalent interactions such as hydrophobic interactions, hydrogen bonding, π–π stacking, electrostatic interactions, and van der Waals forces within the gelator networks not only helps to overcome the shortcomings of physical films based on weak interactions such as lower stability, but also enables them to be used in both gaseous and solution phases provided only a poor solvent is used.
To construct fluorescent sensing films for organic amines, PBI and cholesterol were selected as functional units. On the one hand, PBI is an attractive molecule and exhibits excellent photochemical and thermal stability, a high fluorescence quantum yield and novel optoelectronic properties. In fact, PBI and its derivatives have found applications in many fields such as liquid crystals, organogels, photoinduced electron transfer systems and organic electronic devices, etc. Furthermore, the energy levels of the highest occupied (π) and lowest unoccupied (π*) molecular orbitals (HOMO and LUMO) of PBI can be tuned to match those of some organic amines and facilitate electron transfer from the amines to their photoexcited state.67 Accordingly, rationally designed PBI derivatives are useful for the detection of organic amines, which is usually achieved by regulating the π-stacking of the PBI backbone. On the other hand, cholesterol derivatives are versatile building blocks with preferable self-assembly properties and are some of the most studied low-molecular-mass gelators.50 Hence, it is anticipated that the combination of PBI and cholesterol would produce compounds that are not only capable of self-assembly but are also sensitive to some organic amines.
Based on the above considerations, compounds 1–3 were designed and synthesized.26,60,61Fig. 8 shows the molecular structures and some of the self-assembled networked structures of the three compounds. As expected, the compounds were able to form self-assembled structures with the preferable porosity. Film 1 is characterized by ordered nanofibers with a diameter of ca. 80 nm (Fig. 8a);60 film 2 is composed of an abundance of spherical nanoparticles (∼150 nm) (Fig. 8b);61 film 3 comprises fibrous networks of a size of about 100 nm (Fig. 8c).26 Fluorescence sensing studies demonstrated that these films exhibited a fast response (a few seconds) (Fig. 9b), high sensitivity, and good reversibility (Fig. 9c) in the detection of organic amines in the vapor phase. The films were stable and their fluorescence emissions decreased slightly after several hours' illumination by the light source of the fluorescence instrument used (Fig. 9d). However, there were some differences in their sensing performance. The emission of film 1 could be quenched by ten commonly found organic amines, which are important contaminants in air. The quenching efficiencies varied from ∼60% to ∼80%.60 Film 2 was also sensitive to the presence of most of the organic amines being tested, but the quenching efficiencies varied from ∼40% to ∼85%.61 Film 3 was excellent in sensing N-methamphetamine (MAPA), which is one of the most widely abused drugs. The detection limit for MAPA could be as low as 5.5 ppb.26
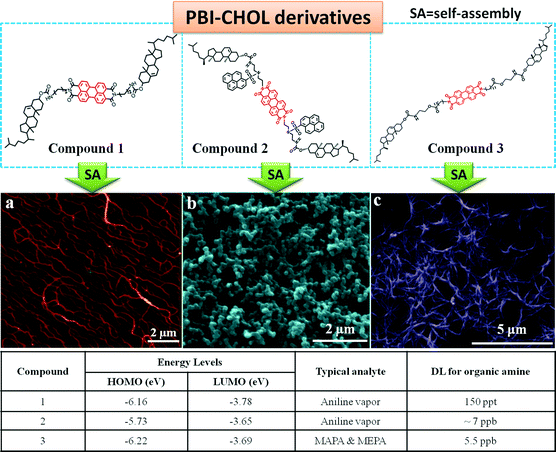 | ||
| Fig. 8 Molecular structures of compounds 1–3 and morphologies of films 1–3: AFM image of film 1 (a), SEM image of film 2 (b) and SEM image of film 3 (c). Reproduced with permission from ref. 26, 60 and 61. | ||
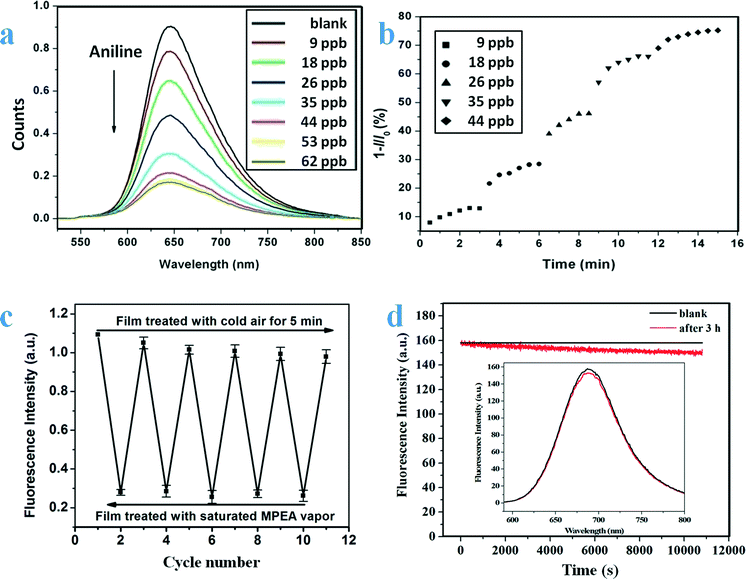 | ||
| Fig. 9 (a): Fluorescence emission spectra of film 1 in the presence of different concentrations of aniline (from top to bottom 0, 9, 18, 26, 35, 44, 53, 62 ppb; data error ±4%). The fluorescence intensity of the film decreased when aniline was introduced into the system. (b): Time-dependent quenching efficiency of the film exposed to different concentrations of aniline; for each concentration, each measurement was conducted every 30 seconds and it can be seen that the equilibrium time was about 3 min. (c): Reversible response of film 3 to saturated MPEA vapor. The fluorescence intensity of film 3 alternated between ∼1.0 and ∼0.3 when the film was treated with either cold air or MPEA vapor (λex = 467 nm; λem = 678 nm). (d): Photostability of film 3. The red line represents the fluorescence intensity (λex = 467 nm; λem = 678 nm) of film 3 at different time points and the black line represents the control. The inset shows the fluorescence emission spectra of film 3 before (black) and after (red) excitation at 467 nm for 3 hours. Reproduced with permission from ref. 26 and 60. | ||
To understand the sensing mechanism of the as-prepared SA-3D films, the energy levels (HOMO and LUMO) of the three molecules were calculated, and the results are shown in the table in Fig. 8. It is seen that their HOMO energies are in between the HOMO energies of aniline and phenols. This result explains why phenols displayed little interference in the detection of the organic amines. A further test with a film that was prepared by directly coating a routine solution of the fluorescent compound onto the surface of a glass plate demonstrated very poor sensing ability, which suggests that both the structure of the sensing fluorophore and the internal structure of the film are the key factors in determining the sensing performance of a film. Specifically, the molecular structure determines the binding strength or the thermodynamics between the analyte and the sensing sites of the film, as well as the photophysical interaction between them, while the film structure decides the mass transfer or dynamics of the sensing process.
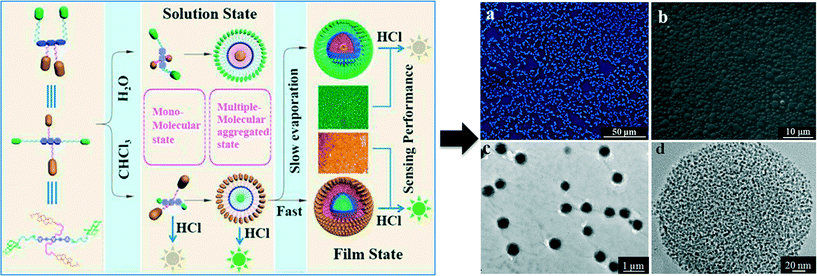 | ||
| Fig. 10 Left: Aggregation behavior of compound 4 in different mediums or films dried by different methods and the corresponding responses of the compound to HCl in different states. Fluorescence (a) and SEM (b) images of film 4. TEM (c) and HRTEM images (d) of the spherical particles in film 4. Reproduced with permission from ref. 62. | ||
Based on the unique self-assembly properties of compound 4, two SA-3D fluorescent films, film 4 and film 5, were fabricated by using two solutions of different concentrations of the compound, of which film 4 was obtained from a solution with a concentration of 1 × 10−3 mol L−1, and film 5 from a solution with a concentration of 1 × 10−4 mol L−1. SEM studies revealed that film 4 was composed of well-defined spherical particles of a diameter between 1 and 3 μm (Fig. 10b), whereas film 5 comprised spherical pores of an average diameter of ∼10 μm. Furthermore, it is surprising to find that the spherical particles in film 4 were composed of hundreds of loosely packed small particles (about 1–3 nm, Fig. 10c and d), which is beneficial for mass transfer. What is more interesting is that the two films exhibited very different properties in the sensing of HCl. Film 4 was proved to be reversible and sensitive to HCl vapor with a detection limit of 0.4 ppb (Fig. 11a), whereas HCl at ppb levels had no effect on the fluorescence emission of film 5 (Fig. 11b). The presence of HCl in concentrations between 88 and 2860 ppm only resulted in a decrease of less than 25% in the original fluorescence emission of film 5 (Fig. 11b). These studies demonstrated that the introduction of auxiliary structures with opposite properties into a typical fluorophore is an efficient strategy for developing fluorescent supramolecular motifs with plentiful assembly properties and wide application potential. Moreover, a study of the sensing performance of film 4 and film 5 further confirmed the aforementioned statement that both the molecular structure and the film morphology are pivotal issues in the construction of a fluorescent sensing film.
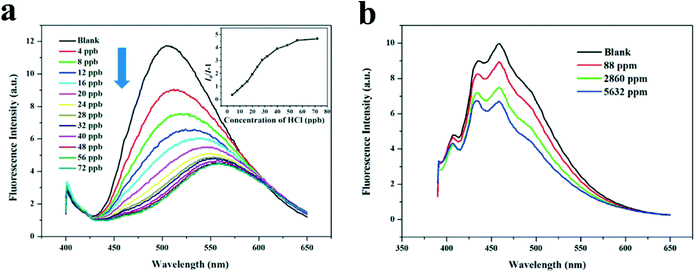 | ||
| Fig. 11 Fluorescence response of film 4 and film 5 to HCl. (a): Fluorescence emission spectra and Stern–Volmer plot (inset) of film 4 in the presence of different concentrations of HCl (data error: ±3%). It is observed that the fluorescence intensity of film 4 gradually decreased when the concentration of HCl vapor was increased. (b): Fluorescence emission spectra of film 5 in the presence of different concentrations of HCl vapor (data error: ±3%), which show that the fluorescence intensity of film 5 is not sensitive to HCl vapor. It is obvious that film 4 behaves much better than film 5 in the sensing of HCl vapor. Reproduced with permission from ref. 62. | ||
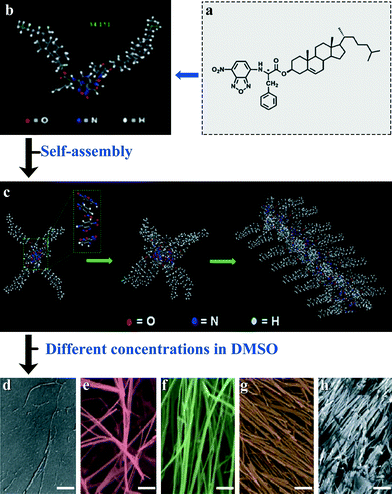 | ||
| Fig. 12 Structure of compound 5 (a and b) and the proposed self-assembly mechanism of the compound (c). SEM images of the gel system of compound 5 in DMSO with different concentrations (w/v): (d) 0.05%, (e) 0.50%, (f) 1.00%, (g) 2.50%, and (h) 5.00%. Scale bar = 3 μm. Reproduced with permission from ref. 25. | ||
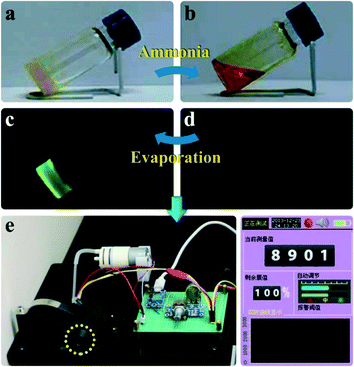 | ||
| Fig. 13 Reversible gel–sol phase transition of the gel of compound 5 in DMSO via the injection and evaporation of gaseous ammonia (a–d). (a) and (b) are photos taken in sunlight showing the reversible transition between the yellow gel (a) and the copper-brown solution (b) via the injection or evaporation of NH3 vapor. (c) and (d) are the corresponding images of the reversible gel–sol transformation under UV light. The two images at the bottom (e) show one of our typical conceptual sensor devices. The left image shows the detailed structure of the sensor device and the yellow circle highlights where the fluorescent sensing film is fixed. The right image shows the display unit connected to the sensor device. Images (a)–(d) are reproduced with permission from ref. 25. | ||
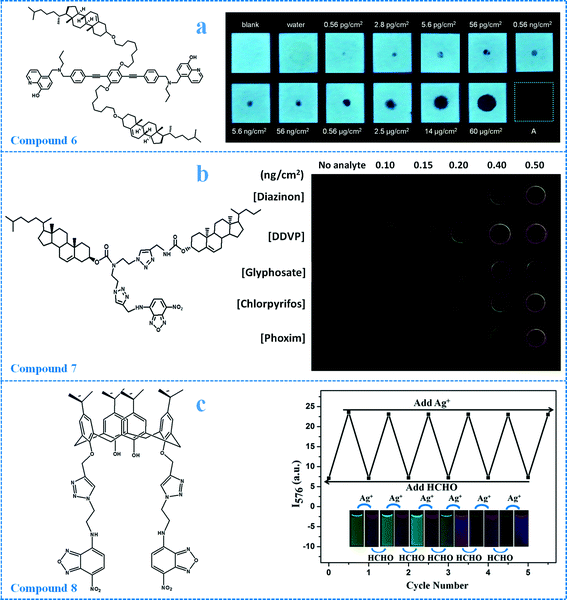 | ||
| Fig. 14 Molecular structures of compounds 6–8 and their sensing performance. a): Images of test strips coated with compound 6 (1 × 10−3 mol L−1, 20 μL) in the presence of different amounts of diethyl chlorophosphate in contact mode when viewed under UV illumination at 365 nm. When drops of diethyl chlorophosphate solution were loaded onto the coated test strips, non-fluorescent black spots appeared. It can be observed that the areas of the black spots increased when more diethyl chlorophosphate was loaded. b): The fluorescence of compound 7 can be selectively quenched by Hg2+ and the addition of OPs will recover the fluorescence. Based on this, the complex (compound 7-Hg2+) was used for the sensing of OPs in both solution and film states. The images in b) show TLC plates coated with the complex upon exposure to different amounts of OPs under excitation at 365 nm. The plates coated with the complex show little fluorescence (left column), but noticeable fluorescence was observed when the five OPs (from top to bottom: diazinon, dimethyl dichlorovinyl phosphate, glyphosate, chlorpyrifos and phoxim) were added. It is seen that the fluorescence of the plates increased with an increase in the concentration of the OPs (columns 2 to 5). c): The fluorescence of compound 8 changed from green (∼526 nm) to red (∼576 nm) when Ag+ was added, whereas the fluorescence reverted to green upon the addition of formaldehyde (the inset image). In addition, the response of compound 8 to Ag+ and formaldehyde was reversible and the process could be repeated for more than 5 cycles. The fluorescence intensity (λex = 460 nm; λem = 576 nm) of compound 8 varied from ∼25 to ∼7 when Ag+ or formaldehyde was added to the system (the plot on the right). The concentration of compound 8 was 2 × 10−6 mol L−1 and 50 μL Ag+ (4 × 10−3 mol L−1) or 55 μL formaldehyde (4 × 10−3 mol L−1) was added to the system successively. Reproduced with permission from ref. 27, 63 and 64. | ||
4. Conclusion and outlook
Owing to the characteristics of fluorescence techniques and the advantages of film sensing, fluorescent films have shown great potential in sensing applications and sensor development. During at least the last ten years, a variety of fluorescent sensing molecules have been reported with significant progress. However, there is still a long way to go from a sensing molecule to a sensor device. The fabrication of a fluorescent film is a critical step in the transformation from a sensing molecule into a sensor device, in which molecular design and film fabrication are equally important issues.With the aim of producing usable fluorescent sensors, fluorescent sensing films of different constitutions and structures were designed and fabricated: conventional films, SA-2D films, and SA-3D films. Each type of fluorescent sensing film has its advantages and disadvantages. The intended use, molecular structure and film fabrication strategy should be considered together in the design of a fluorescent film. Conventional films are advantageous in terms of their production but their internal structures need further optimization. Some SA-2D films exhibit good sensitivity, response speed and reversibility. However, the efficiency of their surface reaction is low and it is difficult to obtain uniform surface structures, which restricts their mass production. Regarding molecular gel-based SA-3D films, although they have their advantages, as discussed, and at the same time retain the advantages of conventional films such as being easier to fabricate, which overcomes the serious limitations of monolayer chemistry-based SA-2D films, it is to be noted that these kinds of film have their own limitations. Firstly, SA-3D films are often mechanically weak, which may limit their applications in some circumstances, especially in solution-phase sensing. Secondly, knowledge of the relationship between the structure of a gelator and that of an SA-3D film is extremely limited and this leads to unpredictability in the internal structure of SA-3D films, which is crucial for the sensing performance of the films. Thirdly, studies of the creation and sensing performance of fluorescent SA-3D films are in their early stage, and the number of chemicals that could be sensitively and selectively sensed by them is limited. Therefore, much effort needs to be devoted to creating more desirable fluorescent sensing films, to investigating the relationship between the structures (of both the sensing molecules and the films) and the sensing properties, and to developing new strategies to improve the sensing performance and photochemical stability of films. In addition, the investigation of the inner relationship between the structure of a fluorescent site and that of the corresponding sensing film is also of great importance for the ultimate control of the fabrication of high-performance fluorescent sensing films. Moreover, comprehensive studies of the sensing mechanism that include not only the interactions between the sensing sites and the analyte but also the sensing dynamics relevant to mass transfer are also required. Furthermore, it is worth mentioning that greater challenges will occur when the as-developed films are employed for the manufacture of sensor devices and in real-life applications. Studies in this emerging and highly attractive area are urgently needed.
Acknowledgements
We acknowledge the funding from the Natural Science Foundation of China (21273141, 20206089, 21527802), the 111 project (B14041), and the Program for Changjiang Scholars and Innovative Research Team in University (IRT-14R33).References
- Y. Xie, T.-H. Tsai, A. Konneker, B.-I. Popa, D. J. Brady and S. A. Cummer, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 10595 CrossRef CAS PubMed.
- Y. Cui, Q. Wei, H. Park and C. M. Lieber, Science, 2001, 293, 1289 CrossRef CAS PubMed.
- O. S. Wolfbeis, Angew. Chem., Int. Ed., 2013, 52, 9864 CrossRef CAS PubMed.
- A. T. Sage, J. D. Besant, B. Lam, E. H. Sargent and S. O. Kelley, Acc. Chem. Res., 2014, 47, 2417 CrossRef CAS PubMed.
- K.-R. Lin, C.-H. Chang, T.-H. Liu, S.-W. Lin and C.-H. Lin, J. Biomech., 2011, 44, 1879 CrossRef PubMed.
- F. Kashfi and J. Draper, Microelectron. J., 2014, 45, 500 CrossRef.
- H. Tsai, Y. Lin, H. W. Chang and C. B. Fuh, Biosens. Bioelectron., 2008, 24, 485 CrossRef CAS PubMed.
- C. McDonagh, C. S. Burke and B. D. MacCraith, Chem. Rev., 2008, 108, 400 CrossRef CAS PubMed.
- O. S. Wolfbeis, BioEssays, 2015, 37, 921 CrossRef CAS PubMed.
- R. C. Hamilton, S. K. Sahoo, S. Kamila, N. Singh, N. Kaur, B. W. Hylanda and J. F. Callan, Chem. Soc. Rev., 2015, 44, 4415 RSC.
- A. T. Aron, K. M. Ramos-Torres, J. A. Cotruvo Jr. and J. C. Christopher, Acc. Chem. Res., 2015, 48, 2434 CrossRef CAS PubMed.
- M. Burnworth, S. J. Rowan and C. Weder, Chem. – Eur. J., 2007, 13, 7828 CrossRef CAS PubMed.
- X. Wang and O. S. Wolfbeis, Chem. Soc. Rev., 2014, 43, 3666 RSC.
- B. G. Imsick, J. R. Acharya and E. E. Nesterov, Adv. Mater., 2013, 25, 120 CrossRef CAS PubMed.
- L. Basabe-Desmonts, D. N. Reinhoudt and M. Crego-Calama, Chem. Soc. Rev., 2007, 36, 993 RSC.
- S. W. Thomas, G. D. Joly and T. M. Swager, Chem. Rev., 2007, 107, 1339 CrossRef CAS PubMed.
- L. Ding and Y. Fang, Chem. Soc. Rev., 2010, 39, 4258 RSC.
- W. Guan, W. Zhou, J. Lu and C. Lu, Chem. Soc. Rev., 2015, 44, 6981 RSC.
- E. Kim, Y. Lee, S. Lee and S. B. Park, Acc. Chem. Res., 2015, 48, 538 CrossRef CAS PubMed.
- J. Chen, Y.-H. Chan, T. Yang, S. E. Wark, D. H. Son and J. D. Batteas, J. Am. Chem. Soc., 2009, 131, 18204 CrossRef CAS PubMed.
- J. Lu, J.-X. Yang, J. Wang, A. Lim, S. Wang and K. P. Loh, ACS Nano, 2009, 3, 2367 CrossRef CAS PubMed.
- T. Joshi, B. Graham and L. Spiccia, Acc. Chem. Res., 2015, 48, 2366 CrossRef CAS PubMed.
- S. Guo and E. Wang, Nano Today, 2011, 6, 240 CrossRef CAS.
- G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184 CrossRef CAS PubMed.
- H. Yu, Y. Lü, X. Chen, K. Liu and Y. Fang, Soft Matter, 2014, 10, 9159 RSC.
- M. He, H. Peng, G. Wang, X. Chang, R. Miao, W. Wang and Y. Fang, Sens. Actuators, B, 2016, 227, 255 CrossRef CAS.
- Y.-Y. Qi, X.-H. Sun, X.-M. Chang, R. Kang, K.-Q. Liu and Y. Fang, Acta Phys.-Chim. Sin., 2016, 32, 373 CAS.
- Y. Matsunaga and J.-S. Yang, Angew. Chem., Int. Ed., 2015, 54, 7985 CrossRef CAS PubMed.
- D. T. McQuade, A. E. Pullen and T. M. Swager, Chem. Rev., 2000, 100, 2537 CrossRef CAS PubMed.
- Y. Tian, B. R. Shumway, W. Gao, C. Youngbull, M. R. Holl, R. H. Johnson and D. R. Meldrum, Sens. Actuators, B, 2010, 150, 579 CrossRef CAS PubMed.
- Y. Tian, E. Fuller, S. Klug, F. Lee, F. Su, L. Zhang, S. Chao and D. R. Meldrum, Sens. Actuators, B, 2013, 188, 1 CrossRef CAS PubMed.
- Y. Tian, B. R. Shumway and D. R. Meldrum, Chem. Mater., 2010, 22, 2069 CrossRef CAS PubMed.
- Y. Tian, B. R. Shumway, A. C. Youngbull, Y. Li, A. K.-Y. Jen, R. H. Johnson and D. R. Meldrum, Sens. Actuators, B, 2010, 147, 714 CrossRef CAS PubMed.
- X. Zhou, F. Su, Y. Tian, R. H. Johnson and D. R. Meldrum, Sens. Actuators, B, 2011, 159, 135 CrossRef CAS PubMed.
- L. Zhang, F. Su, S. Buizer, H. Lu, W. Gao, Y. Tian and D. Meldrum, Biomaterials, 2013, 34, 9779 CrossRef CAS PubMed.
- G. He, N. Yan, H. Cui, T. Liu, L. Ding and Y. Fang, Macromolecules, 2011, 44, 7096 CrossRef CAS.
- G. He, N. Yan, J. Yang, H. Wang, L. Ding, S. Yin and Y. Fang, Macromolecules, 2011, 44, 4759 CrossRef CAS.
- D. H. Zhao and T. M. Swager, Macromolecules, 2005, 38, 9377 CrossRef CAS.
- S. Rochat and T. M. Swager, Angew. Chem., Int. Ed., 2014, 53, 9792 CrossRef CAS PubMed.
- Z. Sun, F. Lv, L. Cao, L. Liu, Y. Zhang and Z. Lu, Angew. Chem., Int. Ed., 2015, 54, 7944 CrossRef CAS PubMed.
- G. He, H. Peng, T. Liu, M. Yang, Y. Zhang and Y. Fang, J. Mater. Chem., 2009, 19, 7347 RSC.
- Y. Fang, G. Ning, D. Hu and J. Lu, J. Photochem. Photobiol., A, 2000, 135, 141 CrossRef CAS.
- G. He, N. Yan, H. Kong, S. Yin, L. Ding, S. Qu and Y. Fang, Macromolecules, 2011, 44, 703 CrossRef CAS.
- H. Cui, G. He, H. Wang, X. Sun, T. Liu, L. Ding and Y. Fang, ACS Appl. Mater. Interfaces, 2012, 4, 6935 CAS.
- H. Wang, G. He, X. Chen, T. Liu, L. Ding and Y. Fang, J. Mater. Chem., 2012, 22, 7529 RSC.
- L. You, D. Zha and E. V. Anslyn, Chem. Rev., 2015, 115, 7840 CrossRef CAS PubMed.
- N. Yan, Z. Xu, K. K. Diehn, S. R. Raghavan, Y. Fang and R. G. Weiss, J. Am. Chem. Soc., 2013, 135, 8989 CrossRef CAS PubMed.
- N. Yan, Z. Xu, K. K. Diehn, S. R. Raghavan, Y. Fang and R. G. Weiss, Langmuir, 2013, 29, 793 CrossRef CAS PubMed.
- J. Yan, J. Liu, P. Jing, C. Xu, J. Wu, D. Gao and Y. Fang, Soft Matter, 2012, 8, 11697 RSC.
- J. Liu, P. He, J. Yan, X. Fang, J. Peng, K. Liu and Y. Fang, Adv. Mater., 2008, 20, 2508 CrossRef CAS.
- N. Yan, H. Zhang, Z. Xu and Y. Fang, Chin. Sci. Bull., 2012, 57, 4310 CrossRef CAS.
- Z. Xu, J. Peng, N. Yan, H. Yu, S. Zhang, K. Liu and Y. Fang, Soft Matter, 2013, 9, 1091 RSC.
- K. Liu, T. Liu, X. Chen, X. Sun and Y. Fang, ACS Appl. Mater. Interfaces, 2013, 5, 9830 CAS.
- A. Wada, S. Tamaru, M. Ikeda and I. Hamachi, J. Am. Chem. Soc., 2009, 131, 5321 CrossRef CAS PubMed.
- M. Ikeda, T. Yoshii, T. Tanida, T. Matsui, H. Komatsu and I. Hamachi, J. Am. Chem. Soc., 2011, 133, 1670 CrossRef CAS PubMed.
- S. Bhowmik, B. N. Ghosh, V. Marjomäki and K. Rissanen, J. Am. Chem. Soc., 2014, 136, 5543 CrossRef CAS PubMed.
- K. K. Kartha, S. Babu, S. Srinivasan and A. Ajayaghosh, J. Am. Chem. Soc., 2012, 134, 4834 CrossRef CAS PubMed.
- C. Yu, M. Xue, K. Liu, G. Wang and Y. Fang, Langmuir, 2014, 30, 1257 CrossRef CAS PubMed.
- T. Liu, L. Ding, K. Zhao, W. Wang and Y. Fang, J. Mater. Chem., 2012, 22, 1069 RSC.
- H. Peng, L. Ding, T. Liu, X. Chen, L. Li, S. Yin and Y. Fang, Chem. – Asian J., 2012, 7, 1576 CrossRef CAS PubMed.
- G. Wang, X. Chang, J. Peng, K. Liu, K. Zhao, C. Yu and Y. Fang, Phys. Chem. Chem. Phys., 2015, 17, 5441 RSC.
- X. Sun, Y. Qi, H. Liu, J. Peng, K. Liu and Y. Fang, ACS Appl. Mater. Interfaces, 2014, 6, 20016 CAS.
- Y. Lü, Q. Sun, B. Hu, X. Chen, R. Miao and Y. Fang, New J. Chem., 2016, 40, 1817 RSC.
- S. Zhang, H. Yang, Y. Ma and Y. Fang, Sens. Actuators, B, 2016, 227, 271 CrossRef CAS.
- B. Hu, K. Liu, X. Chen and Y. Fang, Sci. China: Chem., 2014, 57, 1544 CrossRef CAS.
- Y. Ma, C. Yu, K. Liu, X. Cai, S. Zhang and Y. Fang, Yingxiang Kexue Yu Guang Huaxue, 2015, 33, 67 CAS.
- D. Görl, X. Zhang, V. Stepanenko and F. Würthner, Nat. Commun., 2015, 11, 7009 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2016 |



