Hierarchical architectures of Co3O4 ultrafine nanowires grown on Co3O4 nanowires with fascinating electrochemical performance†
Lei
An
a,
Li
Yu
b,
Yunjiu
Cao
ac,
Wenyao
Li
ad,
Kaibing
Xu
a,
Tao
Ji
ac,
Rujia
Zou
ae and
Junqing
Hu
*a
aState Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Materials Science and Engineering, Donghua University, Shanghai 201620, China. E-mail: hu.junqing@dhu.edu.cn
bIan Wark Research Institute, University of South Australia, Mawson Lakes, SA 5095, Australia
cSchool of Fundamental Studies, Shanghai University of Engineering Science, Shanghai 201620, China
dSchool of Material Engineering, Shanghai University of Engineering Science, Shanghai 201620, China
eCenter of Super-Diamond and Advanced Films (COSDAF), Department of Physics and Materials Science, City University of Hong Kong, Hong Kong
First published on 2nd November 2015
Abstract
A facile method to synthesize hierarchical architectures of Co3O4 nanowires@Co3O4 ultrafine nanowires grown on Ni foam was developed here. The unique architectures consisting of numerous ultrafine Co3O4 nanowires (shell) well grown on the surface of a Co3O4 nanowire (core) delivered remarkable electrochemical performance with ultrahigh specific capacitance (1640 F g−1 at a current density of 2 mA cm−2), superior rate capability (66% retention of the initial capacitance from 2 mA cm−2 to 50 mA cm−2) and outstanding cycling stability (∼99.03% retention of the initial capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles). Such fascinating capacitive behaviors can make these hierarchical architectures of Co3O4 nanowires@Co3O4 ultrafine nanowires promising electrode materials in electrochemical applications.
000 cycles). Such fascinating capacitive behaviors can make these hierarchical architectures of Co3O4 nanowires@Co3O4 ultrafine nanowires promising electrode materials in electrochemical applications.
Introduction
In recent years, unremitting efforts have been contributed to the exploration of high-performance electrode materials for electrochemical capacitors (ECs), one of the most effective and practical devices for electrochemical energy storage.1–6 There are two kinds of supercapacitors based on different ways of charge storage: electrical double-layer capacitors (EDLCs), which store charges via reversible ion absorption at the electrode/electrolyte interface, and pseudocapacitors, in which charge is stored based on fast and reversible surface redox reactions taking place on electrode surfaces, demonstrating prominently higher specific capacitance than EDLCs.7–10Among various electrode materials of supercapacitors, cobaltosic oxide (Co3O4) can be one of the advanced electrode materials due to its high theoretical capacitance (3560 F/g), low cost, controllable size and shape, high availability, and good corrosion resistance in alkaline solutions.11–17 To achieve out-bound electrochemical performance, it is significant to possess more electroactive sites for faradic redox reaction and enhance the kinetics of electron and ion transport on electrodes and the electrode–electrolyte interface. Recently, numerous nanostructured Co3O4 morphologies have been investigated to meet such requirements i.e. nanoparticles,18 dendrite-like nanostructures,19 nanocubes,20 and rhombic dodecahedral structures.21 However, most of the reported literature usually focused on the enhancement of their absolute specific capacitance values, and little attention has been paid to other attributes, such as the improvement of high-rate capabilities, an important factor to enhance the electrochemical performance of an electrode material. Severe capacitance degradations at high current densities were discovered in above-mentioned reports for Co3O4 material. The recession may be attributed to insufficient active material involved in redox reaction with the increase of current density.22 It is reported that the diffusion distance of electrolytes in electrodes is only ∼20 nm and active materials over this value can make less contribution to the total capacitance during the charge/discharge process.23 Thus, ultrafine nanowires with a diameter below 10 nm can be investigated for use in high-performance supercapacitors. Additionally, integrated architectures with core/shell or hierarchical nanostructures have been fabricated as hybrid supercapacitor electrodes and show good electrochemical properties as the core can offer an efficient way of transporting the ions or electrons, while the shell offers small voids for high ion accessibility.24–26 Although hierarchical structures have been prepared from metal oxides, the controllable synthesis of a Co3O4 material with desirable hierarchical architecture possessing an ultrafine nanostructure and predominant electrochemical performance with high specific capacitance, superior rate capability and long lifespan simultaneously still remains a great challenge.
Herein, we have reported a facile effective hydrothermal method for the growth of hierarchical architectures of Co3O4 nanowires@Co3O4 ultrafine nanowires on Ni foam and investigated their electrochemical performance as electrode materials for supercapacitors by means of cyclic voltammetry, the galvanostatic charge–discharge method and impedance spectroscopy and compared the performance with those of conventional Co3O4 nanowires without ultrafine nanostructures. The hierarchical architectures of Co3O4 nanowires@ nanowires exhibited a high specific capacitance of 1640 F g−1 at 2 mA cm−2, which is superior to 560 F g−1 of the conventional Co3O4 nanowires at the same current density. More importantly, these unique microstructures delivered a high specific capacitance (1083 F g−1) at a current density as high as 50 mA cm−2, a superior rate performance (∼66% retention of the initial capacitance from 2 mA cm−2 to 50 mA cm−2) and a long lifespan (∼99.03% retention of the initial capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles). Such intriguing capacitive behavior is ascribed to the unique core/shell hierarchical configuration with ultrafine nanostructures, leading Co3O4 nanowires@Co3O4 ultrafine nanowires to be promising electrode materials in electrochemical applications.
000 cycles). Such intriguing capacitive behavior is ascribed to the unique core/shell hierarchical configuration with ultrafine nanostructures, leading Co3O4 nanowires@Co3O4 ultrafine nanowires to be promising electrode materials in electrochemical applications.
Experimental characterization
All the chemicals were of analytical grade and used without further purification.Synthesis
All the chemicals were of analytical grade and used without further purification. Firstly, one segment of Ni foam (∼3 cm × 1 cm) was carefully cleaned with 6 M HCl solution in an ultrasound bath for 20 min to remove a NiO layer from the surface, and then rinsed with deionized water and absolute ethanol several times until neutral. Secondly, 1 mmol of Co(NO3)2·6H2O and 15 mmol of urea were dissolved in 40 mL of deionized water to form a homogeneous magenta solution. The solution and as pre-treated Ni foam were transferred into a 50 mL Teflon-lined stainless-steel autoclave, which was sealed and maintained at 110 °C for 5 h. After being cooled down to room temperature, the products were collected and washed with deionized water and absolute ethanol several times, then vacuum dried at 60 °C for 4 h. Finally, the products were calcined at 350 °C in a normal N2 atmosphere with a ramping rate of 1 °C min−1 for 2 h. Conventional Co3O4 nanowires were prepared according to a previous publication.23Characterization
X-ray diffraction (XRD) patterns were obtained using a D/max-2550 PC X-ray diffractometer (XRD; Rigaku, Cu-Kα radiation). The morphology of the materials was examined using a scanning electron microscope (SEM; S-4800) equipped with an energy dispersive X-ray spectrometer (EDX) and a transmission electron microscope (TEM; JEM-2100F). The mass of electrode materials was weighed on an XS analytical balance (Mettler Toledo; δ = 0.01 mg).Electrochemical measurement
Electrochemical measurements were performed on an Autolab Electrochemical Workstation (PGSTAT302N) using a three electrode electrochemical cell and 2 M KOH as the electrolyte. The Ni-foam-supported electrode materials (∼1 cm × 1 cm, ∼1.18 and ∼0.82 mg cm−2 for hierarchical Co3O4 nanowires@nanowires and conventional Co3O4 nanowires, respectively) were used directly as the working electrode. A saturated calomel electrode (SCE) was used as the reference electrode and a platinum (Pt) sheet electrode was used as the counter electrode. All potentials were referenced to the reference electrode. The specific capacitance and current density were calculated based on the mass of these electroactive materials.Results and discussion
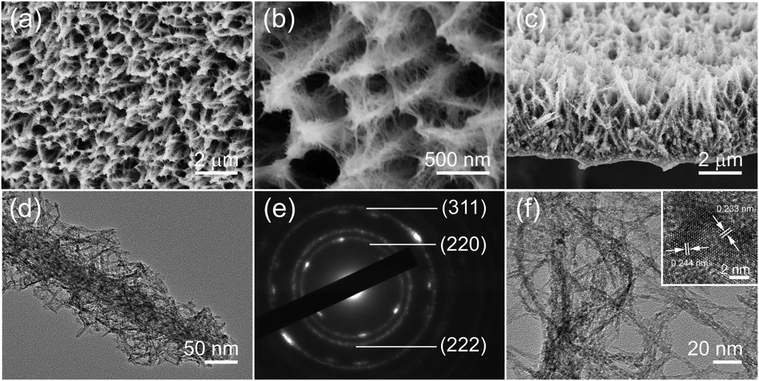
The Co-based intermediates are first obtained from the magenta hydrothermal reaction, and their morphology was characterized by SEM, with the results shown in Fig. S1 (ESI†). It can be clearly identified from Fig. S1a (ESI†) that the intermediates with high density are uniformly distributed on Ni foam, forming an ordered array with an opened-up network. Higher-magnification SEM images shown in Fig. S1b (ESI†) clearly display that the internal nanowires directly grew on Ni foam along with the external multidirectional ultrafine nanowires grounded on the internal nanowires. The XRD pattern (Fig. S2, ESI†) shows that all the identified peaks can be assigned to cobalt carbonate hydroxide hydrate (Co(CO3)0.5·0.11H2O) crystals (JCPDS card no. 48-0083).In order to obtain pure Co3O4 material, we subsequently thermally treated the as-prepared Co(CO3)0.5·0.11H2O product at 350 °C for 2 h in a normal N2 atmosphere. As seen from different magnification SEM images, Fig. 1a and b, the original morphology of the Co(CO3)0.5·0.11H2O products is retained perfectly, and the ultrafine nanowires still grew on the major nanowires, exhibiting a unique core/shell hierarchical feature with suitable rooms among neighbouring nanounits. Fig. 1c shows the hierarchical microstructure arrays homogeneously aligned from the side view and their height is ∼5 μm. Such unique hierarchical microstructures possessing more electroactive sites can improve electrolyte diffusion efficiency and increase electron transport when used as electrodes for supercapacitors, resulting in the promotion of electrochemical performance. In contrast, the SEM image of conventional Co3O4 nanowires (Fig. S3, ESI†) prepared according to previous publication27 reveals crowded space among adjacent nanowires, which may render difficult the diffusion of the electrolyte into the inner region.
The morphology and structure of the hierarchical nanowires@ultrafine nanowires are further elucidated by TEM. As shown in Fig. 1d–f, Fig. 1d shows the TEM image of the Co3O4 nanowires@nanowire samples scraped off from the Ni foam. Clearly, the internal nanowires were used as the backbone material, while the external ultrafine nanowires were grown on the backbone, which can agree with that of the preceding SEM images. The diameter of the internal nanowire is estimated to be about 50 nm. The corresponding selected-area electron diffraction (SAED) pattern (Fig. 1e) of the whole hierarchical microstructure shows well-defined diffraction rings, which correspond to the (222), (220) and (311) planes, demonstrating that the as-synthesized sample is polycrystalline. A high-magnification TEM image of the ultrafine nanowires, as shown in Fig. 1f, exhibits a diameter of ∼2–3 nm. These ultrafine nanowires would be highly accessible to the electrolyte and participate in the reversible faradic reaction adequately due to the presence of convenient diffusion paths. Besides, the corresponding HRTEM image (inset) reveals lattice fringes with d-spacings of 0.244 and 0.233 nm, corresponding to the distance of the (311) and (222) planes, respectively, of the Co3O4 crystal.
In order to eliminate the strong impact of the Ni foam substrate on the XRD peak signals, the nanowires@nanowire powder was scratched from the Ni foam for XRD analysis. As indicated in Fig. 2a, the XRD pattern confirms that all the diffraction peaks can be indexed to the Co3O4 phase (JCPDS no. 42-1467). The relatively high peak intensities indicate that the Co3O4 sample was of high crystallinity, which is consistent with the prior TEM results. In order to better understand the chemical composition of the as-prepared nanowires@nanowires, the energy dispersive X-ray (EDX) spectrometry characterization of the as-prepared nanowires@nanowire microstructures was also conducted. In Fig. 2b, it was found that the microstructure consists of Co, Ni, O, and Al elements, in which the Al peaks were derived from the aluminum platform and the Ni signal comes from Ni foam, further indicating the formation of the Co3O4 crystal.
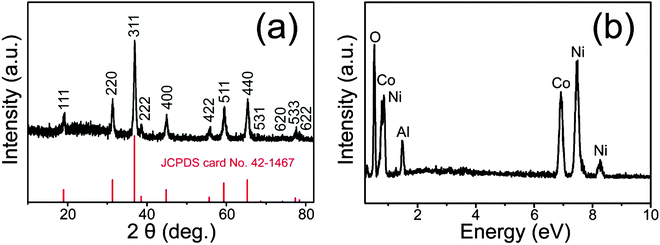 | ||
| Fig. 2 (a) XRD patterns of the hierarchical Co3O4 nanowires@nanowires scraped off from the Ni foam. (b) The EDX spectrum of the Co3O4 nanowires@nanowires on the Ni foam. | ||
We next demonstrate the formation mechanism of our synthetic system via modifying the reaction time to reveal different reaction stages. Fig. 3a–d are the SEM images of the samples obtained at various growth times, indicating a transformation of the structure and morphology (intermediate) into Co3O4 nanowire@nanowire arrays finally. After the initial 1 h reaction, many nanoparticles nucleated on the surface of the Ni foam and some nanowires grew based on these crystal nuclei, as shown in Fig. 3a. By prolonging the reaction time to 2 h, these crystal nuclei were developed into Co-based intermediate nanowires completely with high density on the substrate (Fig. 3b). Meanwhile, many ultrafine and short nanowires appeared and grew on the surface of the backbone nanowires. By unremittingly extending the reaction time to 5 h, these nanowires became long and dense (Fig. 3c). It can be concluded that the backbone internal nanowires can give rise to multiple directional ultrafine microstructures on the surface. These ultrafine microstructures are interconnected with each other, leading to a unique core/shell hierarchical feature with rational interspaces among adjacent nanostructures. Further increasing the reaction time to 14 h, the nanowires got extremely dense and longer with deficient interspace (Fig. 3d), which may make the diffusion of the electrolyte into the inner region difficult when used as electrodes. This evolution process seems to be a similar circumstance found in other material systems.28,29
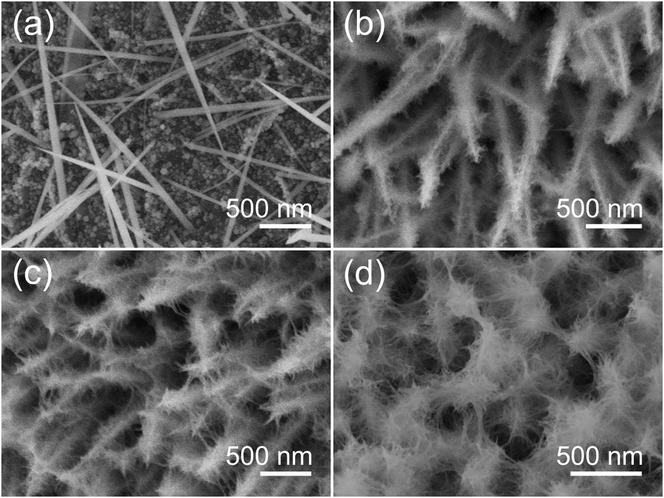 | ||
| Fig. 3 SEM images of the hierarchical Co3O4 nanowires@nanowires grown via different reaction times: (a) 1 h, (b) 2 h, (c) 5 h, and (d) 14 h. | ||
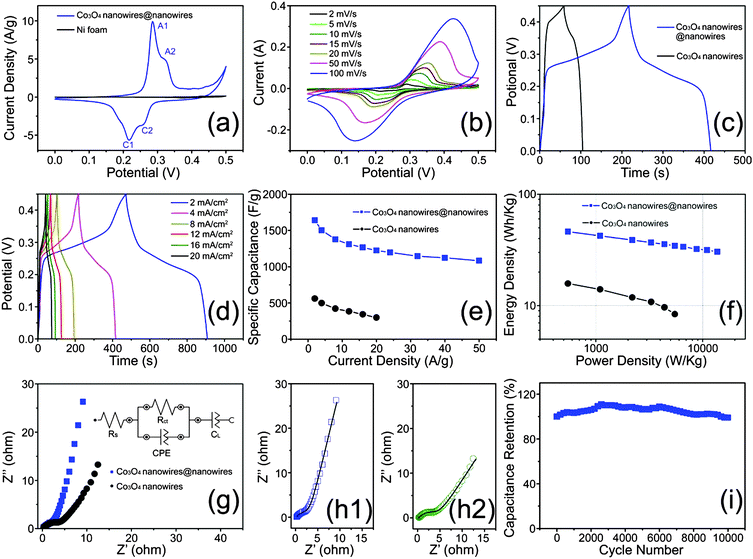
The electrochemical tests were carried out in a three-electrode configuration using a Pt counter electrode and a SCE reference electrode in a 2 M KOH aqueous electrolyte. Fig. 4a shows the CV curves of the Ni foam supported-Co3O4 nanowires@nanowires and pure Ni foam at a scan rate of 1 mV s−1. Two pairs of well-defined redox peaks indicate good super-capacitive characteristics, similar to previous literatures of Co3O4 electrodes.30–32 The first redox couple A1/C1 is ascribed to the conversion between Co3O4 and CoOOH, illustrated as follows:33,34
| Co3O4 + OH + H2O ↔ 3CoOOH + e− | (1) |
The second redox peak of A2/C2 corresponds to the change between CoOOH and CoO2, represented by the following reaction:33,34
| CoOOH + OH− ↔ CoO2 + H2O + e− | (2) |
Galvanostatic charge–discharge (CD) measurements were also conducted on the hierarchical Co3O4 nanowires@nanowires at different current densities. Fig. 4c depicts the comparison of CD curves at a stable potential window between 0 and 0.45 V for the Co3O4 nanowires@nanowires and conventional Co3O4 nanowire electrodes at the same current density of 4 mA cm−2. As expected, the Co3O4 nanowires@nanowire electrode reveals a much longer discharging time compared with that of the conventional Co3O4 nanowires. This means that the as-synthesized hierarchical Co3O4 nanowires@nanowires demonstrate higher specific capacitance values than the conventional Co3O4 nanowire electrode. Further CD curves of the Co3O4 nanowires@nanowire electrode were recorded with various current densities ranging from 2 to 50 mA cm−2, as shown in Fig. 4d and Fig. S5 (ESI†). However, it is obvious that all shapes of these CD curves are not strictly but approximately symmetric to their corresponding discharge counterparts, also indicating their good reversibility, which is consistent with the CV investigations. A similar phenomenon also occurred in the conventional Co3O4 nanowires (Fig. S6, ESI†).
It is considered that the CD measurement is more accurate than that of the CV measurement.35 Thus, the specific capacitance of the Co3O4 nanowires@nanowires and conventional Co3O4 nanowires in this study was calculated based on their corresponding CD curves and the typical results are shown in Fig. 4e. The Co3O4 nanowires@nanowires possess high specific capacitances up to 1640, 1503, 1377, 1308, 1266, 1225, 1198, 1145, 1122 and 1083 F g−1 at 2, 4, 8, 12, 16, 20, 24, 32, 40 and 50 mA cm−2, respectively, while the conventional Co3O4 nanowires only show specific capacitances of 560 and 298 F g−1 at 2 and 20 A g−1, respectively. It is well accepted that the rate capability is a vital factor for the supercapacitors in high power applications. The decline of the specific capacitances along with the increase of current density may be ascribed to the incremental voltage drop and insufficient active material involved in redox reaction. As can be seen, the Co3O4 nanowires@nanowire electrode preserved 74.7% of its specific capacitance (from 1640 to 1225 F g−1) with the current increasing from 2 to 20 mA cm−2. However, the specific capacitance of the conventional Co3O4 nanowires can only preserve 53.2% under the same change interval of current density, much less than the Co3O4 nanowires@nanowire electrode material on the Ni foam. Furthermore, even at a higher current density of 50 mA cm−2, the Co3O4 nanowires@nanowires can still preserve 66% of the specific capacitance at 2 mA cm−2. The predominant specific capacitance of our sample is mainly due to its ability to have short paths for ion diffusion in the Co3O4 nanowire interconnected network, and sufficient utilization of active material due to its ultrafine microstructures.36 It is noteworthy that both of the specific capacitance and rate capability reported here were remarkable compared with those pure Co3O4 as well as Co3O4-based hybrid electrode materials reported in previous literature,18–20,37–48 as summarized in Table S1 (ESI†).
Fig. 4f shows the Ragone plot (power density vs. energy density) for the two electrodes at the potential window of 0–0.45 V. At a power density of 549 W kg−1, the Co3O4 nanowires@nanowires delivered an energy density as high as 46.12 W h kg−1, which is almost 3 times larger than that of the conventional Co3O4 nanowires (15.76 W h kg−1). The energy density of the Co3O4 nanowires@nanowire electrode materials is higher than those of the reported nanostructured Co3O4 electrodes, such as Co3O4 nanowire arrays (31.68 W h kg−1),49 hollow Co3O4 microspheres (34.51 W h kg−1),50 multi-shelled Co3O4 hollow microspheres (13.7 W h kg−1),51 mesoporous Co3O4 nanoflake arrays (18.91 W h kg−1),52 3D hierarchical mesoporous Co3O4 nanoparticles (15.24 W h kg−1)53 and 3D enoki mushroom-like Co3O4 hierarchitectures (22.13 W h kg−1).54 More importantly, the energy density of the Co3O4 nanowires@nanowire electrode materials can still reach 30.46 W h kg−1 even at a high power density of 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 720 W kg−1.
720 W kg−1.
Nyquist plots of the two Co3O4 electrodes were also investigated and are shown in Fig. 4g. The two impedance curves are similar in form, with an arc in the high-frequency region and a spike at lower frequencies. The inset of Fig. 4g shows the equivalent fitting circuit analysis of the as-synthesized Co3O4 electrodes using a complex nonlinear least-squares fitting method.55 The equivalent series resistance (Rs) value of the Co3O4 nanowires@nanowires was only 0.26 Ω, which was lower than that (0.30 Ω) of the conventional Co3O4 nanowires. In particular, the Co3O4 nanowires@nanowires have a smaller charge transfer resistance (Rct) value (2.93 Ω) than that of the conventional Co3O4 nanowires (4.54 Ω), which demonstrates that the unique microstructure can provide an ideal pathway for electron and ion transport without kinetic limitations. It is worth mentioning that the as-fitted equivalent circuits have higher accuracy as the as-obtained fitting curves of these two Co3O4 electrodes can match the original testing impedance curves very well, as shown in Fig. 4h. The durability of electrode material is also a critical factor except higher specific capacitance as well as superior rate capability for practical applications. The cycling stability of the Co3O4 nanowires@nanowires was measured by the repeated CV measurement at a scan rate of 50 mV s−1, as shown in Fig. 4i. The final specific capacitance of the Co3O4 nanowires@nanowires is 99.03% of its initial value after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. There is a minor damage of the Co3O4 nanowires@nanowire electrode active materials after experiencing harsh and frequent phase variations during the redox reactions (shown in Fig. S7, ESI†). The outstanding lifespan of the Co3O4 nanowires@nanowire electrode material was mainly ascribed to the unique core/shell structure consisting of ultrafine nanowires which provides more active sites for efficient electrolyte ion transportation.
000 cycles. There is a minor damage of the Co3O4 nanowires@nanowire electrode active materials after experiencing harsh and frequent phase variations during the redox reactions (shown in Fig. S7, ESI†). The outstanding lifespan of the Co3O4 nanowires@nanowire electrode material was mainly ascribed to the unique core/shell structure consisting of ultrafine nanowires which provides more active sites for efficient electrolyte ion transportation.
The above electrochemical measurements indicate that the unique hierarchical Co3O4 nanowires@nanowires can deliver fascinating electrochemical performance with high specific capacitance, superior rate capability as well as remarkable cycling stability synchronously. The outstanding electrochemical properties could be related to the following structural and morphological peculiarities. (i) Co3O4 is a kind of good super-capacitive metal oxide material and Co3O4 microstructures grown on the Ni foam directly could avoid “dead” volume caused by the tanglesome process of mixing active materials with binders and carbon powder; (ii) the reasonable room among the interconnected hierarchical crystalline nanounits possessing more electroactive sites allows for easy diffusion of the electrolyte into the inner region of the electrodes; (iii) the core (interior nanowires) can offer an efficient way of transporting the ions or electrons, while the shell (external ultrafine nanowires) can result in short paths for electron and ion transport as well as species diffusion. Thus, the as-obtained hierarchical Co3O4 nanowires@nanowires can engage in the reversible faradic reaction adequately and deliver fascinating electrochemical performance.
Conclusions
A facile, low-cost and effective hydrothermal process was developed to synthesize Co3O4 nanowires@Co3O4 ultrafine nanowires directly grown on the Ni foam with high electrochemical properties as supercapacitor electrodes. The as-synthesized Co3O4 hierarchical microstructure electrode on the Ni foam demonstrated excellent electrochemical performances with high specific capacitance (1640 F g−1 at 2 mA cm−2), desirable rate capability (66% retention from 2 to 50 mA cm−2) and superior long lifespan (99.03% of the initial value after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) simultaneously. Such a high electrochemical behavior can be attributed to the unique structural and morphological peculiarities of the Co3O4 nanowires@Co3O4 ultrafine nanowire core/shell hierarchical microstructures. It is expected that the Co3O4 nanowires@Co3O4 ultrafine nanowires can be promising electrode materials for supercapacitor applications.
000 cycles) simultaneously. Such a high electrochemical behavior can be attributed to the unique structural and morphological peculiarities of the Co3O4 nanowires@Co3O4 ultrafine nanowire core/shell hierarchical microstructures. It is expected that the Co3O4 nanowires@Co3O4 ultrafine nanowires can be promising electrode materials for supercapacitor applications.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grants 21171035, 51302035, 51472049, and 11204030), the Key Grant Project of Chinese Ministry of Education (Grant 313015), the PhD Programs Foundation of the Ministry of Education of China (Grants 20110075110008 and 20130075120001), the China Postdoctoral Science Foundation (2014M550203), the Science and Technology Commission of Shanghai Municipality (Grant 13ZR1451200), the Program Innovative Research Team in University (Grant IRT1221), the Shanghai Leading Academic Discipline Project (Grant B603), the Program of Introducing Talents of Discipline to Universities (Grant 111-2-04), the Fundamental Research Funds for the Central Universities, the Foundation of Shanghai University of Engineering Science (E1-0501-15-0105) and the Project of Shanghai universities young teacher training scheme (ZZGCD15037).Notes and references
- J. R. Miller and P. Simon, Science, 2008, 321, 651 CrossRef CAS PubMed.
- G. Nystrom, A. Marais, E. Karabulut, L. Wagberg, Y. Cui and M. M. Hamedi, Nat. Commun., 2015, 6, 7259 CrossRef PubMed.
- N. S. Choi, Z. Chen, S. A. Freunberger, X. Ji, Y. K. Sun, K. Amine, G. Yushin, L. F. Nazar, J. Cho and P. G. Bruce, Angew. Chem., Int. Ed., 2012, 51, 9994 CrossRef CAS PubMed.
- X. H. Lu, T. Y. Liu, T. Zhai, G. M. Wang, M. H. Yu, S. L. Xie, Y. C. Ling, C. L. Liang, Y. X. Tong and Y. Li, Adv. Energy Mater., 2014, 4, 1300994 Search PubMed.
- C. Guan, J. L. Liu, Y. D. Wang, L. Mao, Z. X. Fan, Z. X. Shen, H. Zhang and J. Wang, ACS Nano, 2015, 9, 5198 CrossRef CAS PubMed.
- D. Pech, M. Brunet, H. Durou, P. Huang, V. Mochalin and Y. Gogotsi, Nat. Nanotechnol., 2010, 5, 651 CrossRef CAS PubMed.
- J. Jiang, Y. Li, J. Liu, X. Huang, C. Yuan and X. W. Lou, Adv. Mater., 2012, 24, 5166 CrossRef CAS PubMed.
- C. Guan, X. L. Li, Z. L. Wang, X. H. Cao, C. Soci, H. Zhang and H. J. Fan, Adv. Mater., 2012, 24, 4186 CrossRef CAS PubMed.
- G. H. Yu, L. B. Hu, M. Vosgueritchian, H. L. Wang, X. Xie, J. R. McDonough, X. Cui, Y. Cui and Z. N. Bao, Nano Lett., 2011, 11, 2905 CrossRef CAS PubMed.
- W. B. Zhang, L. B. Kong, X. J. Ma, Y. C. Luo and L. Kang, New J. Chem., 2014, 38, 3236 RSC.
- R. B. Rakhi, W. Chen, D. K. Cha and H. N. Alshareef, Nano Lett., 2012, 12, 2559 CrossRef CAS PubMed.
- G. X. Wang, X. P. Shen, J. Horvat, B. Wang, H. Liu, D. Wexler and J. Yao, J. Phys. Chem. C, 2009, 113, 4357 CAS.
- T. Y. Wei, C. H. Chen, K. H. Chang, S. Y. Lu and C. C. Hu, Chem. Mater., 2009, 21, 3228 CrossRef CAS.
- Y. H. Xiao, S. J. Liu, F. Li, A. Q. Zhang, J. H. Zhao, S. M. Fang and D. Z. Jia, Adv. Funct. Mater., 2012, 22, 4052 CrossRef CAS.
- Q. Y. Liao, N. Li, S. X. Jin, G. W. Yang and C. X. Wang, ACS Nano, 2015, 9, 5310 CrossRef CAS PubMed.
- X. H. Xia, J. P. Tu, X. L. Wang, C. D. Gu and X. B. Zhao, Chem. Commun., 2011, 47, 5786 RSC.
- Y. T. Hao, H. W. Wang, Z. H. Hu, L. H. Gan and Z. J. Xu, New J. Chem., 2015, 39, 68 RSC.
- S. Vijayakumar, A. Kiruthika Ponnalagi, S. Nagamuthu and G. Muralidharan, Electrochim. Acta, 2013, 106, 500 CrossRef CAS.
- H. Pang, F. Gao, Q. Chen, R. M. Liu and Q. Y. Lu, Dalton Trans., 2012, 41, 5862 RSC.
- X. M. Liu, Q. Long, C. H. Jiang, B. B. Zhan, C. Li, S. J. Liu, Q. Zhao, W. Huang and X. C. Dong, Nanoscale, 2013, 5, 6525 RSC.
- Y. Z. Zhang, Y. Wang, Y. L. Xie, T. Cheng, W. Y. Lai, H. Pang and W. Huang, Nanoscale, 2014, 6, 14354 RSC.
- Y. J. Cao, W. Y. Li, K. B. Xu, Y. X. Zhang, T. Ji, R. J. Zou, J. M. Yang, Z. Y. Qin and J. Q. Hu, J. Mater. Chem. A, 2014, 2, 20723 CAS.
- F. Z. Deng, L. Yu, G. Cheng, T. Lin, M. Sun, F. Ye and Y. F. Li, J. Power Sources, 2014, 251, 202 CrossRef CAS.
- L. Su, Y. Jing and Z. Zhou, Nanoscale, 2011, 3, 3967 RSC.
- J. Duay, S. A. Sherrill, Z. Gui, E. Gillette and S. B. Lee, ACS Nano, 2013, 7, 1200 CrossRef CAS PubMed.
- X. H. Xia, J. P. Tu, Y. Q. Zhang, X. L. Wang, C. F. D. Gu, X. B. Zhao and H. J. Fan, ACS Nano, 2012, 6, 5531 CrossRef CAS PubMed.
- J. Jiang, J. P. Liu, X. T. Huang, Y. Y. Li, R. M. Ding, X. X. Ji, Y. Y. Hu, Q. B. Chi and Z. H. Zhu, Cryst. Growth Des., 2010, 10, 70 CAS.
- X. Y. Liu, Y. Q. Zhang, X. H. Xia, S. J. Shi, Y. Lu, X. L. Wang, C. D. Gu and J. P. Tu, J. Power Sources, 2013, 239, 157 CrossRef CAS.
- W. Zhu, Z. Y. Lu, G. X. Zhang, X. D. Lei, Z. Chang, J. F. Liu and X. M. Sun, J. Mater. Chem. A, 2013, 1, 8327 CAS.
- M. J. Deng, F. L. Huang, I. W. Sun, W. T. Tsai and J. K. Chang, Nanotechnology, 2009, 20, 175602 CrossRef PubMed.
- C. W. Kung, H. W. Chen, C. Y. Lin, R. Vittal and K. C. Ho, J. Power Sources, 2012, 214, 91 CrossRef CAS.
- X. W. Lou, D. Deng, J. Y. Lee and L. A. Archer, J. Mater. Chem., 2008, 18, 4397 RSC.
- H. W. Shim, A. H. Lim, J. C. Kim, E. J. Jang, S. D. Seo, G. H. Lee, T. D. Kim and D. W. Kim, Sci. Rep., 2013, 3, 2325 Search PubMed.
- H. T. Wang, L. Zhang, X. H. Tan, C. M. B. Holt, B. Zahiri, B. C. Olsen and D. Mitlin, J. Phys. Chem. C, 2011, 115, 17599 CAS.
- L. Zhao, L. Z. Fan, M. Q. Zhou, H. Guan, S. Y. Qiao, M. Antonietti and M. M. Titirici, Adv. Mater., 2010, 22, 5202 CrossRef CAS PubMed.
- H. Jiang, T. Zhao, J. Ma, C. Yan and C. Li, Chem. Commun., 2011, 47, 1264 RSC.
- W. Du, R. M. Liu, Y. W. Jiang, Q. Y. Lu, Y. Z. Fan and F. Gao, J. Power Sources, 2013, 227, 101 CrossRef CAS.
- X. Zhang, Y. Q. Zhao and C. L. Xu, Nanoscale, 2014, 6, 3638 RSC.
- M. X. Liao, Y. F. Liu, Z. H. Hu and Q. Yu, J. Alloys Compd., 2013, 562, 106 CrossRef CAS.
- W. L. Yanga, Z. Gao, J. Ma, J. Wang, B. Wang and L. H. Liu, Electrochim. Acta, 2013, 112, 378 CrossRef.
- K. Wang, Z. Q. Shi, Y. Y. Wang, Z. G. Ye, H. Y. Xia, G. W. Liu and G. J. Qiao, J. Alloys Compd., 2015, 624, 85 CrossRef CAS.
- K. W. Qiu, H. L. Yan, D. Y. Zhang, Y. Lu, J. B. Cheng, M. Lu, C. L. Wang, Y. H. Zhang, X. M. Liu and Y. S. Luo, J. Solid State Electrochem., 2015, 19, 391 CrossRef CAS.
- J. P. Liu, J. Jiang, C. W. Cheng, H. X. Li, J. X. Zhang, H. Gong and H. J. Fan, Adv. Mater., 2011, 23, 2076 CrossRef CAS PubMed.
- K. B. Wang, Z. Y. Zhang, X. B. Shi, H. J. Wang, Y. N. Lu and X. Y. Ma, RSC Adv., 2015, 5, 1943 RSC.
- D. P. Cai, H. Huang, D. D. Wang, B. Liu, L. L. Wang, Y. Liu, Q. H. Li and T. H. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 15905 CAS.
- L. B. Ma, H. Zhou, X. P. Shen, Q. R. Chen, G. X. Zhu and Z. Y. Ji, RSC Adv., 2014, 4, 53180 RSC.
- S. Balasubramanian and P. K. Kamaraj, Electrochim. Acta, 2015, 168, 50 CrossRef CAS.
- X. W. Wang, S. Q. Liu, H. Y. Wang, F. Y. Tu, D. Fang and Y. H. Li, J. Solid State Electrochem., 2012, 16, 3593 CrossRef CAS.
- X. H. Xia, J. P. Tu, Y. Q. Zhang, Y. J. Mai, X. L. Wang, C. D. Gu and X. B. Zhao, RSC Adv., 2012, 2, 1835 RSC.
- C. Feng, J. F. Zhang, Y. D. Deng, C. Zhong, L. Liu and W. B. Hu, RSC Adv., 2015, 5, 42055 RSC.
- Y. P. Wang, A. Q. Pan, Q. Y. Zhu, Z. W. Nie, Y. F. Zhang, Y. Tang, S. Q. Liang and G. Z. Cao, J. Power Sources, 2014, 272, 107 CrossRef CAS.
- A. G. Xiao, S. B. Zhou, C. G. Zuo, Y. B. Zhuan and X. Ding, Mater. Res. Bull., 2014, 60, 674 CrossRef CAS.
- Y. F. Fan, G. J. Shao, Z. P. Ma, G. L. Wang, H. B. Shao and S. Yan, Part. Part. Syst. Charact., 2014, 31, 1079 CrossRef CAS.
- F. L. Luo, J. Li, Y. Lei, W. Yang, H. Y. Yuan and D. Xiao, Electrochim. Acta, 2014, 135, 495–502 CrossRef CAS.
- Z. Fan, J. Yan, T. Wei, L. Zhi, G. Ning, T. Li and F. Wei, Adv. Funct. Mater., 2011, 21, 2366 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5nj02112j |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 |