Integrated nanotechnology for synergism and degradation of fungicide SOPP using micro/nano-Ag3PO4†
Jingzhe
Xue
a,
Guangtao
Zan
ab,
Qingsheng
Wu
*ab,
Baolin
Deng
c,
Yahui
Zhang
a,
Hongqin
Huang
a and
Xiaochen
Zhang
a
aDepartment of Chemistry, Key Laboratory of Yangtze River Water Environment, Ministry of Education, Tongji University, Shanghai 200092, P. R. China. E-mail: qswu@tongji.edu.cn; Fax: +86-21-65982620; Tel: +86-21-65982620
bSchool of Materials Science and Engineering, Tongji University, Shanghai 200092, P. R. China
cDepartment of Civil & Environmental Engineering, University of Missouri, Columbia, MO 65211, USA
First published on 6th January 2016
Abstract
The pursuit of food safety and environmental protection has encouraged the development of new antifungal agents to replace traditional fungicides. Here we present an integrated green nanotechnology using inorganic materials, Ag3PO4 micro/nano-crystals, which could enhance the efficiency of fungicide sodium o-phenyl phenolate (SOPP) but without its residue remaining. The experiments demonstrate that the micro/nano Ag3PO4 was effective in inhibiting fungal hyphae growth against Phytophthora capsici and Botrytis cinerea. After being combined with Ag3PO4 micro/nanocrystals, the antifungal activities of fungicides SOPP and cyproconazole were enhanced. More importantly, it was found that over 90% of the SOPP was decomposed by the Ag3PO4 at a dose of 1.6 g L−1 under simulated sunlight irradiation within 2 h, exhibiting a much better photocatalytic activity than ZnO nanoparticles (NPs). These achievements demonstrate that this green nanotechnology could reduce fungicide usage without compromising on pathogen control and provide a residue-free effect under natural environmental conditions. Furthermore, it was found that the antifungal activity of Ag3PO4 was not due to the production of ROS but strongly related to interaction with fungal cells and the release of Ag+ ions. The mechanism for the synergistic enhanced antifungal effect was speculated from three possible aspects: (a) Ag3PO4 micro/nano-crystals and Ag+ ions promoting the penetration of OPP ions into the cell interior; (b) formation of a Ag3PO4–SOPP composite; and (c) multiple modes of antifungal action of the Ag3PO4–SOPP system.
Introduction
Due to the continued growth of the population, there is a critical need to enhance food production and maintain food security in the world.1 Plant pathogenic fungi could significantly threaten crop production,1,2 thus, fungicides are commonly applied to prevent spreading of pathogenic fungi. There are, however, concerns about the negative impact of fungicides on human health and the environment.3,4 Much effort has been devoted to developing alternative approaches, including organic agriculture for the control of fungicides; nevertheless, low crop yields and the high cost associated with these approaches have limited their application,5–7 and fungicides are still considered irreplaceable.With the fast advent of inorganic chemistry, there is great opportunity to develop new strategies for the control of plant pathogenic fungi. Some inorganic nanomaterials with effective antimicrobial activities are also potential antifungal agents, for example, Ag and ZnO nanoparticles (NPs) were reported to have inhibitory effects on fungal hyphae growth.8–10 Other studies have demonstrated that some semiconductor nanomaterials have excellent photocatalytic properties.11,12 Recently, Ag3PO4 materials have been intensively studied for their photocatalytic applications. Ag3PO4 has excellent photooxidative capabilities for water splitting and organic pollutant decomposition under visible light irradiation.13–16 Its photocatalytic activity is almost the highest among the known visible light photocatalysts.17,18 Interestingly, Ag3PO4 was also found to have antibacterial activities against Escherichia coli and Bacillus subtilis.19,20 These findings encouraged us to develop a residue-free antifungal nanotechnology in which Ag3PO4 is applied to synergistically enhance the antifungal activity of the fungicides and eliminate the fungicide residue by photocatalysis.
Sodium o-phenyl phenolate (SOPP) is a widely used fungicide for commercial and consumer purposes. It is almost always applied to fruit for mould prevention and disinfection during fruit storage. Since several studies have shown that SOPP is able to induce carcinogenicity in non-target organisms,21,22 the SOPP residues on fruit and other food products have caused significant public concern. From this point of view, the goal of this study is to establish a nanotechnology which could remove the SOPP residue and guarantee public health without compromising the antifungal effect.
We have prepared Ag3PO4 micro/nano-crystals using a simple ion exchange method and demonstrated that the material could enhance the antifungal properties of SOPP. At the same time, the SOPP residue could be degraded through Ag3PO4 photocatalysis. To the best of our knowledge, this is the first report concerning the antifungal activity of Ag3PO4, a green technology that may provide highly efficient plant protection without fungicide residue. It is noted that this green nanotechnology could offer great benefits for crop production and human society.
Experimental section
Materials, characterization and preparation of the composite antifungal system
Fungicide thiram, silver nitrate (AgNO3) and disodium hydrogen phosphate (Na2HPO4) were purchased from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China) and used without any purification. Fungicides sodium o-phenylphenolate (SOPP) and cyproconazole were obtained from TCI (Shanghai) Development Co., Ltd. ZnO NPs were purchased from Tianxin Zinc Industry Co., Ltd (Baoji, Shanxi, China). Ag3PO4 micro/nano-crystals were prepared using an ion-exchange method:15 0.4 M AgNO3 was added into 0.15 M Na2HPO4 dropwise. The yellow mixture was washed with deionized water and ethanol several times, then dried at 60 °C overnight.A series of composite antifungal systems with various Ag3PO4/fungicide mass ratios were prepared for the residue-free antifungal nanotechnology. Typically, Ag3PO4 micro/nano-crystal water suspensions were firstly subjected to ultrasonication for 10 min to disaggregate the particles and then diluted to the required concentration. Next, SOPP water solutions were added to the Ag3PO4 suspensions with vigorous shaking at room temperature (22 ± 2 °C) for full dispersion, and then the samples were subjected to ultrasonication for 20 min to assemble SOPP on the surface of the Ag3PO4. In our study, the mass ratios of Ag3PO4/SOPP were set up to be 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 25
20, 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, 25
40, 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, 25
80, 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160, 50
160, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 and 100
40 and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40. The composite systems were then used for the antifungal tests without additional treatment. For characterization, the assembled Ag3PO4–SOPP composite was separated by centrifugation for 10 min at 10
40. The composite systems were then used for the antifungal tests without additional treatment. For characterization, the assembled Ag3PO4–SOPP composite was separated by centrifugation for 10 min at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and washed with a little deionized water after being filtered with Millipore membranes. The composite antifungal systems of Ag3PO4–thiram and Ag3PO4–cyproconazole were prepared using a similar method.
000 rpm and washed with a little deionized water after being filtered with Millipore membranes. The composite antifungal systems of Ag3PO4–thiram and Ag3PO4–cyproconazole were prepared using a similar method.
The as-synthesized Ag3PO4 samples and Ag3PO4–SOPP composites were subjected to a series of characterization experiments. X-ray diffraction (XRD) experiments were carried out with a Bruker D8 Advance X-ray diffractometer (Cu Kα radiation, λ = 0.154056 Å). Scanning electron microscopy (SEM, Hitachi S-4800, Japan) and energy dispersive spectroscopy (EDS, Horiba EMAX) were used to examine the morphology and constituent elements of the samples. The particle size distribution was measured using a Malvern Zetasize Nano zs90. Fourier transform infrared (FTIR) spectra of the samples were collected using a Nicolet NEXUS 6700 FTIR spectrometer. X-ray photoelectron spectroscopy (XPS) analysis was performed with a Kratos AXIS Ultra DLD. UV-Vis spectrophotometer studies were conducted with an Agilent UV-Vis 8453. UV-Visible diffuse reflectance absorbance and Raman spectra of the sample were recorded with a UV-vis diffuse reflectance spectrometer (DRS, BWS003, Newark, DE) and a Raman spectrometer (Invia, Renishow), respectively.
Antifungal tests
The plant pathogenic fungi Phytophthora capsici and Botrytis cinerea used in our investigation were obtained from China Agricultural University and Huazhong Agricultural University, respectively. A carrot agar (CA) medium (carrot juice made from 200 g fresh carrot, 13 g agar, 1000 mL distilled water) and potato dextrose agar (PDA) medium (extracted from 200 g boiled potato, 20 g glucose, 15 g agar and 1000 mL distilled water) were used in the antifungal tests for Phytophthora capsici and Botrytis cinere, respectively. The antifungal tests were performed to enable comparison of the fungal hyphae growth rates in the presence and absence of the antifungal agents. Ag3PO4, the fungicides and their combinations were added into the melted medium at about 45 ± 5 °C. The medium with added deionized water was used as a control. After the medium solidified, the cultures were incubated with a 7-day-old fungal strain at the center of each Petri dish. The diameters of the fungal colonies were recorded after 4 days of incubation. The antifungal efficacies were calculated as follows:| Antifungal efficacy = (Dc − Dt) × 100%/Dc | (1) |
| %Cexp = A + B − (A × B/100) | (2) |
| SF = %Cobs/%Cexp | (3) |
Photocatalytic degradation experiments
Photocatalytic experiments with the Ag3PO4 micro/nano-crystals were conducted for the decomposition of 40 mg L−1 SOPP, using a XPA-Photochemical Reactor (Xujiang Electromechanical Plant, Nanjing, China). A 500 W Xenon lamp was used to simulate sunlight irradiation (wavelength: 200–2500 nm). Desired amounts of the Ag3PO4 micro/nano-crystals were added into 40 mL of a SOPP aqueous solution in quartz tubes. After ultrasonication for 20 min, the suspension was placed inside the reactor and kept for 30 min to achieve adsorption equilibrium before irradiation. A series of aqueous samples were then collected at given intervals, and after centrifuging to remove the catalyst, the concentration of SOPP was determined by measuring its absorption at 282 nm with a UV-Vis spectrophotometer (Agilent 8453).Inhibitory effect of an antioxidant on the Ag3PO4 micro/nano-crystals antifungal activity
The effects of an antioxidant, histidine, were determined to investigate the relationship between the antifungal mechanism of Ag3PO4 and the production of reactive oxygen species (ROS). Histidine was added into CA medium containing 0.025 and 0.05 g L−1 Ag3PO4. Then, the fungi hyphae were inoculated and cultivated at 25 °C in the dark for 4 days. The diameters of the fungal colonies were measured and the inhibition rates were calculated.Detection of Ag+ release from Ag3PO4
Flasks containing a 0.025, 0.05 and 0.1 g L−1 Ag3PO4 water suspension were placed in a shaker at 100 shakes per min at 25 °C for 60 min. The samples were centrifugated three times, at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min each time, to remove the Ag3PO4 micro/nano-crystals. 10 mL supernatants of each sample were digested with 4% HNO3 for 30 min and then subjected to ICP analysis with an inductively coupled plasma emission spectrometer (ICP, Agilent 720ES). Before that, 1.0 and 10.0 mg L−1 standard Ag+ solutions were prepared with AgNO3 (Guaranteed reagent) for setting up a standard curve.
000 rpm for 15 min each time, to remove the Ag3PO4 micro/nano-crystals. 10 mL supernatants of each sample were digested with 4% HNO3 for 30 min and then subjected to ICP analysis with an inductively coupled plasma emission spectrometer (ICP, Agilent 720ES). Before that, 1.0 and 10.0 mg L−1 standard Ag+ solutions were prepared with AgNO3 (Guaranteed reagent) for setting up a standard curve.
Results and discussion
Characterization of the materials synthesized for the residue-free antifungal nanotechnology
The XRD pattern of the as-prepared Ag3PO4 micro/nano-crystals (Fig. 1c) matched well with a cubic Ag3PO4 phase (JCP DS no. 06-05 05), demonstrating the formation of pure Ag3PO4 crystals. The SEM images showed that the Ag3PO4 particles had an irregular spherical shape (Fig. 1a). Particle size distribution analysis displayed that the Ag3PO4 micro/nano-crystals have a size distribution range mainly within 400–800 nm, with an average diameter of 603.7 nm (Fig. 1b and d). Further characterization results can be seen in the ESI Fig. 1,† and the results confirmed that the as-synthesized sample consisted of Ag3PO4 micro/nano-crystals and indicated potential photocatalytic properties of the Ag3PO4 sample in the visible light wavelength range.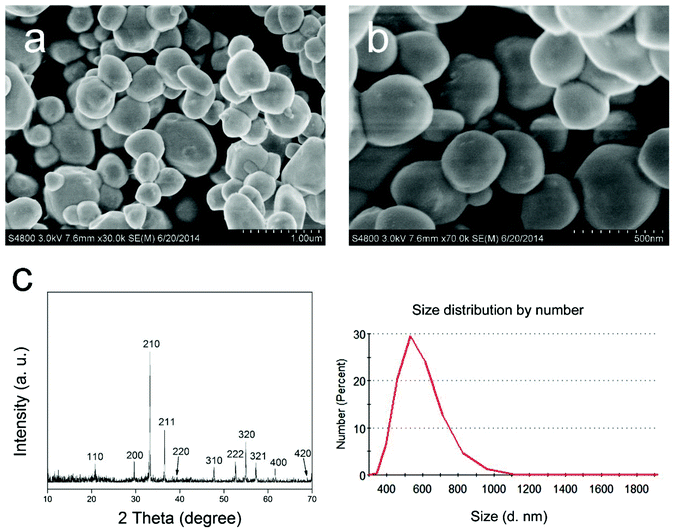 | ||
| Fig. 1 SEM (a, b), XRD (c) and particle size distribution (d) images of the Ag3PO4 micro/nano-crystals. | ||
From the SEM image (Fig. 2a), it can be seen that the Ag3PO4–SOPP composite system also had an irregular spherical shape. While Fig. 2b shows that the Ag3PO4–SOPP composite has a diameter distribution range quite similar to the Ag3PO4 micro/nano-crystals, with a slightly increased average diameter of 606.1 nm. This approximate 1.2 nm increase in the average diameter indicates the thickness of the assembled fungicide. EDS was used to examine the elemental composition of the composite. Fig. 2c displays that obvious carbon peaks existed for the composite system, suggesting the presence of the organic fungicide SOPP. Meanwhile, similar results can be seen in the EDS elemental mapping images (ESI Fig. 2†), clearly showing the presence and distribution of carbon elements which originate from the benzene ring structure of SOPP. In the FTIR spectrum (Fig. 2d), strong absorption bands at 550 and 1007 cm−1 in the Ag3PO4–SOPP spectrum can be assigned to the vibrations of PO43− groups,24 demonstrating the presence of Ag3PO4. Moreover, several absorption bands can be identified that belong to the unique structure of SOPP. For example, the absorption bands at 1467 and 1584 cm−1 can be assigned to skeleton vibrations of benzene. The absorption band from deformation vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in benzene derivatives can be seen at 1662 cm−1. The absorption band at 1282 cm−1 can be assigned to stretching vibrations of C–O. These specific absorption bands demonstrate the presence of SOPP in the Ag3PO4–SOPP composite. Compared with the pure Ag3PO4 or SOPP spectrum, the bands were shifted several wavenumbers in the Ag3PO4–SOPP composite spectrum, which provides evidence for the weak connection between Ag3PO4 and SOPP. Moreover, a UV-Vis spectrophotometry investigation further shows the absorption of SOPP onto the Ag3PO4 particles. From the ESI Fig. 3,† it can be seen that after introducing Ag3PO4 micro/nano-crystals, the concentration of SOPP in the solution decreased, indicating part of the SOPP molecules were absorbed by Ag3PO4. As presented above, it can be concluded that the Ag3PO4–SOPP composite was formed by assembling SOPP on the Ag3PO4 micro/nano-crystals. The full XPS results are shown in Fig. 2e, and demonstrate the existence of Ag, P, O, Na and C elements. Comparing the signal intensity for C and Ag, it can be found that carbon occupies an even larger portion of the sample than silver, indicating the presence of a layer of C-based substance on the Ag3PO4 micro/nano-crystals. Due to the XPS technique generally providing surface (1–5 nm) information for the sample, this result also implies that the thickness of the assembled SOPP was no larger than 5 nm, which is in consistence with the particle distribution test results. Fig. 2f shows the resolved C 1s signal. The obvious peaks at 284.8 and 286.1 eV correspond to the C–C and C–O in the SOPP structure, respectively.25 The very weak satellite peak at 291.7 eV came from the π–π* transitions of the aromatic carbons.26 As O2 may oxidize SOPP during the experimental procedure, a peak at 287.1 eV corresponding to C
C in benzene derivatives can be seen at 1662 cm−1. The absorption band at 1282 cm−1 can be assigned to stretching vibrations of C–O. These specific absorption bands demonstrate the presence of SOPP in the Ag3PO4–SOPP composite. Compared with the pure Ag3PO4 or SOPP spectrum, the bands were shifted several wavenumbers in the Ag3PO4–SOPP composite spectrum, which provides evidence for the weak connection between Ag3PO4 and SOPP. Moreover, a UV-Vis spectrophotometry investigation further shows the absorption of SOPP onto the Ag3PO4 particles. From the ESI Fig. 3,† it can be seen that after introducing Ag3PO4 micro/nano-crystals, the concentration of SOPP in the solution decreased, indicating part of the SOPP molecules were absorbed by Ag3PO4. As presented above, it can be concluded that the Ag3PO4–SOPP composite was formed by assembling SOPP on the Ag3PO4 micro/nano-crystals. The full XPS results are shown in Fig. 2e, and demonstrate the existence of Ag, P, O, Na and C elements. Comparing the signal intensity for C and Ag, it can be found that carbon occupies an even larger portion of the sample than silver, indicating the presence of a layer of C-based substance on the Ag3PO4 micro/nano-crystals. Due to the XPS technique generally providing surface (1–5 nm) information for the sample, this result also implies that the thickness of the assembled SOPP was no larger than 5 nm, which is in consistence with the particle distribution test results. Fig. 2f shows the resolved C 1s signal. The obvious peaks at 284.8 and 286.1 eV correspond to the C–C and C–O in the SOPP structure, respectively.25 The very weak satellite peak at 291.7 eV came from the π–π* transitions of the aromatic carbons.26 As O2 may oxidize SOPP during the experimental procedure, a peak at 287.1 eV corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O can be identified.25 The above information provides further evidence for SOPP assembling on the Ag3PO4 micro/nano-crystals.
O can be identified.25 The above information provides further evidence for SOPP assembling on the Ag3PO4 micro/nano-crystals.
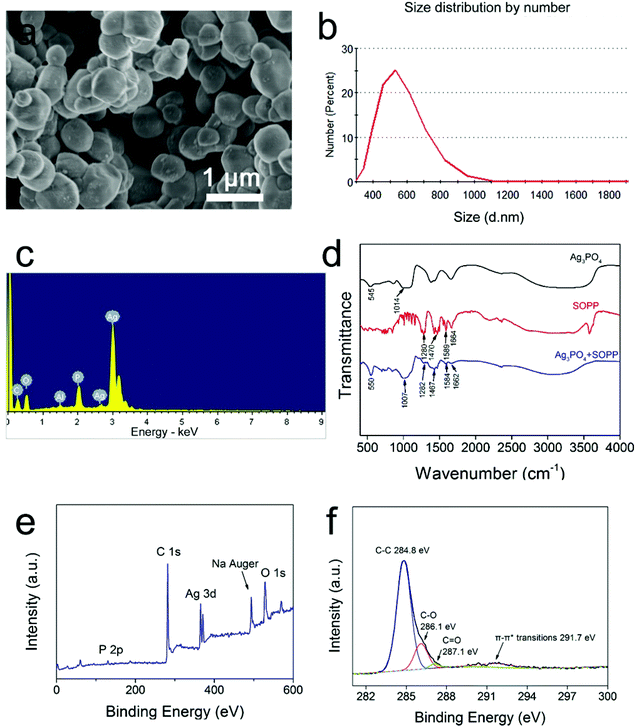 | ||
| Fig. 2 SEM (a), particles size distribution (b), EDS (c), FTIR (d) and XPS (e: full spectra, f: C 1s bands) images of the Ag3PO4-SOPP composite system. | ||
Due to the bands in the FTIR spectra of Ag3PO4 and SOPP remaining after assembling the composites, it was unlikely to form new chemical bonds which could obviously change the absorption bands. Meanwhile, the UV-Vis absorption peaks of SOPP remained after interaction with Ag3PO4, and it can be deduced that the composite assembled mainly via physicochemical interactions. Considering the structure of the two agents, hydrogen bonds may be formed between the Ag3PO4 surface O atoms and H atoms in the SOPP molecules. As seen from the electrical properties of the two agents, the Ag3PO4 micro/nano-particles were negatively charged (−25.4 ± 2.5 mV) under weakly acidic conditions according to the zeta potential test. Due to our antifungal system being weakly acidic (pH 5.5–6), the SOPP molecules dissolved in water may form a positive SOPP-H+ structure by combining protons onto the hydroxyl oxygen. As a result, electrostatic adsorption may happen to assemble the Ag3PO4–SOPP composite. Except for the aforesaid, given that the van der Waals force always exists, such an intermolecular force may lead to the weak connection between Ag3PO4 and SOPP. In conclusion, the assembling of the Ag3PO4–SOPP composite was the result of various non-chemical bond intermolecular forces.
Antifungal activity of the residue-free nanotechnology
The antifungal activity was evaluated against the plant pathogen fungi Phytophthora capsici and Botrytis cinerea (Fig. 3). On a CA or PDA medium, the fungal hyphae growth rates were dramatically reduced in the presence of the Ag3PO4 micro/nano-crystals. For Phytophthora capsici (Fig. 3a), the minimum inhibitory concentration (MIC) of Ag3PO4 was 0.4 g L−1, which is obviously lower than the MIC of the ZnO NPs (2.0 g L−1) we tested before.27 Meanwhile, even when the concentration of the Ag3PO4 was decreased to 0.025 g L−1, it still retained a 60% inhibition rate for fungal hyphae growth. In contrast, the ZnO NPs at such a concentration did not show any antifungal effect. For Botrytis cinerea (Fig. 3b), the MIC of Ag3PO4 was 0.4 g L−1, and the Ag3PO4 also attained a 60% inhibition rate for hyphae growth at 1/16 of the MIC. These results indicate that the Ag3PO4 micro/nano-crystals can be an effective potential antifungal agent in plant protection.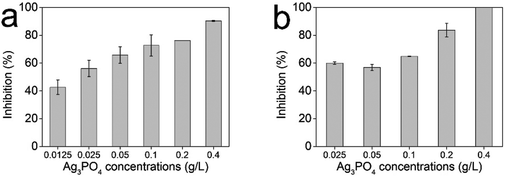 | ||
| Fig. 3 Antifungal activities of the Ag3PO4 micro/nano-crystals against the plant pathogen fungi Phytophthora capsici (a) and Botrytis cinerea (b). Error bars are standard errors with n = 3. | ||
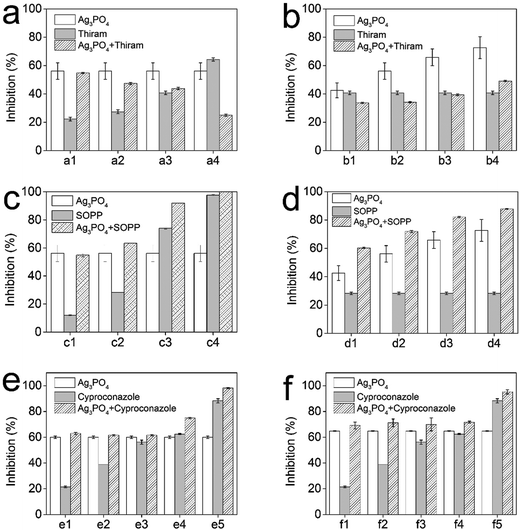
The antifungal activity of the Ag3PO4–fungicide composite antifungal system was further explored. Because thiram could exhibit an obvious improvement in antifungal performance with ZnO NPs,27 in this study we firstly investigated the combined antifungal effect of Ag3PO4 with thiram against Phytophthora capsici. Unlike the combination of ZnO with thiram, the antifungal effect of Ag3PO4 with thiram was not enhanced but even decreased significantly (Fig. 4a). Though the antifungal effect of thiram increased with an increasing concentration, the combined effects of 0.025 g L−1 Ag3PO4 with thiram decreased with the increasing of the thiram dosage (Fig. 4a). When the thiram concentration reached 0.02 g L−1, the antifungal activity of 0.025 g L−1 Ag3PO4 with thiram was even lower than Ag3PO4 or thiram alone (Fig. 4a). The synergy factors for 0.025 g L−1 Ag3PO4 with 0.0025, 0.005, 0.01 and 0.02 g L−1 thiram were 0.7, 0.57, 0.45 and 0.21, respectively, decreasing with the increase of the thiram concentration and eventually dropping to below 0.5. The results demonstrate an antagonistic action of Ag3PO4 combined with thiram. It can also be concluded that the concentration of thiram was the key factor in defining the interactions of Ag3PO4 and thiram. The influence of the Ag3PO4 dosage on the combined effect was also studied (Fig. 4b). The antifungal activity of Ag3PO4 combined with thiram did not increase with increasing Ag3PO4 concentration. The synergy factors for 0.0125, 0.025, 0.05 and 0.1 g L−1 Ag3PO4 with 0.01 g L−1 thiram were 0.41, 0.35, 0.37 and 0.43, respectively, which demonstrates an antagonistic effect. The antagonistic effect means that the combination has an overall effect that is less than the sum of their individual effects, or even less than the effect of one of the components alone. This “1 + 1 < 2” or “1 + 1 < 1” result makes it undesirable to combine Ag3PO4 and thiram for fungal control.
The combined antifungal effect of Ag3PO4 and SOPP against Phytophthora capsici was then investigated. From Fig. 4c and d, it can be observed that the combination lead to an increase in the overall antifungal effect. The combined antifungal effect increased with the increasing of the Ag3PO4 and SOPP concentrations. The synergy factors for 0.025 g L−1 Ag3PO4 with 0.02, 0.04, 0.08 and 0.16 g L−1 SOPP were 0.77, 0.73, 0.68 and 0.65, respectively. When considering 0.0125, 0.025, 0.05 and 0.1 g L−1 Ag3PO4 with 0.04 g L−1 SOPP, the synergy factors were 0.85, 0.85, 0.87 and 0.87 respectively. These results show that the combination could bring extra antifungal effects compared with SOPP alone. For example, with the help of 0.025 g L−1 Ag3PO4, 0.08 g L−1 SOPP could provide a similar antifungal effect compared with 0.16 g L−1 SOPP. This enhanced effect indicates that Ag3PO4 could increase the SOPP antifungal activity, and at the same time reduce the fungicide usage as well as delay the development of resistance.
The enhanced antifungal activity of the fungicide cyproconazole with Ag3PO4 was studied. Because cyproconazole was ineffective in controlling Phytophthora capsici, Botrytis cinerea was selected as the test fungal strain. According to the antifungal test, the addition of Ag3PO4 enhanced the antifungal activity of cyproconazole (Fig. 4e and f). However, the synergy factors for Ag3PO4–cyproconazole were all lower than for the Ag3PO4–SOPP combinations (ESI Table 1†), demonstrating that the enhanced effect in the antifungal activity was relatively small. Taking into account that the obtained extra antifungal activity was limited, the Ag3PO4–cyproconazole combination was not selected for further studies.
The residue-free effect of the antifungal nanotechnology: photocatalytic degradation of the fungicide residue after the antifungal application
As Ag3PO4 could enhance the antifungal activity of SOPP, the photocatalytic degradation of SOPP by Ag3PO4 was studied under simulated sunlight irradiation to establish a residue-free antifungal nanotechnology. From Fig. 5, it can be observed that the photocatalytic degradation rates increased as the Ag3PO4 concentration increased from 0.8 g L−1 to 1.2 g L−1 and 1.6 g L−1. After 2 h of simulated sunlight irradiation, over 90% of the SOPP was decomposed by 1.6 g L−1 Ag3PO4. In contrast, the remaining SOPP in the presence of 0.25 g L−1 ZnO NPs was still nearly 100% after 2 h of irradiation. This significant difference in photocatalytic activity of the above two materials could be explained using the following reasons: firstly, the Ag3PO4 micro/nano-crystals utilized sunlight more efficiently than the ZnO NPs. The light source in our experiments was a 500 W Xenon lamp for the purpose of simulating the actual light irradiation present in fields. The light was primarily constituted of visible light, with only a small part from the ultraviolet range, and such a light irradiation cannot be efficiently utilized by ZnO NPs. In contrast, the as-prepared Ag3PO4 micro/nano-crystals showed strong absorption of visible light, and thus could better utilize the simulated sunlight. Secondly, according to a previous report, the quantum efficiency of Ag3PO4 micro/nano-crystals was over 90%, which was much higher than ZnO with values at around 20%.14 In comparison with UV photocatalytic materials, the high photocatalytic efficiency of the Ag3PO4 micro/nano-crystals under visible light irradiation makes this approach more applicable to natural environmental conditions.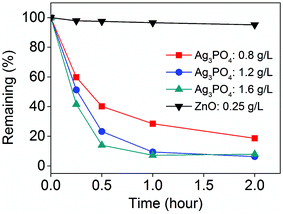 | ||
| Fig. 5 Photocatalytic activities of the Ag3PO4 micro/nano-crystals for fungicide SOPP degradation under simulated sunlight irradiation. | ||
Combining the enhanced antifungal activity and the successive highly photocatalytic degradation of SOPP, the residue-free green antifungal nanotechnology for fungicide SOPP using Ag3PO4 micro/nano-crystals was established. A schematic diagram of the nanotechnology is depicted in Fig. 6. Owing to the enhanced antifungal effect, the usage of the fungicide SOPP could be reduced from 0.16 g L−1 to 0.08 g L−1 without compromising pathogen control. More importantly, the complete photocatalytic degradation enables the removal of excess SOPP after the antifungal process and consequently reduces the fungicide residue to within the government administrations standard. Such a significant decline in fungicide usage and successive degradation could offer great benefits for crop production and human society as well as environmental protection.
Mechanism study of the enhanced antifungal activity of SOPP with Ag3PO4 micro/nano-crystals
It is important to establish the antifungal mechanism of the Ag3PO4 micro/nano-crystals before discussing the synergistic enhanced effect. As the generation of reactive oxygen species (ROS) by NPs is regarded as a common mechanism which can cause cellular toxicity,28–31 we therefore studied whether ROS were produced by a Ag3PO4 micro/nano-crystal suspension. Because histidine is a known scavenger of ROS such as hydroxyl radicals (˙OH) and singlet oxygen (O2˙−),27,32 it was therefore added to the CA culture to examine its effect on the Ag3PO4 micro/nano-crystals antifungal activity. However, in the presence of histidine as an antioxidant, the fungal hyphae growth in the presence of the Ag3PO4 micro/nano-crystals was still inhibited (Fig. 7). The inhibition of fungal growth was even promoted with the addition of 1.0 and 2.0 g L−1 histidine. These results demonstrate that the antifungal activity of the Ag3PO4 micro/nano-crystals may not rely on directly producing ROS.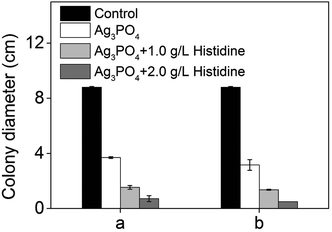 | ||
| Fig. 7 Effect of the antioxidant histidine on the antifungal activity of the Ag3PO4 micro/nano-crystals. a, Ag3PO4: 0.025 g L−1. b, Ag3PO4: 0.05 g L−1. Error bars are standard errors with n = 3. | ||
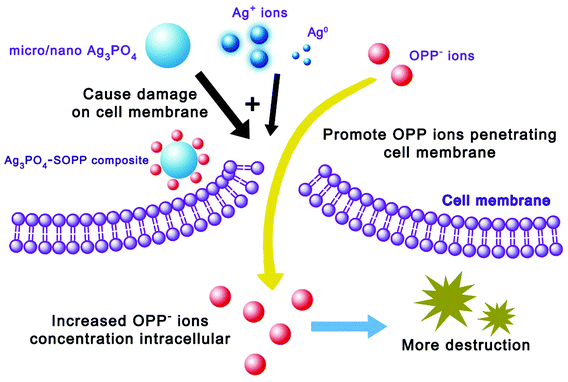
The direct interaction of NPs with microbial cells was considered as another main mechanism.33,34 In our system, Ag3PO4 micro/nano-crystals are able to come into contact with fungal cells during the growth of the hyphae, and therefore could disrupt the integrity of the cell membrane, leading to leakage of the cellular content and interruption of fungal growth.35–37 Furthermore, the surface silver phosphate of the micro/nano-crystals is able to release Ag+ ions in the presence of water or during interaction with the cell membrane. Our experiments found that there are 0.00235, 0.00303 and 0.00322 g L−1 Ag+ ions leached from 0.025, 0.05 and 0.1 g L−1 Ag3PO4 micro/nano-crystal suspensions respectively (Table 1), and demonstrated that adding Ag+ ions (source: AgNO3) to CA medium could induce a certain degree of antifungal activity (ESI Fig. 4†). These results demonstrate the contribution of Ag+ ions to the antifungal action, and are in consistence with previous reports that released Ag+ induces antimicrobial activity.38–40 The Ag+ ions could develop their antimicrobial activity from several pathways. For example, Ag+ ions were reported to interact with transport or respiratory chain enzymes inducing collapse of the proton motive force and disruption of ATP production as well as disruption of the cell membrane.41,42 Moreover, due to possessing some degree of oxidizability (oxidation–reduction potential: +0.798 eV), part of the Ag+ ions could be reduced to form Ag0 from the presence of organic matter in the culture medium or by light irradiation during experimental procedures.43,44 In order to obtain more information on the reduction of the Ag+ ions, we first conducted ICP analysis on the AgNO3 solution after subjecting it to illumination for 24 hours (500 W Xenon lamp). The results showed that the Ag+ concentration decreased from 19.1 mg L−1 to 17.7 mg L−1, providing evidence for the reduction of Ag+ ions. An XPS technique was also employed to analyse the solid content of the Ag3PO4 suspension after illumination. The full XPS spectrum (Fig. 8a) demonstrates the existence of Ag, O, and P elements in the solid content of the Ag3PO4 micro/nano-crystal suspension. In Fig. 8b, the two peaks at approximately 367.9 eV and 373.9 eV can be ascribed to the binding energies of Ag 3d5/2 and Ag 3d3/2, respectively.45 The two peaks can be further divided to yield four different peaks at 367.8 eV, 368.9 eV, 373.8 eV and 374.9 eV. According to previous reports, the peaks at 368.9 eV and 374.9 eV can be attributed to Ag0 whereas the peaks at 367.8 eV and 373.8 eV can be attributed to Ag+ (Ag3PO4).45,46 These XPS results confirm the reduction of Ag+ to Ag0 and are in consistence with the results of the UV-Vis spectrophotometer study. Because the freshly reduced Ag0 species are highly active, they could directly attack the cell membrane or enter the cellular interior which also contributed to the inhibition of the fungal growth, so reduction of part of the Ag+ ions will not obviously change the antifungal activity of the Ag3PO4. From the above full investigation, it can be concluded that the Ag3PO4 micro/nano-crystals inhibited the fungal growth together with Ag+ ion and fresh active Ag0.
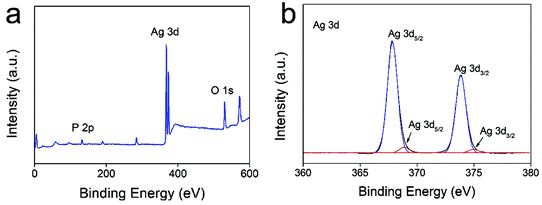 | ||
| Fig. 8 The full XPS spectra for the solid content of the Ag3PO4 suspension after illumination (a) and the high-resolution XPS spectrum of Ag 3d (b). | ||
| Ag3PO4 (g L−1) | Ag+ released from Ag3PO4 (g L−1) |
|---|---|
| 0.025 | 0.00235 |
| 0.05 | 0.00303 |
| 0.1 | 0.00322 |
Though scientists have established the synergistic antimicrobial mechanism for various antibiotics,47,48 there has not been a universal explanation for all circumstances. For this study, SOPP is known to release the ions of o-phenylphenate (OPP) in solution which exert an antifungal activity after penetrating microbial cells.49 As fungal cells exposed to the Ag3PO4 micro/nano-crystals, Ag+ ions and freshly active Ag0 suffer disruption of the integrity of the cell membrane, it is reasonable to speculate that the OPP− ions penetrating the cell membrane is facilitated by the above agents. As is shown in Fig. 9, this mechanism may lead to an increase of the intracellular OPP− concentration and therefore cause more destruction, showing the enhanced antifungal activity. Meanwhile, the formation of a Ag3PO4–SOPP composite could lead to an increasing local SOPP concentration near to the hyphae where Ag3PO4 is in contact with fungal cells and therefore cause more destruction. Additionally, due to the multiple attacking actions from SOPP, Ag3PO4 micro/nano-crystals, released Ag+ ions and reduced Ag0, the microbe cells were destroyed inevitably. Thus, this situation also plays an important role in the enhanced antifungal activity as developing multiple resistance mechanisms simultaneously is very difficult for cells. In summary, damage of cell membranes by Ag3PO4, Ag+ and Ag0, the formation of a Ag3PO4–SOPP composite, plus the multiple antifungal mechanisms of the antifungal system finally jointly result in an enhanced antifungal effect (Fig. 9).
Conclusions
Our work presents an integrated green nanotechnology using Ag3PO4 micro/nano-crystals to enhance the efficiency of the fungicide SOPP but without its residue remaining. The results demonstrate that the as-prepared Ag3PO4 micro/nano-crystals could reduce the usage of fungicide SOPP by 50 percent without compromising on pathogen control. More importantly, it was found that over 90% of the SOPP was decomposed by the Ag3PO4 micro/nano-crystals under simulated sunlight irradiation within 2 h, showing that the SOPP residue could be eliminated. It is noted that this high-efficiency and residue-free nanotechnology could offer great benefits for crop production and human society. Furthermore, according to investigation, the antifungal activity of the Ag3PO4 is not due to the production of ROS but strongly related to interaction with fungal cells and the release of Ag+ ions. The mechanism for the synergistic enhanced antifungal effect was speculated from three possible aspects: (a) Ag3PO4 micro/nano-crystals and Ag+ ions promoting the penetration of OPP ions into the cell interior; (b) formation of a Ag3PO4–SOPP composite; and (c) multiple modes of antifungal action of the Ag3PO4–SOPP system. To the best of our knowledge, the above frontier achievements in inorganic chemistry demonstrate that our approach outperforms state-of-the-art antifungal techniques.Conflict of interest
The authors declare that they have no conflict of interest.Acknowledgements
This work is supported by the National Natural Science Foundation of China (no. 21471114), and the State Major Research Plan (973) of China (no. 2011CB932404).References
- P. J. P. Verger and A. R. Boobis, Science, 2013, 341, 717–718 CrossRef CAS PubMed.
- J. Popp, K. Pet and J. Nagy, Agron. Sustainable Dev., 2013, 33, 243–255 CrossRef.
- D. Tilman, K. G. Cassman, P. A. Matson, R. Naylor and S. Polasky, Nature, 2002, 418, 671–677 CrossRef CAS PubMed.
- F. P. Carvalho, Environ. Sci. Policy, 2006, 9, 685–692 CrossRef.
- E. A. Stockdale, N. H. Lampkin, M. Hovi, R. Keatinge, E. K. M. Lennartsson, D. W. Macdonald, S. Padel, F. H. Tattersall, M. S. Wolfe and C. A. Watson, in Advances in Agronomy, Academic Press, 2001, pp. 261–327 Search PubMed.
- P. M. Der, A. Flie Bach, D. Dubois, L. Gunst, P. Fried and U. Niggli, Science, 2002, 296, 1694–1697 CrossRef PubMed.
- D. W. Lotter, J. Sustainable Agric., 2003, 21, 59–128 CrossRef.
- L. He, Y. Liu, A. Mustapha and M. Lin, Microbiol. Res., 2011, 166, 207–215 CrossRef CAS.
- J. S. Min, K. S. Kim, S. W. Kim, J. H. Jung, K. Lamsal, S. B. Kim, M. Jung and Y. S. Lee, Plant Pathology J., 2009, 25, 376–380 CrossRef CAS.
- P. Patra, S. Mitra, N. Debnath and A. Goswami, Langmuir, 2012, 28, 16966–16978 CrossRef CAS.
- H. Tong, S. Ouyang, Y. Bi, N. Umezawa, M. Oshikiri and J. Ye, Adv. Mater., 2012, 24, 229–251 CrossRef CAS PubMed.
- M. Pelaez, N. T. Nolan, S. C. Pillai, M. K. Seery, P. Falaras, A. G. Kontos, P. S. M. Dunlop, J. W. J. Hamilton, J. A. Byrne, K. O'Shea, M. H. Entezari and D. D. Dionysiou, Appl. Catal., B, 2012, 125, 331–349 CrossRef CAS.
- Y. Bi, H. Hu, S. Ouyang, G. Lu, J. Cao and J. Ye, Chem. Commun., 2012, 48, 3748 RSC.
- Y. Bi, S. Ouyang, N. Umezawa, J. Cao and J. Ye, J. Am. Chem. Soc., 2011, 133, 6490–6492 CrossRef CAS.
- Z. Yi, J. Ye, N. Kikugawa, T. Kako, S. Ouyang, H. Stuart-Williams, H. Yang, J. Cao, W. Luo, Z. Li, Y. Liu and R. L. Withers, Nat. Mater., 2010, 9, 559–564 CrossRef CAS.
- H. Wang, Y. Bai, J. Yang, X. Lang, J. Li and L. Guo, Chem. – Eur. J., 2012, 18, 5524–5529 CrossRef CAS PubMed.
- S. Bai, X. Shen, H. Lv, G. Zhu, C. Bao and Y. Shan, J. Colloid Interface Sci., 2013, 405, 1–9 CrossRef CAS PubMed.
- N. Umezawa, O. Shuxin and J. Ye, Phys. Rev. B: Condens. Matter, 2011, 83 Search PubMed.
- J. Liu, C. Luo, J. Wang, X. Yang and X. Zhong, CrystEngComm, 2012, 14, 8714 RSC.
- A. Wu, C. Tian, W. Chang, Y. Hong, Q. Zhang, Y. Qu and H. Fu, Mater. Res. Bull., 2013, 48, 3043–3048 CrossRef CAS.
- P. Henschke, E. Almstadt, L. X. FC, S. Ttgert and K. E. Appel, Arch. Toxicol., 2000, 73, 607–610 CrossRef CAS PubMed.
- K. Hiraga and T. Fujii, Food Cosmet. Toxicol., 1981, 19, 303–310 CrossRef CAS PubMed.
- U. Gisi, Phytopathology, 1996, 86, 1273–1279 CAS.
- P. Ma, A. Chen, Y. Wu, Z. Fu, W. Kong, L. Che and R. Ma, J. Colloid Interface Sci., 2014, 417, 293–300 CrossRef CAS PubMed.
- H. Valdés, M. Sánchez-Polo, J. Rivera-Utrilla and C. A. Zaror, Langmuir, 2002, 18, 2111–2116 CrossRef.
- A. A. Farah, J. P. Bravo-Vasquez, R. A. Alvarez-Puebla, J. Cho and H. Fenniri, Small, 2009, 5, 1283–1286 CrossRef CAS PubMed.
- J. Xue, Z. Luo, P. Li, Y. Ding, Y. Cui and Q. Wu, Sci. Rep., 2014, 4 Search PubMed.
- R. Brayner, R. Ferrari-Iliou, N. Brivois, S. Djediat, M. F. Benedetti and F. Fiévet, Nano Lett., 2006, 6, 866–870 CrossRef CAS PubMed.
- Y. Li, W. Zhang, J. Niu and Y. Chen, ACS Nano, 2012, 6, 5164–5173 CrossRef CAS PubMed.
- A. Nel, T. Xia, L. M. A. Dler and N. Li, Science, 2006, 311, 622–627 CrossRef CAS.
- E. Malka, I. Perelshtein, A. Lipovsky, Y. Shalom, L. Naparstek, N. Perkas, T. Patick, R. Lubart, Y. Nitzan, E. Banin and A. Gedanken, Small, 2013, 9, 4069–4076 CrossRef CAS PubMed.
- A. Lipovsky, Y. Nitzan, A. Gedanken and R. Lubart, Nanotechnology, 2011, 22, 105101 CrossRef.
- M. J. Hajipour, K. M. Fromm, A. AkbarAshkarran, D. Jimenez De Aberasturi, I. R. D. Larramendi, T. Rojo, V. Serpooshan, W. J. Parak and M. Mahmoudi, Trends Biotechnol., 2012, 30, 499–511 CrossRef CAS PubMed.
- A. Thill, O. Zeyons, O. Spalla, F. Chauvat, J. Rose, M. Auffan and A. M. Flank, Environ. Sci. Technol., 2006, 40, 6151–6156 CrossRef CAS.
- W. K. Jung, H. C. Koo, K. W. Kim, S. Shin, S. H. Kim and Y. H. Park, Appl. Environ. Microb., 2008, 74, 2171–2178 CrossRef CAS PubMed.
- Q. L. Feng, J. Wu, G. Q. Chen, F. Z. Cui, T. N. Kim and J. O. Kim, J. Biomed. Mater. Res., 2000, 52, 662–668 CrossRef CAS.
- X. Yang, J. Qin, Y. Jiang, R. Li, Y. Li and H. Tang, RSC Adv., 2014, 4, 18627 RSC.
- X. Yang, J. Qin, Y. Jiang, K. Chen, X. Yan, D. Zhang, R. Li and H. Tang, Appl. Catal., B, 2015, 166–167, 231–240 CrossRef CAS.
- J. Xu, Z. Gao, K. Han, Y. Liu and Y. Song, ACS Appl. Mater. Interfaces, 2014, 6, 15122–15131 CAS.
- C. Marambio-Jones and E. M. V. Hoek, J. Nanopart. Res., 2010, 12, 1531–1551 CrossRef CAS.
- C. Lok, C. Ho, R. Chen, Q. He, W. Yu, H. Sun, P. K. Tam, J. Chiu and C. Che, J. Proteome. Res., 2006, 5, 916–924 CrossRef CAS PubMed.
- K. B. Holt and A. J. Bard, Biochemistry, 2005, 44, 13214–13223 CrossRef CAS.
- L. Zhang, J. C. Yu, H. Y. Yip, Q. Li, K. W. Kwong, A. Xu and P. K. Wong, Langmuir, 2003, 19, 10372–10380 CrossRef CAS.
- M. Sathishkumar, K. Sneha, S. W. Won, C. W. Cho, S. Kim and Y. S. Yun, Colloids Surf., B, 2009, 73, 332–338 CrossRef CAS PubMed.
- W. Wang, H. Du, R. Wang, T. Wen and A. Xu, Nanoscale, 2013, 5, 3315 RSC.
- H. Zhang, G. Wang, D. Chen, X. Lv and J. Li, Chem. Mater., 2008, 20, 6543–6549 CrossRef CAS.
- S. Croes, E. E. Stobberingh, K. N. J. Stevens, M. L. W. Knetsch and L. H. Koole, ACS Appl. Mater. Interfaces, 2011, 3, 2543–2550 CAS.
- I. S. Hwang, J. H. Hwang, H. Choi, K. J. Kim and D. G. Lee, J. Med. Microbiol., 2012, 61, 1719–1726 CrossRef CAS PubMed.
- A. Apelbaum and R. Barkai Golan, Food Addit. Contam., 1987, 4, 317–324 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5qi00186b |
| This journal is © the Partner Organisations 2016 |