Multi-shelled LiMn2O4 hollow microspheres as superior cathode materials for lithium-ion batteries†
Feng
Wang‡
a,
Jiangyan
Wang‡
bd,
Hao
Ren
a,
Hongjie
Tang
b,
Ranbo
Yu
*a and
Dan
Wang
*bc
aDepartment of Physical Chemistry, School of Metallurgical and Ecological Engineering, University of Science and Technology Beijing, No. 30, Xueyuan Road, Haidian District, Beijing 100083, P. R. China. E-mail: ranboyu@ustb.edu.cn
bNational Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, No. 1 Beiertiao, Zhongguancun, Beijing 100190, P. R. China. E-mail: danwang@ipe.ac.cn
cCentre for Clean Environment and Energy, Gold Coast Campus Griffith University, Queensland 4222, Australia
dUniversity of Chinese Academy of Sciences, No.19A Yuquan Road, Beijing 100049, P. R. China
First published on 22nd December 2015
Abstract
Owing to its environmental-benignity, low-cost and abundance, spinel LiMn2O4 has long been considered as a promising cathode material for lithium-ion batteries (LIBs). However, the low electronic conductivity, small lithium diffusion coefficient and poor capacity retention hindered its further development and application. Herein, we report the synthesis of multi-shelled LiMn2O4 hollow microspheres through a hard template method, with the composition, shell number, shell thickness and porosity accurately controlled. Benefitting from the structural superiorities of multi-shelled hollow structures, the triple-shelled LiMn2O4 hollow microsphere exhibits a better cycling stability than all the reported results based on un-coated or un-doped LiMn2O4 (the capacity fading rate is 0.10% per cycle).
Introduction
Lithium-ion batteries (LIBs), owing to their remarkable advantages such as light-weight, no memory effect, and environmental friendliness, have been considered as the most promising candidate to meet the energy and environmental requirements in the 21st century.1,2 The commercially available cathode materials are mostly LixCoO2 with a specific capacity of 140 mA h g−1 based on a practical value of x from 1 to 0.5. However, the toxicity, high-cost and scarcity of Co limit its large-scale application in LIBs.3 As a result, it's urgently needed to develop other cathode materials to meet the further development of next-generation LIBs. Spinel-structured lithium manganese oxide (LiMn2O4), with a high potential of 4 V and a theoretical specific capacity of 148 mA h g−1, has long been studied as an attractive cathode material for high-power LIBs due to the non-toxicity, low-cost and abundance of Mn.4–8 Unfortunately, the practical application of LiMn2O4 has been impeded by its kinetic problems such as low electronic conductivity and small lithium diffusion coefficient, also poor capacity retention caused by Mn dissolution in the electrolyte.9–12 One effective strategy to overcome these drawbacks is building hollow micro/nanostructured materials to replace the bulk one as cathodes of LIBs. The advantages of adopting hollow micro/nanostructures are obvious and listed below:13–15 (1) the enhanced surface-to-volume ratio can provide more effective specific surface area, thus a higher specific capacity; (2) the improved activity and shortened transport length for both ions and electrons lead to an improved rate capability and power density; (3) more importantly, with the multi-shelled hollow structures, different shells can support each other and the exterior shell protects the interior shells from dissolution, leading to an improved structural stability and better cycling performance.Given the above considerations, great efforts have been devoted to the synthesis of hollow micro/nanostructured cathode materials, such as hollow spheres,16–18 hollow octahedra,19 hollow spindles20 and hollow boxes.21 However, all the achieved hollow structures are only single-shelled or single-shelled dominated in the final products.22,23 Recently, though some other metal oxide multi-shelled hollow microspheres have been synthesized using carbonaceous microspheres (CMSs) as templates,24–28 most of the products contain a single metal. There are a few products containing two kinds of metal elements, yet only restricted to metal elements with similar qualities. Due to the distinct nature of Li and Mn elements, the synthesis of multi-shelled LiMn2O4 hollow microspheres (MS-LiMn2O4-HMSs) still remains rather challenging. Herein, we report an effective approach to produce MS-LiMn2O4-HMSs with the composition, shell number (triple or quadruple), shell thickness and porosity accurately controlled. Profiting from the superiorities of multi-shelled hollow nanostructures, MS-LiMn2O4-HMSs as cathode materials for LIBs exhibit highly improved lithium-storage performance: the triple-shelled one exhibits a better cycling stability than all the reported results based on un-coated or un-doped LiMn2O4 (the capacity fading rate is only 0.10% per cycle even to 200 cycles) (see Table S1†).
Results and discussion
As Fig. S1† shows, the compositions of the final products are quite different at different ratios of the Li precursor (LiAc) to Mn precursor (Mn(NO3)2·4H2O). Only when the adding ratio of the Li precursor to Mn precursor is around 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 can LiMn2O4 products be achieved (Fig. 1e). It's also worth mentioning that the ratio of Li to Mn in the precursor solution is much higher than that in the final products. The reason may be that CMSs are more inclined to adsorb Mn(II) ions compared to Li (I) ions, since the former has more charges than the latter, leading to a stronger electrostatic interaction between Mn(II) ions and negatively-charged CMS templates.29 Besides, the empty d orbitals of Mn2+ are well ready to accept the p electrons donated by oxygen (O) in the CMSs, making strong coordination interaction between Mn(II) ions and CMSs, thus Mn(II) ions can stay stable in CMSs. Moreover, more metal precursor will be adsorbed at a larger concentration. Therefore, to guarantee that enough Li is adsorbed by CMSs, the concentration of the Li precursor should be much higher than that of the Mn precursor.
1 can LiMn2O4 products be achieved (Fig. 1e). It's also worth mentioning that the ratio of Li to Mn in the precursor solution is much higher than that in the final products. The reason may be that CMSs are more inclined to adsorb Mn(II) ions compared to Li (I) ions, since the former has more charges than the latter, leading to a stronger electrostatic interaction between Mn(II) ions and negatively-charged CMS templates.29 Besides, the empty d orbitals of Mn2+ are well ready to accept the p electrons donated by oxygen (O) in the CMSs, making strong coordination interaction between Mn(II) ions and CMSs, thus Mn(II) ions can stay stable in CMSs. Moreover, more metal precursor will be adsorbed at a larger concentration. Therefore, to guarantee that enough Li is adsorbed by CMSs, the concentration of the Li precursor should be much higher than that of the Mn precursor.
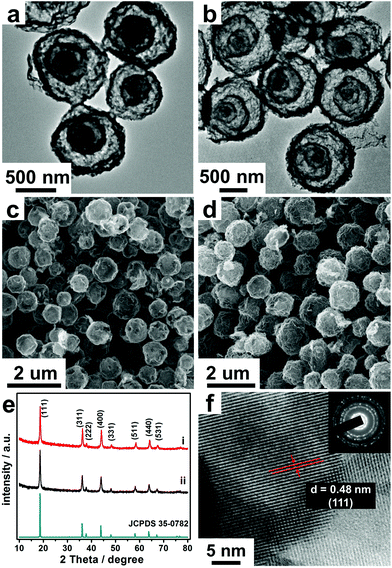
The annealing process is also a key parameter which controls the morphology and structure of the final microsphere products, wherein the heating rate is one of the most important factors which can cause great discrepancy between the rate of metal oxide shell formation (v1) and CMS contraction (v2). Normally, the slow heating rate can benefit the shell number. Under a slow heating rate (1 °C min−1), both the speed of oxide-crystallization and the difference between v1 and v2 decrease. And the outer oxide will shrink along with the shrinking of the inner CMS core during the early period of annealing as there is no enough oxide to sustain the formation of the oxide shell, thus accumulating to form a thick shell. Then the thick shell further shrinks and separates to double shells, and finally results in triple-shelled hollow microspheres with relatively small diameters (3S-HMSs) (Fig. 1a and c). By contrast, bigger double-shelled hollow microspheres (2S-HMSs) were obtained at a higher heating rate (10 °C min−1) (Fig. S2†) (yet the phase is not pure LiMn2O4).
Besides the heating rate, the adsorption duration also matters a lot in controlling the morphology and structure of the products. It's easy to understand that the adsorption amount and the penetration depth into the CMSs increase along with the increase of the adsorption duration. As a result, by prolonging the adsorption duration and further slowing the heating rate (0.5 °C min−1), quadruple-shelled hollow microspheres (4S-HMSs) are obtained (Fig. 1b and d) (see Experimental details in Table S2†).
Through the above methods, triple- and quadruple-shelled LiMn2O4 hollow microspheres with high purity were prepared. From the transmission electron microscopy (TEM) (Fig. 1a and b) and scanning electron microscopy (SEM) (Fig. 1c and d) characterization, we can see that the size is uniform, with diameters of about 0.9 and 1.2 μm for 3S- and 4S-LiMn2O4-HMSs respectively. The X-ray diffraction (XRD) patterns (Fig. 1e) show that both the products correspond to spinel type LiMn2O4 (JCPDS card no. 35-0782). Besides, the peaks are rather sharp and no additional impurity peaks were observed, indicating good crystallinity and purity of the products.30,31 From the high resolution TEM (HRTEM) image (Fig. 1f), we can also see that the product is highly crystallized, with a d-space of 0.48 nm commonly observed, corresponding to the (111) crystal facet of LiMn2O4. Considering the weight loss stopped at about 348 °C according to TGA-DTA (thermogravimetric analysis–differential thermal analysis) data (Fig. S3†), the excellent crystallinity of the products can be ascribed to the optimal annealing treatment conditions (600 °C, 2 h) which guarantee the complete combustion of CMS templates and crystallization of LiMn2O4. To further test the chemical composition of the as-prepared MS-LiMn2O4-HMSs, X-ray photoelectron spectroscopy (XPS) was carried out (Fig. S4†). The two peaks located at 641.1 eV and 642.55 eV correspond to Mn3+, while the other peak located at 643.1 eV corresponds to Mn4+.12
The continuous shells are composed of small nanoparticles according to the structural observation of the typical 3S-LiMn2O4-HMS (Fig. S5†). The nitrogen adsorption–desorption analysis (Fig. S6†) shows that the specific surface area of 4S-LiMn2O4-HMS is larger than that of the triple-shelled (47.04 vs. 31.69 m2 g−1) (Table S3†). Fig. S7† demonstrates that nanopores of 2.5 and 4 nm mainly exist in the triple- and quadruple-shelled hollow microspheres, respectively. The electrolyte can directly penetrate into the interior of the hollow microspheres through these pores, thus shortening the transfer path and improving the kinetic characters.
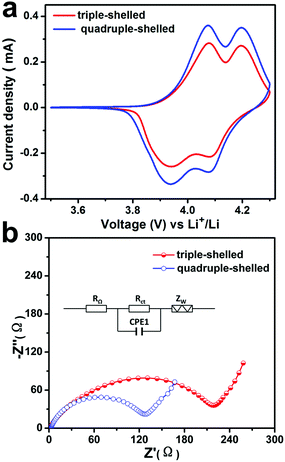
The cyclic voltammograms (CV) curves of the MS-LiMn2O4-HMSs samples at a scan rate of 1 mV s−1 in a potential range from 3.5 to 4.3 V are shown in Fig. 2a. Two typical redox peaks around 3.95/4.05 V and 4.1/4.2 V (vs. Li+/Li) are clearly observed for all the LiMn2O4 products. Two anode peaks at about 4.05 and 4.2 V are corresponding to Li-extraction from the crystal lattice and the formation of Li0.5Mn2O4 and λ-MnO2 respectively, while the two cathode peaks at around 3.95 and 4.1 V indicate the reversible Li-insertion process, which is similar to the previously reported results.32,33 Moreover, with the same loading amount, the current density of 4S-LiMn2O4-HMSs is larger than that of 3S-LiMn2O4-HMSs, demonstrating a faster charge separation and better electron conductivity. The Nyquist plots in Fig. 2b also support this hypothesis. Both the samples display a semicircle in the high frequency region and a sloping straight line in the low frequency range, presenting the charge transfer resistance (Rct) and diffusion of lithium in the solid (Zw), respectively.34 The smaller semicircle diameter for the 4S-HMS based electrode shows a smaller Rct compared to the 3S-HMS based electrode, indicating quicker charge transfer. This discrepancy may be ascribed to the thinner shell and larger surface area of 4S-LiMn2O4-HMSs, which can enhance the contact area of electrolyte–electrode, and facilitate the electrolyte transport and lithium ion diffusion. By contrast, limited by the thick shell and small specific surface area, the triple-shelled one shows the smaller charge transfer resistance.
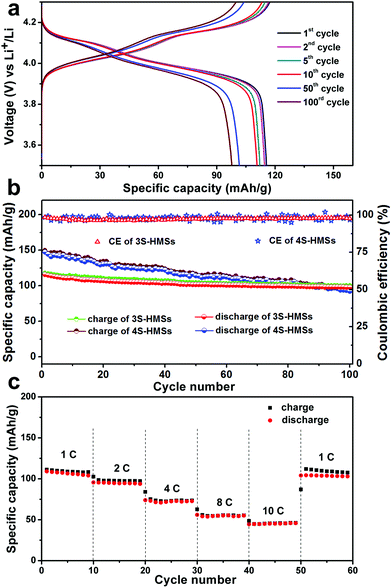
The lithium-storage properties of the MS-LiMn2O4-HMSs as cathode materials for LIBs are tested by fabricating a standard LiMn2O4/Li half-cell configuration. Taking 3S-LiMn2O4-HMS as an example, two plateaus located at about 4.10 and 3.95 V in the discharge curves, and another two plateaus at around 4.05 and 4.2 V in the charge curves are obviously noticed (Fig. 3a and S8†), which is consistent with the above CV results. Moreover, the plateaus can still be observed even after 100 cycles, indicating good reversibility of the 3S-LiMn2O4-HMSs.
The specific capacity and cycling performance of LiMn2O4 hollow microspheres with different shell numbers are compared in Fig. 3b. As expected by CV and EIS results, the 4S-LiMn2O4-HMSs exhibit a higher initial discharge specific capacity as high as 143.4 mA h g−1, while that of the 3S-HMSs is 115.5 mA h g−1. The reason for the specific capacity of the 4S-LiMn2O4-HMSs being superior to that of the 3S-LiMn2O4-HMSs may be the fact that a larger specific surface area of the 4S-LiMn2O4-HMSs can provide more surface lithium-storage sites, a larger electrode/electrolyte contact area, and thus improved kinetic behaviour. However, the excessively large specific surface area of the 4S-LiMn2O4-HMSs is a double-edged sword since it can cause the drastic dissolution of the surface Mn, inducing the Jahn–Teller effect and active material loss.35,36 The Jahn–Teller effect will give rise to structural change, resulting in the increase of the Li-insertion/extraction impedance. As Fig. S9† shows, the Rct of both the types of multi-shelled hollow microspheres increases after 100 cycles, whereas that of the 4S-HMSs increases more and even becomes larger than that of the 3S-HMSs. Together with the capacity loss caused by active material dissolution, the 4S-LiMn2O4-HMSs show a poorer cycling stability. The 3S-LiMn2O4-HMSs, which have a moderate specific surface area with the thick outer shell protecting the inner shells from direct electrochemical dissolution, exhibit a much better cycling stability. After 100 cycles, the discharge specific capacities of triple- and quadruple-shelled are 97.9 and 92.1 mA h g−1, respectively. Impressively, even after 200 consecutive cycles, the capacity of 3S-HMSs still remains 91.2 mA h g−1 with a high coulombic efficiency of over 95% (Fig. S10†), demonstrating a fading rate of only 0.10% per cycle, which is superior to all the reported results based on the LiMn2O4 cathode without carbon-coating or ion-doping (Table S1†). To further testify the structural integrity of 3S-HMSs, samples after cycling were examined by SEM. It turns out that the morphology and structure of the 3S-LiMn2O4-HMSs remain good after 100 cycles (Fig. S11†).
Given that the rate capability is also very important for the practical application of LIBs, the cycling performances of the 3S-LiMn2O4-HMSs at different charge/discharge rates were studied (Fig. 3c). The specific capacity decreases along with the increase of the current rate, which is common among all the electrode materials. Even so, the 3S-LiMn2O4-HMSs can still deliver good capacity at a high current density of 10 C. It should also be noted that a high and steady capacity of over 103 mA h g−1 can be attained when lowering the current rate back to 1 C, validating the indeed “breathable” structure of multi-shelled hollow microspheres for high performance LIB cathode materials.
Conclusions
In summary, uniform multi-shelled LiMn2O4 hollow microspheres with a high purity have been successfully synthesized through a hard-template method. By controlling the ratio of Li precursor and Mn precursor, the composition of the product is accurately controlled. Moreover, the structural parameters of the products such as the shell number, shell thickness and porosity are accurately controlled, by adjusting the heating rate and adsorption duration. When tested as cathode materials for LIBs, 3S-LiMn2O4-HMSs demonstrate a better cycling stability than all the reported results based on un-coated or un-doped LiMn2O4 (the fading rate is only 0.10% per cycle for 200 cycles). The superior lithium-storage is benefited from the merits of the multi-shelled hollow microstructures, including enhanced reaction activity, increased lithium-storage sites, shortened ion/electron diffusion path, and improved structural stability with multiple shells supporting each other and the exterior shell protecting the inner shells from electrochemical dissolution. Considering the facile synthesis and the improved performance, one can expect that these MS-LiMn2O4-HMSs will develop a new avenue for the next generation of LIBs with lower cost and better cycling stability.Acknowledgements
This work was supported financially by the National Natural Science Foundation of China (no. 51172235, 21203201, 51202248, 21201167, 51272165, 51372245, 51302266, 51472244 and 21401199, 51362024) and the National Science Fund for Distinguished Young Scholars (no. 21325105).References
- Y. Idota, T. Kubota, A. Matsufuji, Y. Maekawa and T. Miyasaka, Science, 1997, 276, 1395–1397 CrossRef.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J.-M. Tarascon, Nature, 2000, 407, 496–499 CrossRef PubMed.
- D. Y. Kim, P. Muralidharan, H.-W. Lee, R. Ruffo, Y. Yang, C. K. Chan, H. L. Peng, R. A. Huggins and Y. Cui, Nano Lett., 2008, 8, 3948–3952 CrossRef CAS PubMed.
- M. M. Thackeray, P. J. Johnson, L. A. Depicciotto, P. G. Bruce and J. B. Goodenough, Mater. Res. Bull., 1984, 19, 179–184 CrossRef CAS.
- M. M. Thackeray and A. J. Dekock, J. Solid State Chem., 1988, 74, 414–418 CrossRef CAS.
- M. Jayalakshmi, M. Mohan Rao and F. Scholz, Langmuir, 2003, 19, 8403–8408 CrossRef.
- J. Cabana, T. Valdés-Solís, M. R. Palacín, J. Oró-Solé, A. Fuertes, G. Marbàn and A. B. Fuertes, J. Power Sources, 2007, 166, 492–498 CrossRef CAS.
- J.-Y. Luo, Y.-G. Wang, H.-M. Xiong and Y.-Y. Xia, Chem. Mater., 2007, 19, 4791–4795 CrossRef CAS.
- F. Wu, N. Li, Y. Su, H. Shou, L. Bao, W. Yang, L. Zhang, R. An and S. Chen, Adv. Mater., 2013, 25, 3722–3726 CrossRef CAS.
- M.-J. Lee, S. Lee, P. Oh, Y. Kim and J. Cho, Nano Lett., 2014, 14, 993–999 CrossRef CAS PubMed.
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930–2946 CrossRef CAS PubMed.
- X. Zhao, M. V. Reddy, H. X. Liu, S. Ramakrishna, G. V. S. Rao and B. V. R. Chowdari, RSC Adv., 2012, 2, 7462–7469 RSC.
- X. Y. Lai, J. E. Halpert and D. Wang, Energy Environ. Sci., 2012, 5, 5604–5618 CAS.
- J. Qi, X. Y. Lai, J. Y. Wang, H. J. Tang, H. Ren, Y. Yang, Q. Jin, L. J. Zhang, R. B. Yu, G. H. Ma, Z. G. Su, H. J. Zhao and D. Wang, Chem. Soc. Rev., 2015, 44, 6749–6773 RSC.
- X. W. Lou, L. A. Archer and Z. C. Yang, Adv. Mater., 2008, 20, 3987–4019 CrossRef CAS.
- M. Hu, Y. Tian, J. P. Wei, D. G. Wang and Z. Zhou, J. Power Sources, 2014, 247, 794–798 CrossRef CAS.
- Y. R. Zhong, M. Yang, X. L. Zhou and Z. Zhou, Mater. Horiz., 2015, 2, 553–566 RSC.
- L. W. Su, Y. Jing and Z. Zhou, Nanoscale, 2011, 3, 3967–3983 RSC.
- F. Zou, X. L. Hu, Z. Li, L. Qie, C. C. Hu, R. Zeng, Y. Jiang and Y. H. Huang, Adv. Mater., 2014, 26, 6622–6628 CrossRef CAS PubMed.
- X. N. Fu, Z. R. Chang, K. Chang, B. Li, H. W. Tang, E. Shangguan, X.-Z. Yuan and H. J. Wang, Electrochim. Acta, 2015, 178, 420–428 CrossRef CAS.
- X.-F. Yang, J.-H. Yang, K. Zaghib, M. L. Trudeau and J. Y. Ying, Nano Energy, 2015, 12, 305–313 CrossRef CAS.
- W. Xiang, E.-H. Wang, M.-Z. Chen, H.-H. Shen, S.-L. Chou, H. Chen, X.-D. Guo, B.-H. Zhong and X. L. Wang, Electrochim. Acta, 2015, 178, 353–360 CrossRef CAS.
- Z. X. Chen, L. F. Cao, L. Chen, H. H. Zhou, C. M. Zheng, K. Xie and Y. F. Kuang, J. Power Sources, 2015, 298, 355–362 CrossRef CAS.
- X. Y. Lai, J. Li, B. A. Korgel, Z. H. Dong, Z. M. Li, F. B. Su, J. Du and D. Wang, Angew. Chem., Int. Ed., 2011, 50, 2738–2741 CrossRef CAS PubMed.
- Z. H. Dong, X. Y. Lai, J. E. Halphert, N. L. Yang, L. X. Yi, J. Zhai, D. Wang, Z. Y. Tang and L. Jiang, Adv. Mater., 2012, 24, 1046–1049 CrossRef CAS PubMed.
- J. Y. Wang, N. L. Yang, H. J. Tang, Z. H. Dong, Q. Jin, M. Yang, D. Kisailus, H. J. Zhao, Z. Y. Tang and D. Wang, Angew. Chem., Int. Ed., 2013, 52, 6417–6420 CrossRef CAS PubMed.
- S. M. Xu, C. M. Hessel, H. Ren, R. B. Yu, Q. Jin, M. Yang, H. J. Zhao and D. Wang, Energy Environ. Sci., 2014, 7, 632–637 CAS.
- H. Ren, R. B. Yu, J. Y. Wang, Q. Jin, M. Yang, D. Mao, D. Kisailus, H. J. Zhao and D. Wang, Nano Lett., 2014, 14, 6679–6684 CrossRef CAS PubMed.
- J. Y. Wang, H. J. Tang, H. Ren, R. B. Yu, J. Qi, D. Mao, H. J. Zhao and D. Wang, Adv. Sci., 2014, 1, 1400011 Search PubMed.
- J. Tarascon, W. McKinnon, F. Coowar, T. Bowmer, G. Amatucci and D. Guyomard, J. Electrochem. Soc., 1994, 141, 1421–1431 CrossRef CAS.
- W. Sun, F. Cao, Y. Liu, X. Zhao, X. Liu and J. Yuan, J. Mater. Chem., 2012, 22, 20952–20957 RSC.
- S. Karaal, H. Kose, A. O. Aydin and H. Akbulut, Mater. Sci. Semicond. Process., 2015, 38, 397–403 CrossRef CAS.
- X. He, J. Wang, H. P. Jia, R. Kloepsch, H. D. Liu, K. Beltrop and J. Li, J. Power Sources, 2015, 293, 306–311 CrossRef CAS.
- Y.-M. Lin, P. R. Abel, A. Heller and C. B. Mullins, J. Phys. Chem. Lett., 2011, 2, 2885–2891 CrossRef CAS.
- I. M. Hung, Y.-C. Yang, H.-J. Su and J. Zhang, Ceram. Int., 2015, 41, S779–S786 CrossRef CAS.
- Y. H. Wang, L. Chen, Y. G. Wang and Y. Y. Xia, Electrochim. Acta, 2015, 173, 178–183 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5qi00213c |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2016 |