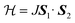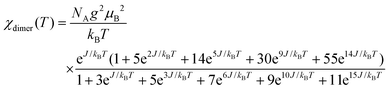Ionothermal synthesis and magnetic study of a new manganese(II) phosphite with an unprecedented Mn/P ratio†
Tan
Su
ab,
Hongzhu
Xing
c,
Yi
Li
 *a,
Junbiao
Wu
ad,
Xiaowei
Song
a,
Takehito
Nakano
*e and
Jihong
Yu
a
*a,
Junbiao
Wu
ad,
Xiaowei
Song
a,
Takehito
Nakano
*e and
Jihong
Yu
a
aState Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P. R. China. E-mail: yili@jlu.edu.cn
bInstitute of Theoretical Chemistry, Jilin University, Changchun 130021, P. R. China
cCollege of Chemistry, Northeast Normal University, Changchun 130024, P. R. China
dDepartment of Chemistry, College of Science, Northeastern University, Shenyang, China
eDepartment of Physics, Graduate School of Science, Osaka University, Toyonaka, Osaka 560-0043, Japan. E-mail: nakano@nano.phys.sci.osaka-u.ac.jp
First published on 12th April 2016
Abstract
A new layered manganese(II) phosphite, |NH4|4[Mn4(PO3H)6] (JIS-10), has been synthesised via the ionothermal method by using 1-pentyl-3-methylimidazolium bromide ([Pmim]Br) as the solvent. JIS-10 is the first manganese phosphite with a Mn/P ratio of 2/3, whose layered framework is built by the alternate connection of Mn2O9 dimers and PO3H pseudo-tetrahedra. JIS-10 represents an antiferromagnetic dimer system with S = 5/2 for each Mn2+ ion.
Inorganic oxides such as zeolites are of significant interest in a number of scientific and industrial fields,1,2 particularly in those associated with catalysis and gas storage.3–6 Conventional routes for the preparation of inorganic oxide materials are hydrothermal and solvothermal syntheses, where the precursor solution is either aqueous or not, respectively. In 2004, Morris and co-workers developed the ionothermal route for the synthesis of inorganic oxide materials, such as aluminophosphate zeolites.7 Ionothermal synthesis employs ionic liquids or eutectic mixtures as the solvent in the reaction, where the cations of the ionic liquid, in many cases, serve as the structure directing agent for the resultant compounds with two-dimensional layered or three-dimensional open frameworks.8–14 The ionothermal synthesis possesses several advantages over the conventional hydro- and solvothermal routes for the preparation of inorganic oxide materials. First, the very large polarity of an ionic liquid provides a distinct ionic atmosphere, which can easily dissolve the inorganic raw materials and is crucial for the resultant structures. Second, the negligible vapour pressure makes the synthesis much safer. More importantly, a tremendous number of ionic liquids can be used as solvents for ionothermal synthesis, providing distinct chemical environment that results in a wide variety of resultant materials. Since the discovery of an ionothermal route, great efforts have been made to prepare new inorganic oxide compounds, such as aluminophosphates, borophosphates, and phosphites, which exhibit unique structural characteristics and interesting properties.15–20 These successes prove that ionothermal synthesis is an efficient route to the discovery of novel inorganic materials.
To date, a number of manganese phosphites have been synthesised hydrothermally or solvothermally. However, the ionothermal synthesis of manganese phosphite has never been investigated. As listed in Table S1,† the reported manganese phosphites with diverse Mn/P ratios exhibit a rich variety of structures. The differences between these structures originate from the different coordination states of Mn and P atoms. So far, the reported Mn/P ratios include 1/1, 1/2, 1/4, 1/6, 2/5, 2/7, 3/4, 5/2 and 11/8 (ESI Table S1†).21–35 Considering the large variety of combinations of Mn- and P-centred polyhedra, one may expect that more manganese phosphites with different Mn/P ratios could be found in the future. Here, we report the synthesis of a new manganese phosphite (NH4)4[Mn4(HPO3)6] (JIS-10) by using 1-pentyl-3-methylimidazolium bromide ([Pmim]Br) as the ionic liquid solvent. Interestingly, JIS-10 possesses a Mn/P ratio of 2/3 that has never been observed among all the known manganese phosphites.
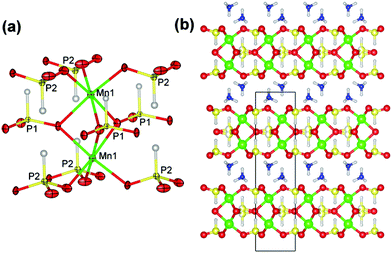
Pink hexagonal-prism-shaped crystals of JIS-10 were obtained from a reaction mixture of MnCl·4H2O (0.07 g, 0.35 mmol), H3PO3 (0.30 g, 3.66 mmol), NH4F (0.15 g, 4.05 mmol), and the ionic liquid of [Pmim]Br (1 mL, 5.09 mmol) heated at 423 K for 7 days with a yield of 70% (calculated on Mn). JIS-10 (|NH4|4[Mn4(PO3H)6]) crystallises in the hexagonal space group P63/mmc with a = 5.4649(1) Å and c = 19.0227(8) Å. It consists of one crystallographically distinct Mn and two crystallographically distinct P atoms (Fig. 1a). Each Mn atom is octahedrally coordinated to three μ2-O and three μ3-O atoms, forming Mn–O–P and Mn–O–Mn linkages, respectively. The Mn–O bond lengths vary in the range of 2.148(4)–2.265(4) Å (ESI Table S2†), which is in agreement with those observed in other manganese phosphites.21,33 Bond valence sum calculation shows that the Mn atom has an oxidation state of +2. Two adjacent MnO6 octahedra are connected via three μ3-O atoms to form a Mn2O9 dimer with a Mn⋯Mn distance of 3.057(3) Å (Fig. 1a). Both the two distinct P atoms have the oxidation state of +3, forming PO3H pseudo-tetrahedra with three bridging O atoms and one terminal H atom. One of the P atoms, P1, is linked to three adjacent Mn2O9 dimers by sharing three μ3-O atoms. Note that P1 is disordered over two crystallographically equivalent sites. The other P atom, P2, is linked to three adjacent Mn2O9 dimers by sharing three μ2-O atoms. The P–O bond lengths in JIS-10 are in the range of 1.511(4)–1.516(6) Å (ESI Table S2†), which is in agreement with those observed in other metal phosphites.27,31 The existence of the P–H with bond lengths of 1.33(5) Å (for P1) and 1.34(5) (for P2) is confirmed by the characteristic absorption bands of phosphite groups [ν(H–P) = 2453 cm−1] in the IR spectrum (ESI Fig. S1†).21 Thus, each Mn2O9 dimer is connected with nine PO3H pseudo-tetrahedra, and each PO3H pseudo-tetrahedron is connected with three Mn2O9 dimers. The strictly alternate linkage of Mn2O9 dimers and PO3H pseudo-tetrahedra leads to a two-dimensional layered framework structure with a Mn/P ratio of 2/3, which has never been observed among the known manganese phosphites.
These manganese phosphite layers are stacked together along the [001] direction (Fig. 1b). The interstitial spaces are occupied by ammonium cations, which not only balance the negative charges in manganese phosphite layers but also donate multiple H-bonds to adjacent layers. The N⋯O distances of these H-bonds vary in the range of 3.014(4) Å and 3.110(8) Å, respectively (ESI Table S3†). Due to these strong interactions, the interstitial ammonium cations could not be completely removed by calcination before the collapse of the layered structure. In situ temperature-dependent powder X-ray data show that the crystalline structure of JIS-10 is stable up to 483 K (ESI Fig. S2†). The thermogravimetric curve shown in ESI Fig. S3† exhibits a total weight loss of 9.3% from 440 K to 720 K (theoretical weight loss: 9.2%). An ion exchange experiment was carried out in a sealed autoclave heated at 353 K for 25 min by using a microwave. The results indicate that about 10% of the ammonium cations could be exchanged by potassium cations.
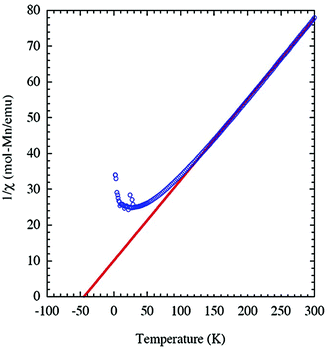
Because of the existence of Mn2O9 dimers in the structure of JIS-10, we wonder whether there will be magnetic interactions within the dimers. The magnetisation of JIS-10 was first measured at 5 T under a direct current (DC) magnetic field. As shown in ESI Fig. S4,† the χmT vs. T curve increases steadily from 2 K to 300 K and reaches a maximum of 3.76 cm3 K mol−1 at room temperature, indicating the antiferromagnetic interaction between Mn atoms in the structure. As shown in Fig. 2, the 1/χmvs. T curve shows a linear dependence of the Curie–Weiss formula when the temperature is higher than 30 K, leading to a Curie constant (C) of 4.435 K emu mol−1 for Mn2+ ions, which is very close to that estimated from isolated Mn2+ ions (C = 4.377 K emu mol−1, g = 2.0, S = 5/2). The negative Weiss temperature of −44.9 K further suggests antiferromagnetic interactions. According to the crystal structure, the Mn2+ ions are dimerised along the crystallographic c-axis in the structure. Therefore a spin-dimer model with S = 5/2 for each Mn2+ ion was built up to elucidate the antiferromagnetic interactions in JIS-10. The Heisenberg Hamiltonian of this dimer model can be expressed as.
where NA is the Avogadro number and kB is Boltzmann constant. As shown in Fig. 3, the model is quite reasonable for the antiferromagnetic interaction of JIS-10 at a higher temperature above 30 K, in which it fits very well with the experimental data; whereas it deviates obviously from the experiment data at a lower temperature. We speculate that this is due to the introduction of a very tiny amount of unbound spins and paramagnetic components obeying the Curie law. In this context, the measured magnetisation was re-elucidated by contributions from both parts of the dimers and paramagnetic components. As shown also in Fig. 3, the experimental data and the calculated one fit perfectly when the Curie constant belonging to the paramagnetic component is calculated to be 0.0594 ± 0.0005 K emu mol−1. The result indicates that the contribution of magnetisation from paramagnetic impurities is very small, i.e. less than 1%. The calculated antiferromagnetic exchange coupling constant (J/kB) is 12.20 ± 0.02 K. Consequently, JIS-10 represents an antiferromagnetic dimer system with S = 5/2 for each Mn2+ ion.36,37
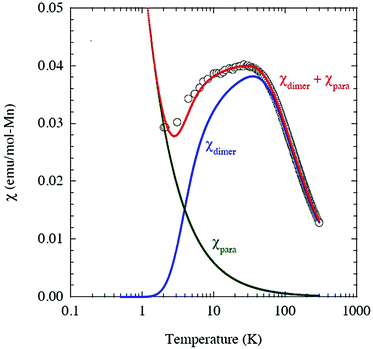 | ||
| Fig. 3 Magnetisation of JIS-10 and the corresponding spin-dimer calculation. χdimer and χpara magnetisations are from spin-dimer and paramagnetic components, respectively. | ||
Conclusions
A new layered manganese(II) phosphite, JIS-10, has been prepared in the ionic liquid of 1-pentyl-3-methylimidazolium bromide ([Pmim]Br). The as-prepared compound possesses a new Mn/P ratio of 2/3, which has not been observed earlier in the family of manganese phosphites. The structure of JIS-10 features Mn2O9 dimers, in which antiferromagnetic interactions have been revealed by magnetisation measurements. The calculation based on a spin-dimer model also reveals that JIS-10 represents an antiferromagnetic dimer system with S = 5/2 for each Mn2+ ion. The successful synthesis of JIS-10 demonstrates that more inorganic materials with new structures and properties could be prepared ionothermally.Acknowledgements
We thank the National Natural Science Foundation of China (Grant No: 21273098, 21473024, and 21501062), the Program for New Century Excellent Talents in University (NCET-13-0246), and the Research Project of the Science and Technology of Jilin Province, China (Grant no: 201413001) for financial support.Notes and references
- M. E. Davis, Nature, 2002, 417, 813 CrossRef CAS PubMed.
- Y. Li and J. Yu, Chem. Rev., 2014, 114, 7168 Search PubMed.
- A. Corma, M. J. Diaz-Cabanas, J. Martinez-Triguero, F. Rey and J. Rius, Nature, 2002, 418, 514 CrossRef CAS PubMed.
- A. Corma, S. Valencia, J. L. Jorda, F. Rey and J. Rius, Nat. Mater., 2003, 2, 493 CrossRef CAS PubMed.
- A. Zecchina, S. Bordiga, J. G. Vitillo, G. Ricchiardi, C. Lamberti, G. Spoto, M. Bjorgen and K. P. Lillerud, J. Am. Chem. Soc., 2005, 127, 6361 CrossRef CAS PubMed.
- P. S. Wheatley, A. R. Butler, M. S. Crane, S. Fox, B. Xiao, A. G. Rossi, I. L. Megson and R. E. Morris, J. Am. Chem. Soc., 2006, 128, 502 CrossRef CAS PubMed.
- E. R. Cooper, C. D. Andrews, P. S. Wheatley, P. B. Webb, P. Wormald and R. E. Morris, Nature, 2004, 430, 1012 CrossRef CAS PubMed.
- E. R. Parnham, P. S. Wheatley and R. E. Morris, Chem. Commun., 2006, 4, 380 RSC.
- E. R. Parnham and R. E. Morris, J. Am. Chem. Soc., 2006, 128, 2204 CrossRef CAS PubMed.
- E. R. Parnham, E. A. Drylie, P. S. Wheatley, A. M. Z. Slawin and R. E. Morris, Angew. Chem., Int. Ed., 2006, 118, 4962 CrossRef PubMed.
- Y. Xu, Z. Tian, S. Wang, Y. Hu, L. Wang, B. Wang, Y. Ma, L. Hou, J. Yu and L. Lin, Angew. Chem., Int. Ed., 2006, 45, 3965 CrossRef CAS PubMed.
- Z. Lin, D. S. Wragg and R. E. Morris, Chem. Commun., 2006, 19, 2021 RSC.
- J. H. Liao, P. C. Wu and Y. H. Bai, Inorg. Chem. Commun., 2005, 8, 390 CrossRef CAS.
- C. Y. Sheu, S. F. Lee and K. H. Lii, Inorg. Chem., 2006, 45, 1891 CrossRef CAS PubMed.
- E. R. Parnham and R. E. Morris, Chem. Mater., 2006, 18(20), 4883 CrossRef.
- E. A. Drylie, D. S. Wragg, E. R. Parnham, P. S. Wheatley, A. M. Z. Slawin, J. E. Warren and R. E. Morris, Angew. Chem., Int. Ed., 2007, 47, 442 Search PubMed.
- R. E. Morris, Angew. Chem., Int. Ed., 2008, 48, 442 CrossRef PubMed.
- H. Xing, J. Li, W. Yan, P. Chen, Z. Jin, J. Yu, S. Dai and R. Xu, Chem. Mater., 2008, 20, 4179 CrossRef CAS.
- Z. Ma, J. H. Yu and S. Da, Adv. Mater., 2010, 22, 261 CrossRef CAS PubMed.
- H. Xing, W. Yang, T. Su, Y. Li, J. Xu, T. Nakano, J. Yu and R. Xu, Angew. Chem., Int. Ed., 2010, 49, 2328 CrossRef CAS PubMed.
- S. Fernandez, J. L. Mesa, J. L. Pizarro, R. Olazcuaga and T. Rojo, Chem. Mater., 2000, 12, 2092 CrossRef CAS.
- S. Fernandez, J. L. Mesa, J. L. Mesa, L. Lezama, M. I. Arriortua, R. Olazcuaga and T. Rojo, Inorg. Chem., 2001, 40, 3476 CrossRef CAS PubMed.
- M. Ebert and J. Eysseltova, Monatsh. Chem., 1974, 105, 1030 CrossRef CAS.
- M. P. Aitfield, R. E. Morris and A. K. Cheetham, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1994, 50, 981 CrossRef.
- U. C. Chunga, J. L. Mesab, J. L. Pizarroa, V. Juberac, L. Lezamab, M. I. Arriortuaa and T. Rojo, J. Solid State Chem., 2005, 178, 2913 CrossRef.
- G. Chen, Y. Su, J. Yu, G. Yang, Y. Zhao and Z. Fu, Z. Anorg. Allg. Chem., 2009, 635, 1463 CrossRef CAS.
- Z. Lin, H. P. Nayek and S. Dehnen, Microporous Mesoporous Mater., 2009, 126, 95 CrossRef CAS.
- F. Hamchaoui, V. Alonzo, T. Roisnel, H. Rebbaha and E. L. Fur, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2009, 65, i33 CAS.
- P. Ramaswamy, S. Mandal and S. Natarajan, J. Solid State Chem., 2009, 182, 2491 CrossRef CAS.
- L. N. Jin, J. M. Hong and Y. H. Ni, Mater. Chem. Phys., 2010, 123, 337 CrossRef CAS.
- L. Li, T. Su, J. Li, J. Xu, J. Yu and R. Xu, Inorg. Chim. Acta, 2011, 370, 65 CrossRef CAS.
- M. Yang, P. Yan, F. Xu, J. Ma and U. W. Biermann, Microporous Mesoporous Mater., 2012, 147, 73 CrossRef.
- L. Liu, D. Luo, D. Lia and Z. Lin, Dalton Trans., 2014, 43, 7695 RSC.
- Y. He, Y. Yan, F. Yang, J. Wu and X. Song, Chem. J Chin. Univ., 2014, 35(9), 1859 CAS.
- L. Liu, W. Zhang, Z. Shi, Y. Chen and Z. Lin, Solid State Sci., 2014, 38, 13 CrossRef CAS.
- A. A. Tsirlin, R. Nath, C. Geibel and H. Rosner, Phys. Rev. B: Condens. Matter, 2008, 77, 104436 CrossRef.
- Z. He, M. H. Wangbo, Y. Ueda, Y. Narumi, K. Kindo, T. Taniyama, M. Itoh and W. Cheng, Chem. – Asian J., 2009, 4, 1530 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterisation details, IR spectrum, thermogravimetric curve, χ–T curve, temperature-dependent XRD, and selected bond distances and angles. CCDC 1441924. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qi00014b |
| This journal is © the Partner Organisations 2016 |