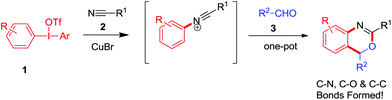
Synthesis of benzo[1,3]oxazines via copper(I)-catalyzed cascade annulation of nitriles, aldehydes and diaryliodonium salts†
Jinyu
Sheng
,
Xiang
Su
,
Chengyao
Cao
and
Chao
Chen
*
Key Laboratory of Bioorganic Phosphorus Chemistry & Chemical Biology (Ministry of Education), Department of Chemistry, Tsinghua University, Beijing 100084, China. E-mail: chenchao01@mails.tsinghua.edu.cn; Tel: +86-10-62773684
First published on 17th February 2016
Abstract
A copper(I)-catalyzed one-pot [2 + 2 + 2] cascade annulation reaction of diaryliodoniums, nitriles, and aldehydes has been developed for the efficient synthesis of 2,4-substituted benzoxazine derivatives.
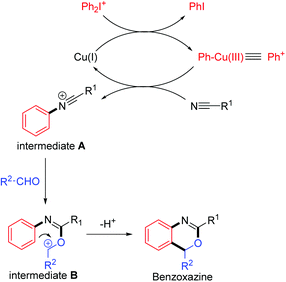
Benzoxazine I, a heterocycle containing two heteroatoms (N,O), is an important backbone in a large variety of derivatives used as antifungal, antiobiotic, and anti-inflammatory agents, as well as C 1r serine protease inhibitors, anticonvulsants, and DNA-binding antitumor agents.1 Furthermore, 2,4-substituted benzoxazine skeletons II show great significance in many pharmaceutical molecules and biologically active natural products (Fig. 1).2 For example, etifoxine, a non-benzodiazepine anticonvulsant drug, is used for the treatment of psychiatric illnesses with great therapeutic efficiency and less toxicity. Therefore, how to synthesize the benzoxazines and their analogues efficiently received large attention and interest among organic chemists. As a result, new elegant methodologies to prepare 2,4-substituted benzoxazines have been developed rapidly in the recent decades.3 However, most of these reactions are intramolecular cyclization initiated by radicals or cations with vinyl amide (or alkynyl amide) and their derivatives as starting materials.4 Herein, we would like to report an intermolecular annulation reaction to synthetize benzo[d][1,3]oxazine derivatives by using readily available substrates including diaryliodonium salts, nitriles and aldehydes. The reaction proceeded smoothly with copper catalysts through the formation of the C–N bond, C–O bond and C–C bond in cascade annulation reactions (Scheme 1).
C–N bond and C–O bond formation reactions are developed fleetly and maturely with transition metal catalysts in the past few decades.5 Moreover, the utilization of the C–N bond and C–O bond formation reactions to construct heterocycles is rapidly realized.6 However, to the best of our knowledge, there are few reports to build N and O contained heterocycles from two different substrates in the cascade annulation reaction. Thus, the development of simple and efficient methods to build several heteroatoms containing heterocycles by the formation of the C–N and C–O bonds is highly attractive.7 For this goal, we exploited diaryliodoniums, simple nitriles and aldehydes as reactants to build up the benzoxazine backbone.8,9
Our study was stemmed with di-p-tolyliodonium triflate (1a), benzonitrile (2a) and benzaldehyde (3a) (Table 1) in the presence of 10% Cu(OTf)2 as a catalyst in DCE (entry 2). Delightfully, we detected 6-methyl-2,4-diphenyl-4H-benzo[d][1,3]oxazine (4aaa) in 56% GC yield. The screening of copper-catalysts showed that CuBr was the most efficient for this transformation (entries 2–6). No copper catalyst or FeCl3 catalyst didn't work for this reaction. To improve the yield of 4aaa, we adjusted the temperature and found that 4aaa was obtained in 90% yield (isolated in 81%) at 120 °C.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Cat. [10%] | Temp. [°C] | Yieldb [%] |
|---|---|---|---|
| a Unless otherwise noted, reactions were performed with 1a (0.3 mmol), 2a (0.3 mmol), 3a (0.3 mmol), and catalyst (10 mol%), in the solvent DCE (1.5 ml) at the preceding temperature. b GC-MS yield using dodecane as an internal standard. c Isolated yield. | |||
| 1 | No | 100 | 6 |
| 2 | Cu(OTf)2 | 100 | 56 |
| 3 | CuBr | 100 | 69 |
| 4 | CuI | 100 | 60 |
| 5 | CuCl | 100 | 67 |
| 6 | CuCl2 | 100 | 51 |
| 7 | FeCl3 | 100 | 0 |
| 8 | CuBr | 100 | 87 |
| 9 | CuBr | 80 | 57 |
| 10 | CuBr | 60 | 42 |
| 11 | CuBr | 120 | 90 (81 ) |
| 12 | CuBr | 130 | 87 |
| 13 | CuBr | 140 | 86 |
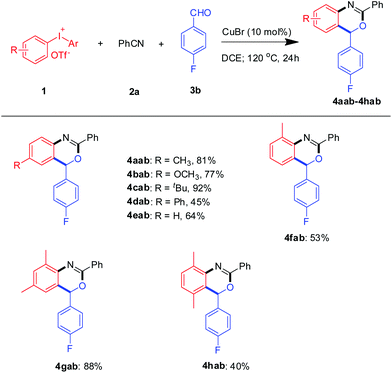
Under the optimal conditions, a range of functionalized diaryliodoniums were examined in this reaction (Scheme 2). To our delight, diaryliodoniums with a range of substituents involving 2- and 4-methyl, 2,4- and 2,5-dimethyl, 4-methoxyl, 4-t-butyl, 4-phenyl and benzo groups all worked well with benzonitrile and 4-fluorobenzaldehyde. Disappointingly, diaryliodoniums with electron-withdrawing, such as 4-CF3 and 4-Cl, did not undergo the benzoxazine annulation. When the unsymmetrical diaryliodonium salt 4-methyl-4′-methoxycarbonyl diaryliodonium was used, only 4aab was obtained in 54% GC yield.10 The above results show that the electronic effect strongly affected this reaction.
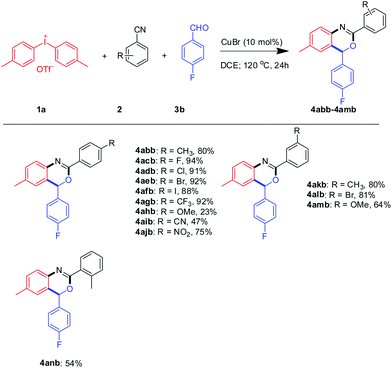
Next, a series of aromatic nitriles, including 4-cyano, 4-nitro, and 4-iodo were examined and in all cases 2,4-substituted benzoxazine was obtained in good yields except for anisonitrile isolated in 23% yields (Scheme 3). The structure of 4aeb was further confirmed by XRD of its single crystal (Fig. 2). Strangely, the use of aliphatic nitriles like valeronitrile only supplied trace amounts of the product, accompanied by quinazoline as the side-product.9c
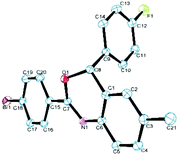 | ||
| Fig. 2 X-ray structure of 4aeb.11 | ||
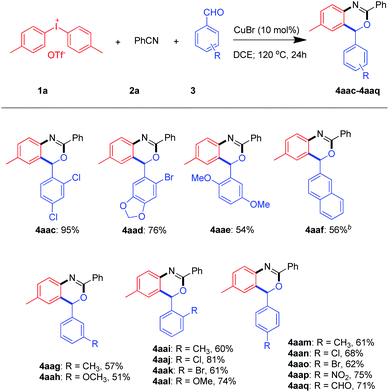
To further broaden the scope of this transformation, a series of aldehydes were examined (Scheme 4). Encouragingly, aromatic aldehydes with a large variety of substituents including the electron-withdrawing and electron-donating groups such as 2-napth, 4-methoxyl and 4-nitro were fit for this reaction. It is worth mentioning that p-phthalaldehyde underwent cyclization once to give the product 4aaq in 71%.
Besides, multiple substituted benzaldehydes like 2-bromo-4,5-methylenedioxybenzaldehyde, 2,4-dichloro-benzaldehyde and 2,5-dimethoxybenzaldehyde proceeded well to give the product in 76%, 95% and 54% yields.
Based on the previous reports, a preliminary mechanism suggested that an Ar–Cu(III) species is involved as shown in Scheme 5.12 Firstly, oxidative addition to CuBr by the diaryliodonium salt (exemplified as Ph2I+) gives a Ph–Cu(III) species, which transferred the phenyl group to the nitrile to give the N-phenylnitrilium intermediate A.9a,c Then N-phenylnitrilium species A is quickly attacked by the benzaldehyde to give the intermediate B, which undergoes an electrophilic substitution on the aryl ring to give the benzoxazine product.
Conclusions
In summary, an one-pot approach to multiply substituted benzoxazines with diaryliodonium salts 1, simple nitriles 2 and aldehydes 3 has been presented. This reaction provided efficient and practical access to diversely functionalized benzoxazine derivatives which could realize the flexible control of the substituents on benzoxazines. The facile construction of the benzoxazine skeleton and simple manipulation might be useful for the design and generation of a benzoxazines’ library.Notes and references
- (a) H. B. Bode, H. Irschik, S. C. Wenzel, H. Reichenbach, R. Muller and G. Hofle, J. Nat. Prod., 2003, 66, 1203 CrossRef CAS PubMed; (b) T. Kline, N. H. Andersen, E. A. Harwood, J. Bowman, A. Malanda, S. Endsley, A. L. Erwin, M. Doyle, S. Fong, A. L. Harris, B. Mendelsohn, K. Mdluli, C. R. H. Raetz, C. K. Stover, P. R. Witte, A. Yabannavar and S. Zhu, J. Med. Chem., 2002, 45, 3112 CrossRef CAS PubMed; (c) M. C. Pirrung, L. N. Tumey, A. L. McClerren and C. R. H. Raetz, J. Am. Chem. Soc., 2003, 125, 1575 CrossRef CAS PubMed; (d) N. Dias, J. F. Goossens, B. Baldeyrou, A. Lansiaux, P. Colson, A. DiSalvo, J. Bernal, A. Turnbull, D. J. Mincher and C. Bailly, Bioconjugate Chem., 2005, 16, 949 CrossRef CAS PubMed; (e) H. Sugiyama, K. Hosoda, Y. Kumagai, M. Takeuchi and M. Okada, US Pat, 4,596,801, 1986 Search PubMed; (f) J. W. Kobzina, US Pat, 4,030,906, 1977 Search PubMed; (g) A. Krantz, R. W. Spencer, T. F. Tam, T. J. Liak, L. L. Copp, E. M. Thomas and S. P. Rafferty, J. Med. Chem., 1990, 33, 464 CrossRef CAS PubMed; (h) S. J. Hays, B. W. Caprathe, J. L. Gilmore, N. Amin, M. R. Emmerling, W. Michael, R. Nadimpalli, R. Nath, K. J. Raser, D. Stafford, D. Watson, K. Wang and J. C. Jaen, J. Med. Chem., 1998, 41, 1060 CrossRef CAS PubMed.
- (a) B. V. S. Reddy, R. A. Babu, M. Ramana Reddy, B. J. M. Reddy and B. Sridhar, RSC Adv., 2014, 4, 44629 RSC; (b) W.-C. Lee, H.-C. Shen, W.-P. Hu, W.-S. Lo, C. Murali, J. K. Vandavasi and J.-J. Wang, Adv. Synth. Catal., 2012, 354, 2218 CrossRef CAS.
- (a) P. M. Fresneda, J. A. Bleda, M. A. Sanz and P. Molina, Synlett, 2007, 1541 CrossRef CAS; (b) K. Kobayashi, Y. Okamura and H. Konishi, Synthesis, 2009, 1494 CrossRef CAS; (c) T. Xiong, Y. Li, X. Bi, Y. Lv and Q. Zhang, Angew. Chem., Int. Ed., 2011, 50, 7140 CrossRef CAS PubMed; (d) Y. Li, Z. Li, T. Xiong, Q. Zhang and X. Zhang, Org. Lett., 2012, 14, 3522 CrossRef CAS PubMed.
- (a) M. Costa, N. Della Ca, B. Gabriele, C. Massera, G. Salerno and M. Soliani, J. Org. Chem., 2004, 69, 2469 CrossRef CAS PubMed; (b) T. Saito, S. Ogawa, N. Takei, N. Katsumura and T. Otani, Org. Lett., 2011, 13, 1098 CrossRef CAS PubMed; (c) W.-C. Lee, H.-C. Shen, W.-P. Hu, W.-S. Lo, C. Murali, J. K. Vandavasi and J.-J. Wang, Adv. Synth. Catal., 2012, 354, 2218 CrossRef CAS; (d) Á. Sinai, Á. Mészáros, T. Gáti, V. Kudar, A. Palló and Z. Novák, Org. Lett., 2013, 15, 5654 CrossRef PubMed; (e) A. L. Stein, F. N. Bilheri, D. F. Back and G. Zeni, Adv. Synth. Catal., 2014, 356, 501 CrossRef CAS; (f) H. Yang, X.-H. Duan, J.-F. Zhao and L.-N. Guo, Org. Lett., 2015, 17, 1998 CrossRef CAS PubMed; (g) Q.-H. Deng, J.-R. Chen, Q. Wei, Q.-Q. Zhao, L.-Q. Lu and W.-J. Xiao, Chem. Commun., 2015, 51, 3537 RSC; (h) J.-F. Zhao, X.-H. Duan, H. Yang and L.-N. Guo, J. Org. Chem., 2015, 80, 11149 CrossRef CAS PubMed; (i) Z.-J. Cai, C. Yang, S.-Y. Wang and S.-J. Ji, Chem. Commun., 2015, 51, 14267 RSC; (j) S. Jana, A. Ashokan, S. Kumar, A. Verma and S. Kumar, Org. Biomol. Chem., 2015, 13, 8411 RSC.
- (a) W.-M. Shi, X.-P. Ma, C.-X. Pan, G.-F. Su and D.-L. Mo, J. Org. Chem., 2015, 80, 11175 CrossRef CAS PubMed; (b) G. Jin, C.-G. Werncke, Y. Escudié, S. S. Etienne and S. Bontemps, J. Am. Chem. Soc., 2015, 137, 9563 CrossRef CAS PubMed; (c) S. A. Morris, T. H. Nguyen and N. Zheng, Adv. Synth. Catal., 2015, 357, 2311 CrossRef CAS PubMed; (d) R. Vanjari, T. Guntreddi and K. N. Singh, Org. Lett., 2013, 15, 4908 CrossRef CAS PubMed.
- (a) M. Zheng, L. Huang, H. Huang, X. Li, W. Wu and H. Jiang, Org. Lett., 2014, 16, 5906 CrossRef CAS PubMed; (b) B. C. Raju, K. V. Prasad, G. Saidachary and B. Sridhar, Org. Lett., 2014, 16, 420 CrossRef PubMed; (c) H. Cao, X. Liu, L. Zhao, J. Cen, J. Lin, Q. Zhu and M. Fu, Org. Lett., 2014, 16, 146 CrossRef CAS PubMed; (d) W. Yuan and K. J. Szabó, Angew. Chem., Int. Ed., 2015, 54, 8533 CrossRef CAS PubMed.
- (a) E. Cahard, N. Bremeyer and M. J. Gaunt, Angew. Chem., Int. Ed., 2013, 52, 9284 CrossRef CAS PubMed; (b) E. Cahard, H. P. J. Male, M. Tissot and M. J. Gaunt, J. Am. Chem. Soc., 2015, 137, 7986 CrossRef CAS PubMed; (c) C. J. Calleja, D. Pla, T. W. Gorman, V. Domingo and B. H. M. J. Gaunt, Nat. Chem., 2015, 7, 1009 CrossRef PubMed.
- For reviews, see: (a) M. S. Yusubov, A. V. Maskaev and V. V. Zhdankin, ARKIVOC, 2011, 370 CAS; (b) E. A. Merritt and B. Olofsson, Angew. Chem., Int. Ed., 2009, 48, 9052 CrossRef CAS PubMed; (c) V. V. Zhdankin and P. J. Stang, Chem. Rev., 2008, 108, 5299 CrossRef CAS PubMed; (d) V. V. Zhdankin and P. J. Stang, Chem. Rev., 2002, 102, 2523 CrossRef CAS PubMed; (e) V. V. Grushin, Chem. Soc. Rev., 2000, 29, 315 RSC; (f) P. J. Stang and V. V. Zhdankin, Chem. Rev., 1996, 96, 1123 CrossRef CAS PubMed.
- For selected examples, see: (a) Y. Wang, C. Chen, J. Peng and M. Li, Angew. Chem., Int. Ed., 2013, 52, 5323 CrossRef CAS PubMed; (b) J. Peng, C. Chen, Y. Wang, Z.-B. Lou, M. Li, C. Xi and H. Chen, Angew. Chem., Int. Ed., 2013, 52, 7574 CrossRef CAS PubMed; (c) X. Su, C. Chen, Y. Wang, J. Peng, Z.-B. Lou and M. Li, Chem. Commun., 2013, 49, 6752 RSC; (d) Y. Wang, X. Su and C. Chen, Synlett, 2013, 2619 CAS; (e) J. Chen, C. Chen, J. Chen, G. Wang and H. Qu, Chem. Commun., 2015, 51, 1356 RSC; (f) J. Peng, C. Chen and C. Xi, Chem. Sci., 2016, 7, 1383–1387 RSC; (g) Y. Wang, C. Chen, S. Zhang, Z. Lou, X. Su, L. Wen and M. Li, Org. Lett., 2013, 15, 4794 CrossRef CAS PubMed; (h) J. Chen, C. Chen, J. Chen, H. Gao and H. Qu, Synlett, 2014, 2721 CrossRef CAS; (i) X. Pang, Z. Lou, M. Li, L. Wen and C. Chen, Eur. J. Org. Chem., 2015, 3361 CrossRef CAS; (j) X. Pang, C. Chen, M. Li and C. Xi, Beilstein J. Org. Chem., 2015, 11, 2365 CrossRef PubMed.
- (a) W. Guo, S. Li, L. Tang, M. Li, L. Wen and C. Chen, Org. Lett., 2015, 17, 1232 CrossRef CAS PubMed; (b) X. Pang, C. Chen, X. Su, M. Li and L. Wen, Org. Lett., 2014, 16, 6228 CrossRef CAS PubMed; (c) J. Xu, P. Zhang, Y. Gao, Y. Chen, G. Tang and Y. Zhao, J. Org. Chem., 2013, 78, 8176 CrossRef CAS PubMed.
- CCDC no. 1420993 (4aeb).
- (a) M. G. Suero, E. D. Bayle, B. S. L. Collins and M. J. Gaunt, J. Am. Chem. Soc., 2013, 135, 5332 CrossRef CAS PubMed; (b) R. J. Phipps, L. McMurray, S. Ritter, H. A. Duong and M. J. Gaunt, J. Am. Chem. Soc., 2012, 134, 10773 CrossRef CAS PubMed; (c) B. Chen, X.-L. Hou, Y.-X. Li and Y.-D. Wu, J. Am. Chem. Soc., 2011, 133, 7668 CrossRef CAS PubMed; (d) B. Xiao, Y. Fu, J. Xu, T.-J. Gong, J.-J. Dai, J. Yi and L. Liu, J. Am. Chem. Soc., 2010, 132, 468 CrossRef CAS PubMed; (e) J. Phipps and M. J. Gaunt, Science, 2009, 323, 1593 CrossRef PubMed; (f) R. J. Phipps, N. P. Grimster and M. J. Gaunt, J. Am. Chem. Soc., 2008, 130, 8172 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1420993. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00012f |
| This journal is © the Partner Organisations 2016 |