A copper-catalyzed cascade reaction of o-bromoarylisothiocyanates with isocyanides leading to benzo[d]imidazo[5,1-b]thiazoles under ligand-free conditions†
Kelu
Yan
,
Daoshan
Yang
*,
Wei
Wei
,
Pengfei
Sun
,
Yunxiang
Lu
and
Hua
Wang
*
The Key Laboratory of Life-Organic Analysis and Key Laboratory of Pharmaceutical Intermediates and Analysis of Natural Medicine, School of Chemistry and Chemical Engineering, Qufu Normal University, Qufu 273165, Shandong, P. R. China. E-mail: yangdaoshan@tsinghua.org.cn; huawang_qfnu@126.com
First published on 29th February 2016
Abstract
A convenient and efficient copper-catalyzed domino method has been initially developed for the synthesis of benzo[d]imidazo[5,1-b]thiazole derivatives via the reactions of readily available substituted o-bromoarylisothiocyanates with isocyanides under ligand-free conditions. This chemistry involves intermolecular [3 + 2] cycloaddition and intramolecular Ullmann-type C–S bond formation.
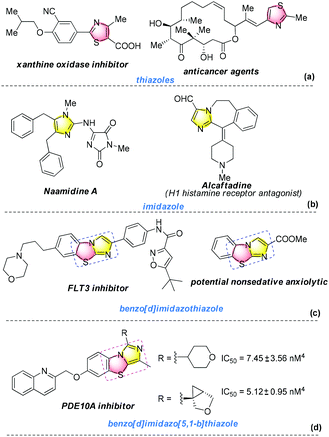
Seeking mild and efficient methods for the C–S bond formation is of fundamental and immense importance in organic chemistry because sulfur-containing frameworks exhibit important functions in organic transformations, and they are also widely used in pharmaceutical, agrochemical, and materials chemistry.1 Thiazoles are an important class of sulfur-containing heterocycles possessing various excellent biological and medicinal activities. For example, they can be used as xanthine oxidase inhibitors,2 antibiotics,3 and anticancer agents4 (Scheme 1a). On the other hand, imidazole is a key core scaffold that also occurs in natural products, drugs, and advanced materials5 (Scheme 1b). The combined structure of thiazole and imidazole frameworks, the benzo[d]imidazothiazole ring system (Scheme 1c), has attracted much attention for its application in the FLT3 inhibitor (phase II clinical trials)6 and potential nonsedative anxiolytics,7 so that some efficient methods for its synthesis can be developed.8 Moreover, the isomer of benzo[d]imidazo[5,1-b]thiazole (Scheme 1d) has recently been found as a core scaffold in phosphodiesterase 10A (PDE10A) inhibitors.9 Surprisingly, few synthetic approaches for its formation have been reported.9,10 In 2013, Gharat et al. reported a four-step method for the construction of benzo[d]imidazo[5,1-b]thiazole skeleton using C2-aminoalkylated benzothiazoles as key intermediates.9 Very recently, Zhu and co-workers developed an efficient copper-promoted cycloaddition of benzothiazoles with isocyanides leading to benzo[d]imidazo[5,1-b]thiazoles at room temperature.10 However, several challenges remain in terms of starting materials and synthesis conditions. It is still highly desirable to develop new strategies to prepare functionalized benzo[d]imidazo[5,1-b]thiazole frameworks by utilizing inexpensive substrates and proceed under mild conditions.
Transition-metal-catalyzed cross-coupling reactions are useful tools in synthetic organic chemistry, since they provide a convenient, and straightforward approach to valuable molecules from readily accessible starting materials under mild conditions.11 In the past few years, with the renaissance of the Ullmann-type reactions, the copper-catalyzed cross-coupling reactions have been demonstrated to be versatile methods for the construction of C(sp2)–X (X = N, O, C, S, P) bonds.12 Although the chemistry of copper-catalyzed C–N, C–O and C–C bond formations has been well explored, methods available for the C–S bond coupling are rather limited due to the deactivation of the metal catalysts by the strong coordinating properties of sulfur.13 As a consequence, there is continued interest to develop new synthetic methodologies for constructing sulfur-containing compounds via the copper-catalyzed Ullmann-type C–S bond formation.
Moreover, isocyanides are easily prepared from readily available chemical materials, which have been widely used as powerful and versatile C1 building blocks possessing nucleophilicity, electrophilicity, and isocyanide insertion properties.14 In recent years, using isocyanides as the starting materials to construct N-heterocycles have caught considerable attention.15 As a part of our continuous efforts for the synthesis of sulfur-containing organic compounds,16 herein we wish to report a ligand-free inexpensive copper-catalyzed approach for the synthesis of benzo[d]imidazo[5,1-b]thiazoles which could possibly possess some important biological activities.
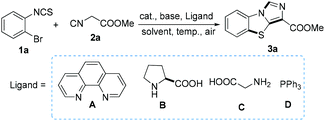
First, 1-bromo-2-isothiocyanatobenzene (1a) and methyl 2-isocyanoacetate (2a) were selected as the model substrates to optimize the reaction conditions including the catalysts, bases, ligands, reaction temperatures and solvents in an air atmosphere. As shown in Table 1, eight catalysts such as CuCl, CuBr, CuI, CuCl2, CuSO4, Cu(OAc)2, FeCl3, and FeCl2 were investigated at 60 °C by using 2.0 equiv. of K3PO4 as the base in 2 mL DMSO, and CuCl2 provided methyl benzo[d]imidazo[5,1-b]thiazole-3-carboxylate (3a) in 80% yield (Table 1, entries 1–8). Notably, the reaction did not proceed without the catalyst (Table 1, entry 9). Furthermore, we compared different bases (Table 1, compare entries 4, 10, 11, 12, and 13). It was found that K3PO4 was superior to the others (entry 1), where no target product 3a was obtained in the absence of a base (entry 14). Moreover, we screened various solvents including DMSO, DMF, DCE, CH3CN, EtOH, 1,4-dioxane, THF, and H2O, and CH3CN was found to be the best choice (Table 1, entry 4 versus entries 15–21). In addition, various temperatures were investigated (Table 1, entries 17, 22–24), and 60 °C was discovered to be more suitable for this transformation (Table 1, entry 17). Of note, elevating the reaction temperature did not enhance the yield (Table 1, entry 22). Furthermore, the effect of ligands was also investigated (entries 24–28), and no obvious increase of the yield was observed. After the optimization process for catalysts, bases, ligands, temperatures, and solvents, various benzo[d]imidazo[5,1-b]thiazole derivatives were synthesized under our standard conditions: 10 mol% CuCl2 as the catalyst, 2 equiv. of K3PO4 as the base (relative to o-bromoarylisothiocyanates), and CH3CN as the solvent, at 60 °C in an air atmosphere.
| Entry | Cat. | Base | Solvent | Yieldb [%] |
|---|---|---|---|---|
| a Reaction conditions: 1-bromo-2-isothiocyanatobenzene (1a) (0.3 mmol), methyl 2-isocyanoacetate (2a) (0.6 mmol), catalyst (0.03 mmol), base (0.6 mmol), solvent (2 mL), and reaction time (18 h). b Isolated yield. c 70 °C. d 50 °C. e 25 °C. f Using A as the ligand. g Using B as the ligand. h Using C as the ligand. i Using D as the ligand. | ||||
| 1 | CuCl | K3PO4 | DMSO | 75 |
| 2 | CuBr | K3PO4 | DMSO | 71 |
| 3 | CuI | K3PO4 | DMSO | 73 |
| 4 | CuCl2 | K3PO4 | DMSO | 80 |
| 5 | CuSO4 | K3PO4 | DMSO | Trace |
| 6 | Cu(OAc)2 | K3PO4 | DMSO | 71 |
| 7 | FeCl3 | K3PO4 | DMSO | Trace |
| 8 | FeCl2 | K3PO4 | DMSO | Trace |
| 9 | None | K3PO4 | DMSO | 0 |
| 10 | CuCl2 | K2CO3 | DMSO | 76 |
| 11 | CuCl2 | Cs2CO3 | DMSO | 77 |
| 12 | CuCl2 | Na2CO3 | DMSO | 71 |
| 13 | CuCl2 | NaHCO3 | DMSO | 69 |
| 14 | CuCl2 | None | DMSO | 0 |
| 15 | CuCl2 | K3PO4 | DMF | 28 |
| 16 | CuCl2 | K3PO4 | DCE | Trace |
| 17 | CuCl 2 | K 3 PO 4 | CH 3 CN | 84 |
| 18 | CuCl2 | K3PO4 | EtOH | Trace |
| 19 | CuCl2 | K3PO4 | 1,4-Dioxane | 79 |
| 20 | CuCl2 | K3PO4 | THF | 73 |
| 21 | CuCl2 | K3PO4 | H2O | 68 |
| 22 | CuCl2 | K3PO4 | CH3CN | 83c |
| 23 | CuCl2 | K3PO4 | CH3CN | 72d |
| 24 | CuCl2 | K3PO4 | CH3CN | 39e |
| 25 | CuCl2 | K3PO4 | CH3CN | 85f |
| 26 | CuCl2 | K3PO4 | CH3CN | 84g |
| 27 | CuCl2 | K3PO4 | CH3CN | 83h |
| 28 | CuCl2 | K3PO4 | CH3CN | 80i |
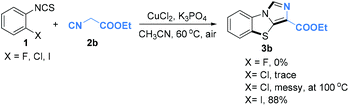
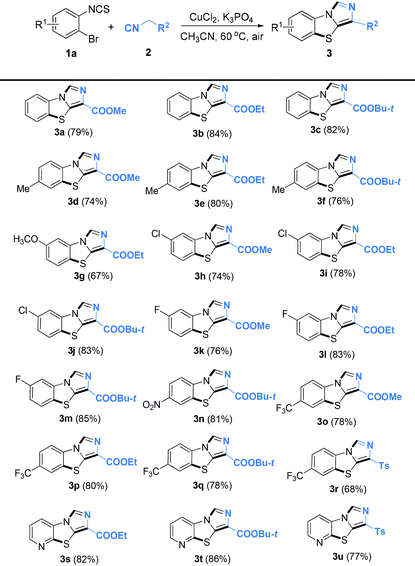
Under the optimized conditions, we next explored the substrate scope with different o-bromoarylisothiocyanates and isocyanides. The results are summarized in Table 2. We were pleased to find that various o-bromoarylisothiocyanate derivatives could be transferred into the benzo[d]imidazo[5,1-b]thiazoles in good to excellent yields. Electron-donating groups such as methyl, methoxy and electron-withdrawing groups such as fluoro-, chloro-, nitro, and trifluoromethyl groups were well-tolerated under the copper-catalyzed reaction conditions. To our delight, 2-bromo-3-isothiocyanatopyridine was also compatible under the standard conditions and the desired products were generated with good yield (3s–3u). Upon changing R2 to tosyl (Ts), the reaction also occurred to give 3r and 3u in yields of 68% and 77%, respectively. The copper-catalyzed cascade reactions could tolerate some functional groups such as C–F bonds, C–Cl bonds, C–Br bonds, nitro, ester, and alkyl groups, which could be used for further transformations. We also attempted cascade reactions of different o-haloarylisothiocyanates with ethyl 2-isocyanoacetate 2b to synthesize 3b under our standard catalytic conditions. Among the substituted o-haloarylisothiocyanates, o-iodoisothiocyanate showed higher reactivity than o-bromoarylisothiocyanate. However, o-fluoroarylisothiocyanate and o-chloroarylisothiocyanate are poor substrates, no desired products were obtained under the standard conditions, even though the reaction temperature was elevated to 100 °C (Scheme 2). Although o-bromoarylisothiocyanates gave a slightly lower yield than o-iodoarylisothiocyanates, they are cheaper and of practical application.
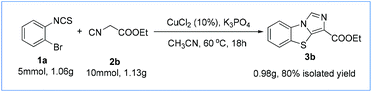
Further, we explored the synthetic applicability of the present method. The gram-scale reaction was performed between 1a and 2b, and the reaction afforded 3b in 80% yield (Scheme 3). Therefore, this simple protocol could serve as an efficient and practical method for the synthesis of various benzo[d]imidazo[5,1-b]thiazole derivatives containing ester groups which could be easily transformed to other useful groups.
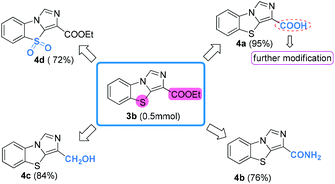
To further demonstrate the utility of the present method in synthesizing various benzo[d]imidazo[5,1-b]thiazole derivatives, the transformations of the ethyl benzo[d]imidazo[5,1-b]thiazole-3-carboxylate 3b obtained above were then investigated. To our satisfaction, benzo[d]imidazo[5,1-b]thiazole-3-carboxylic acid 4a was efficiently obtained by the hydrolysis of 3b under mild basic conditions, which could be used for further modifications (Scheme 4). Furthermore, ammonolysis and reduction of 3b led to amide 4b and alcohol 4c in 76% and 84% yield, respectively. Additionally, the sulfonyl product 4d was directly synthesized in good yield from 3b through oxidative reaction with m-chloroperbenzoic acid (m-CPBA). These representative transformations clearly demonstrate the versatilities of benzo[d]imidazo[5,1-b]thiazole-3-carboxylates in organic chemistry.
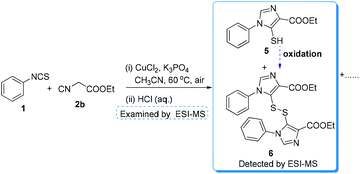
Upon treatment of isothiocyanatobenzene 1 with ethyl 2-isocyanoacetate 2b under the standard conditions, the reaction detected by TLC was messy and no 3b was detected. After acidifying the reaction mixture and examining it by ESI-MS, the intermediates 5 and 6 were found (see Fig. 1 in the ESI†). As shown in Scheme 5, the disulfide 6 might come from 5 under oxidative conditions.17 These preliminary results indicated that B (see Scheme 6) might be the important intermediate in the present transformations.
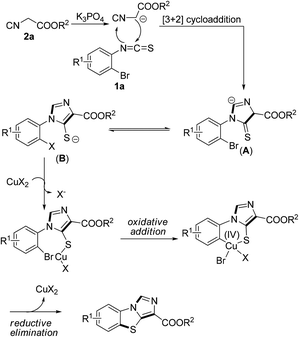
Although many Cu(II)-catalyzed Ullmann-type reactions have been reported so far, the mechanism of these couplings remains rare. In 2009, Reddy and co-workers developed a nanocrystalline CuO-catalyzed coupling of aryl halides with diphenyl diselenide to form diaryl selenide, in which the proposed reaction mechanism was suggested to start from CuIIO and involve Cu(IV) intermediates.18 In 2013, Zeng's group reported a Cu(OAc)2-catalyzed C–S Ullmann cross coupling reaction of thiols with aryl halides. In this work, the proposed reaction mechanism also involves Cu(IV) intermediates.19 Although the mechanism for the present transformation is not yet clear, on the basis of these preliminary results mentioned above together with the previous related literature,17,20 a proposal mechanism would be herein presented (Scheme 6). Initially, the [3 + 2] cycloaddition of isocyanides 2 to o-bromoarylisothiocyanates 1a produces the intermediate A in the presence of a base, which undergoes isomerization to give intermediate B. Subsequently, the intermediate B might proceed in the Ullmann-type pathway leading to the desired products 3. Nevertheless, further investigations on more detailed mechanisms are still ongoing in our laboratory.
In conclusion, we have developed a general and highly efficient method for the synthesis of benzo[d]imidazo[5,1-b]thiazole derivatives via copper-catalyzed cascade couplings of o-haloarylisothiocyanates with isocyanides under mild conditions. The present method shows simple, economical and practical advantages over the previous methods, holding great potential for wide applications in the synthesis of diverse benzo[d]imidazo[5,1-b]thiazoles with sulfur-containing frameworks in organic chemistry and medicinal chemistry.
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (Nos. 21302110, 21302109 and 21375075), the Scientific Research Foundation of Qufu Normal University (BSQD 2012021), the Taishan Scholar Foundation of Shandong Province, the Natural Science Foundation of Shandong Province (ZR2013BQ017), and the Project of Shandong Province Higher Educational Science and Technology Program (J13LD14). We thank Jin Li in this group for reproducing the results of 3c and 3t.
Notes and references
- For selected reviews, see: (a) C. Shen, P. Zhang, Q. Sun, S. Bai, T. S. Andy Hor and X. Liu, Chem. Soc. Rev., 2015, 44, 291 RSC; (b) H. Liu and X. Jiang, Chem. – Asian J., 2013, 8, 2546 CrossRef CAS PubMed; (c) K. Sałat, A. Moniczewski and T. Librowski, Mini-Rev. Med. Chem., 2013, 13, 335 Search PubMed; (d) A. Mishra, C.-Q. Ma and P. Bäuerle, Chem. Rev., 2009, 109, 1141 CrossRef CAS PubMed; (e) J. Zaumseil and H. Sirringhaus, Chem. Rev., 2007, 107, 1296 CrossRef CAS PubMed; (f) M. D. McReynolds, J. M. Dougherty and P. R. Hanson, Chem. Rev., 2004, 104, 2239 CrossRef CAS PubMed; (g) D. J. Ager, Chem. Soc. Rev., 1982, 11, 493 RSC.
- (a) R. A. Hughes and C. J. Moody, Angew. Chem., Int. Ed., 2007, 46, 7996 CrossRef PubMed; (b) Y. Takanoa, K. Hase-Aoki, H. Horiuchi, L. Zhao, Y. Kasahara, S. Kondo and M. A. Becker, Life Sci., 2005, 76, 1835 CrossRef PubMed.
- (a) V. S. Aulakh and M. A. Ciufolini, J. Org. Chem., 2009, 74, 5750 CrossRef CAS PubMed; (b) G. D. Fate, C. P. Benner, S. H. Gride and T. J. Gilbertson, J. Am. Chem. Soc., 1996, 118, 11363 CrossRef CAS.
- (a) Y. Matsuya, T. Kawaguchi, K. Ishihara, K. Ahmed, Q.-L. Zhao, T. Kondo and H. Nemoto, Org. Lett., 2006, 8, 4609 CrossRef CAS PubMed; (b) C. N. Boddy, K. Hotta, M. L. Tse, R. E. Watts and C. Khosla, J. Am. Chem. Soc., 2004, 126, 7436 CrossRef CAS PubMed; (c) S. C. Mutka, J. R. Carney, Y. Liu and J. Kennedy, Biochemistry, 2006, 45, 1321 CrossRef CAS PubMed.
- (a) Z. Jin, Nat. Prod. Rep., 2009, 26, 382 RSC; (b) G. J. Atwell, J.-Y. Fan, K. Tan and W. A. Denny, J. Med. Chem., 1998, 41, 4744 CrossRef CAS PubMed; (c) J. Dietrich, V. Gokhale, X. Wang, L. H. Hurley and G. A. Flynn, Bioorg. Med. Chem., 2010, 18, 292 CrossRef CAS PubMed; (d) T. Yamamoto, T. Uemura, A. Tanimoto and S. Sasaki, Macromolecules, 2003, 36, 1047 CrossRef CAS.
- Q. Chao, K. G. Sprankle, K. M. Grotzfeld, A. G. Lai, T. A. Carter, A. M. Velasco, R. N. Gunawardane, M. D. Cramer, M. F. Gardner, J. James, P. P. Zarrinkar, H. K. Patel and S. S. Bhagwat, J. Med. Chem., 2009, 52, 7808 CrossRef CAS PubMed.
- S. Clements-Jewery, G. Danswan, C. R. Gardner, S. S. Matharu, R. Murdoch, W. R. Tully and R. Westwood, J. Med. Chem., 1988, 31, 122 CrossRef.
- (a) S. Mishra, K. Monir, S. Mitra and A. Hajra, Org. Lett., 2014, 16, 6084 CrossRef CAS PubMed; (b) Z. Wu, Q. Huang, X. Zhou, L. Yu, Z. Li and D. Wu, Eur. J. Org. Chem., 2011, 5242 CrossRef CAS; (c) S. K. Guchhait and V. Chaudhary, Org. Biomol. Chem., 2014, 12, 6694 RSC; (d) M. Bakherad, H. Nasr-Isfahani, A. Keivanloo and G. Sang, Tetrahedron Lett., 2008, 49, 6188 CrossRef CAS.
- A. Banerjee, L. Narayana, F. A. Raje, D. V. Pisal, P. A. Kadam, S. Gullapalli, H. Kumar, S. V. More, M. Bajpai, R. R. Sangana, S. Jadhav, G. S. Gudi, N. Khairatkar-Joshi, R. R. T. Merugu, S. R. Voleti and L. A. Gharat, Bioorg. Med. Chem. Lett., 2013, 23, 6747 CrossRef CAS PubMed.
- J. Wang, J. Li and Q. Zhu, Org. Lett., 2015, 17, 5336 CrossRef CAS PubMed.
- I. Akamura and Y. Yamamoto, Chem. Rev., 2004, 104, 2127 CrossRef PubMed.
- For recent reviews on copper-catalyzed cross couplings, see: (a) D. Ma and Q. Cai, Acc. Chem. Res., 2008, 41, 1450 CrossRef CAS PubMed; (b) F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 6954 CrossRef CAS PubMed; (c) K. Kunz, U. Scholz and D. Ganzer, Synlett, 2003, 16, 2428 CrossRef; (d) G. Evano, N. Blanchard and M. Toumi, Chem. Rev., 2008, 108, 3054–3131 CrossRef CAS PubMed; (e) H. Rao and H. Fu, Synlett, 2011, 22, 745 Search PubMed; (f) Q. Liao, X. Yang and C. Xi, J. Org. Chem., 2014, 79, 8507 CrossRef CAS PubMed.
- (a) C. S. Bryan, J. A. Braunger and M. Lautens, Angew. Chem., Int. Ed., 2009, 48, 7064 CrossRef CAS PubMed; (b) E. Alvaro and J. F. Hartwig, J. Am. Chem. Soc., 2009, 131, 7858 CrossRef CAS PubMed; (c) T. Kondo and T.-A. Mitsudo, Chem. Rev., 2000, 100, 3205 CrossRef CAS PubMed; A. T. Hutton, in Comprehensive Coordination Chemistry, ed. G. Wilkinson, R. D. Gillard and J. A. McCleverty, Pergamon, Oxford, 1984, Vol. 5, p. 1151 Search PubMed; (d) L. L. Hegedus and R. W. McCabe, Catalyst Poisoning, Marcel Dekker, New York, 1984 Search PubMed.
- (a) A. Dömling, Chem. Rev., 2006, 106, 17 CrossRef PubMed; (b) G. Qiu, Q. Ding and J. Wu, Chem. Soc. Rev., 2013, 42, 5257 RSC; (c) E. Ruijter, R. Scheffelaar and R. V. A. Orru, Angew. Chem., Int. Ed., 2011, 50, 6234 CrossRef CAS PubMed.
- For selected examples, see: (a) D. Bonne, M. Dekhane and J. Zhu, Angew. Chem., Int. Ed., 2007, 46, 2485 CrossRef CAS PubMed; (b) A. Fayol and J. Zhu, Angew. Chem., Int. Ed., 2002, 41, 3633 CrossRef CAS; (c) S. Li and J. Wu, Chem. Commun., 2012, 48, 8973 RSC; (d) Y. Li, X. Xu, J. Tan, C. Xia, D. Zhang and Q. Liu, J. Am. Chem. Soc., 2011, 133, 1775 CrossRef CAS PubMed; (e) T. Buyck, Q. Wang and J. Zhu, Angew. Chem., Int. Ed., 2013, 52, 12714 CrossRef CAS PubMed; (f) W. Hao, J. Zeng and M. Cai, Chem. Commun., 2014, 50, 11686 RSC; (g) G. Qiu and J. Wu, Chem. Commun., 2012, 48, 6046 RSC; (h) O. V. Larionov and A. Meijere, Angew. Chem., Int. Ed., 2005, 44, 5664 CrossRef CAS PubMed; (i) W. Hao, J. Huang, S. Jie and M. Cai, Eur. J. Org. Chem., 2015, 6655 CrossRef CAS; (j) J. Peng, J. Zhao, Z. Hu, D. Liang, J. Huang and Q. Zhu, Org. Lett., 2012, 14, 4966 CrossRef CAS PubMed; (k) P. Biossarie, Z. Hamilton, S. Lang, J. Murphy and C. Suckling, Org. Lett., 2011, 13, 6256 CrossRef PubMed.
- (a) D. Yang, K. Yan, W. Wei, J. Zhao, M. Zhang, X. Sheng, G. Li, S. Lu and H. Wang, J. Org. Chem., 2015, 80, 6083 CrossRef CAS PubMed; (b) D. Yang, K. Yan, W. Wei, G. Li, S. Lu, C. Zhao, L. Tian and H. Wang, J. Org. Chem., 2015, 80, 11073 CrossRef CAS PubMed; (c) K. Yan, D. Yang, W. Wei, J. Zhao, Y. Shuai, L. Tian and H. Wang, Org. Biomol. Chem., 2015, 13, 7323 RSC; (d) D. Yang, K. Yan, W. Wei, L. Tian, Q. Li, J. You and H. Wang, RSC Adv., 2014, 4, 48547 RSC; (e) W. Wei, J. Wen, D. Yang, J. Du, J. You and H. Wang, Green Chem., 2014, 16, 2988 RSC; (f) W. Wei, J. Li, D. Yang, J. Wen, Y. Jiao, J. You and H. Wang, Org. Biomol. Chem., 2014, 12, 1861 RSC; (g) K. Yan, D. Yang, P. Sun, W. Wei, Y. Liu, G. Li, S. Lu and H. Wang, Tetrahedron Lett., 2015, 56, 4792 CrossRef CAS.
- (a) A. R. Hajipour, S. E. Mallakpour and H. Adibi, J. Org. Chem., 2002, 67, 8666 CrossRef CAS PubMed; (b) J. P. Tam, C. R. Wu, W. Liu and J. W. Zhang, J. Am. Chem. Soc., 1991, 113, 6657 CrossRef CAS; (c) Y. Liu, H. Wang, C. Wang, J.-P. Wan and C. Wen, RSC Adv., 2013, 3, 21369 RSC.
- V. P. Reddy, A. V. Kumar, K. Swapna and K. R. Rao, Org. Lett., 2009, 11, 951 CrossRef CAS PubMed.
- C. Zong, J. Liu, S. Chen, R. Zeng and J. Zou, Chin. J. Chem., 2014, 32, 212 CrossRef CAS.
- (a) A. V. Lygin, O. V. Larionov, V. S. Korotkov and A. de Meijere, Chem. – Eur. J., 2009, 15, 227 CrossRef CAS PubMed; (b) S. Li and J. Wu, Chem. Commun., 2012, 48, 8973 RSC; (c) C. Kanazawa, S. Kamijo and Y. Yamamoto, J. Am. Chem. Soc., 2006, 128, 10662 CrossRef CAS PubMed; (d) A. V. Gulevich, A. G. Zhdanko, R. V. A. Orru and V. G. Nenajdenko, Chem. Rev., 2010, 110, 5235 CrossRef CAS PubMed; (e) Q. Cai, F. Zhou, T. Xu, L. Fu and K. Ding, Org. Lett., 2011, 13, 340 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6qo00030d |
| This journal is © the Partner Organisations 2016 |