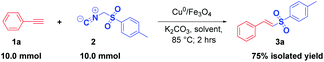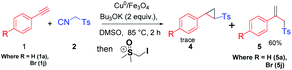Magnetically separable nano-copper catalyzed unprecedented stereoselective synthesis of E-vinyl sulfones from tosylmethyl isocyanide and alkynes: TosMIC as a source of the sulfonyl group†
Mandalaparthi
Phanindrudu
a,
Dipak Kumar
Tiwari
a,
B.
Sridhar
b,
Pravin R.
Likhar
a and
Dharmendra Kumar
Tiwari
*a
aInorganic and Physical Chemistry Division, CSIR-Indian Institute of Chemical Technology, Uppal road, Tarnaka, Hyderabad 500007, India. E-mail: dktiwari.iict@gov.in; dkt80.org@gmail.com; Fax: +91-040-27160921
bX-Ray crystallography Center, CSIR-Indian Institute of Chemical Technology, Uppal road, Tarnaka, Hyderabad, 500007, India
First published on 30th March 2016
Abstract
We have developed an unprecedented efficient and mild catalytic route for stereoselective synthesis of E-vinyl sulfones from terminal alkynes and tosylmethyl isocyanide (TosMIC) in the presence of magnetically separable nano-copper (0) stabilized on Fe3O4. A variety of vinyl sulfones were obtained in moderate to good yields. In this newly developed protocol TosMIC acts as a sulfonyl source. The catalyst was magnetically removed and recycled easily five times without any appreciable loss in activity.
Vinyl sulfones are important key structural units present in a plethora of fine chemicals and pharmaceuticals.1 In addition they are widely used as building blocks for the construction of complex molecular architectures.2 Vinyl sulfones are well recognised as Michael acceptors and utilized in various reactions such as nucleophilic conjugate additions and cycloadditions,3 cyclopropanations,4 epoxidations5 and aza Michael addition.6 Furthermore, they are known to possess various biological inhibitory activities such as cysteine proteases,7 inducible VCAM-1 expression,8 dipeptidyl peptidase I (DPPI),9 transpeptidase10 and neuroprotective agents toward Parkinson's disease therapy.11
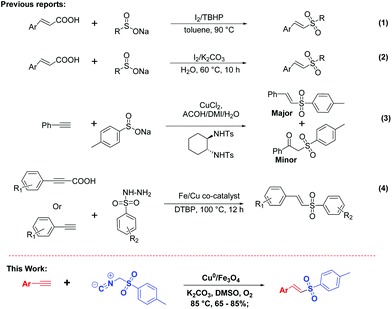
Owing to their importance and utilities, substantial attention has been paid to develop mild and efficient methods for vinyl sulfones.12 Amongst them, a few important methodologies that were recently developed are illustrated in Fig. 1. Shi and co-workers reported the iodine mediated decarboxylative coupling reaction of cinnamic acid and sodium sulfinate in the presence of TBHP as an oxidant (Fig. 1, eqn (1)).13 Furthermore, the Yuan group pursued a green and efficient method by carrying out a similar reaction in aqueous medium (Fig. 1, eqn –(2)).14 In another report, N. Taniguchi developed a copper-catalyzed oxidative hydrosulfonylation of alkynes with sodium sulfinates (Fig. 1, eqn (3)).15 Very recently, Zhang's group described Cu/Fe catalyzed sulfonylation of propiolic acid/phenylacetylene with sulfonyl hydrazides in the presence of di-tert-butyl peroxide (DTBP) (Fig. 1, eqn (4)).16 While all these methods provide access to vinyl sulfones, there are certain limitations associated with them such as the use of external oxidants, expensive ligands, harsh reaction conditions, prolonged reaction time, tedious workup procedures, selectivity and recovery of catalysts. Therefore, the development of a mild and efficient approach to E-vinyl sulfones is still highly desired.
Tosylmethyl isocyanide (TosMIC) has long been renowned for its reactivity in numerous synthetic transformations17,18 including [3 + 2] cycloaddition reaction with substrates containing C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C
N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and C
C, and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C groups, offering access to a large number of chemicals such as oxazoles, pyrroles and imidazoles. As a part of our continuing interest towards developing various biologically important heterocyclic frameworks,19,20 herein we report a novel and efficient stereoselective synthesis of E-vinyl sulfones using TosMIC, alkynes and magnetically recoverable and reusable Cu0/Fe3O4. Notably in this newly developed protocol, TosMIC acts as a source of the sulfonyl group instead of acting as a typical 1,3 dipole. Furthermore, the magnetic separation of catalysts from reaction medium makes this strategy more convenient and practical to access vinyl sulfones.
C groups, offering access to a large number of chemicals such as oxazoles, pyrroles and imidazoles. As a part of our continuing interest towards developing various biologically important heterocyclic frameworks,19,20 herein we report a novel and efficient stereoselective synthesis of E-vinyl sulfones using TosMIC, alkynes and magnetically recoverable and reusable Cu0/Fe3O4. Notably in this newly developed protocol, TosMIC acts as a source of the sulfonyl group instead of acting as a typical 1,3 dipole. Furthermore, the magnetic separation of catalysts from reaction medium makes this strategy more convenient and practical to access vinyl sulfones.
Results and discussion
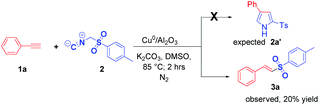
Recently, we reported the nano-copper catalyzed regio selective synthesis of 2,4 disubstituted pyrroles from terminal alkynes and isocyanides.20a When we tried to expand the substrate scope by reacting phenyl acetylene (1a) and tosylmethyl isocyanide (2) under previously optimised conditions, we unexpectedly obtained E-vinyl solfone as the sole product albeit in low yield (20%). Worth mentioning here is that the expected 2,4-disubstituted pyrrole (2a′) was never detected with TosMIC (Scheme 1). At this stage the structure of E-vinyl sulfone (3a) was well characterized using all spectroscopy techniques and a single X-ray diffraction analysis was carried out for one compound (3e, vide infra). Since this reaction represents a new route to access E-vinyl sulfones, we carried out an extensive optimization of the reaction conditions (Table 1).| Entry | Catalyst | Base | Solvent | Temp. [°C] | Yieldd [%] |
|---|---|---|---|---|---|
| Reaction conditions:a Phenyl acetylene 1a (1.0 mmol), TosMIC 2, (1.0 mmol), K2CO3 (1.0 mmol) and Cu0/Al2O3 (40 mg) at 85 °C for 2 h, under a N2 atmosphere.b Under an air atmosphere.c Under an O2 atmosphere.d Isolated yield; n.o. = not observed. | |||||
| 1 | Cu0/Al2O3 | K2CO3 | DMSO | 85 | 20a |
| 2 | Cu0/Al2O3 | K2CO3 | DMSO | 85 | 45b |
| 3 | Cu0/Al2O3 | K2CO3 | DMSO | 85 | 71c |
| 4 | Cu 0 /Fe 3 O 4 | K 2 CO 3 | DMSO | 85 | 84 |
| 5 | Cu0/Fe2O3 | K2CO3 | DMSO | 85 | 67c |
| 6 | Cu0/rGO | K2CO3 | DMSO | 85 | 55c |
| 7 | CuOTf2 | K2CO3 | DMSO | 85 | n.o.c |
| 8 | CuI | K2CO3 | DMSO | 85 | n.o.c |
| 9 | Cu0/Fe3O4 | Na2CO3 | DMSO | 85 | 79c |
| 11 | Cu0/Fe3O4 | Cs2CO3 | DMSO | 85 | 78c |
| 12 | Cu0/Fe3O4 | DBU | DMSO | 85 | 56c |
| 13 | Cu0/Fe3O4 | Et3N | DMSO | 85 | 42c |
| 14 | Cu0/Fe3O4 | t-BuOK | DMSO | 85 | 82c |
| 15 | Cu0/Fe3O4 | K2CO3 | DMF | 85 | 55c |
| 16 | Cu0/Fe3O4 | K2CO3 | DME | 85 | 61c |
| 17 | Cu0/Fe3O4 | K2CO3 | CH3CN | 85 | 41c |
| 18 | Cu0/Fe3O4 | K2CO3 | Toluene | 85 | 15c |
| 19 | Cu0/Fe3O4 | K2CO3 | EtOH | 85 | 55c |
| 20 | Cu0/Fe3O4 | K2CO3 | CHCl3 | 85 | n.o.c |
| 21 | Cu0/Fe3O4 | K2CO3 | DMSO | 100 | 75c |
| 22 | Cu0/Fe3O4 | K2CO3 | DMSO | 60 | 53c |
At this stage we presumed that under these conditions the active methylene group of TosMIC was oxidised to generate a tosyl radical which eventually attacked the terminal carbon acetylene to give an unexpected E-vinyl sulfone (for more details see Scheme 4). The reaction performed in an open air atmosphere gave a better yield (45%) of the desired sulfone 3a (Table 1, entry 2). A further increase in the yield (71%) of 3a was observed by performing the reaction under an oxygen atmosphere (Table 1, entry 3). It is important to note that, under these catalytic conditions Z-vinyl sulfone was never detected. To our delight, a further increase in the yield (84%) of desired 3a was observed when magnetite Cu0/Fe3O4 was employed as a catalyst (Table 1, entry 4) under an oxygen atmosphere. Various other supported nano-catalysts such as commercially available Cu0/Fe2O3 and Cu0/rGO were found to be less efficient, and furnished 3a in 67% and 55% yield respectively (Table 1, entries 5 and 6). During our investigations, several copper salts such as CuOTf2 and CuI were also tested but no product formation was observed (Table 1, entries 7 and 8). Next, several bases such as Na2CO3, Cs2CO3, DBU, Et3N and t-BuOK were screened for the reaction but they all furnished lower yields of the vinyl sulfone 3a (Table 1, entries 9–14). Replacing DMSO with various other solvents such as DMF, DME, CH3CN, toluene and EtOH, produced inferior yield of 3a (Table 1, entries 15–20). We found that the reaction temperature noticeably influenced the yield of the desired product 3a. At an elevated temperature (100 °C), 75% yield of the desired product was achieved (Table 1, entry 21) whereas 53% yield of 3a was observed at a lower temperature (60 °C) (Table 1, entry 22).
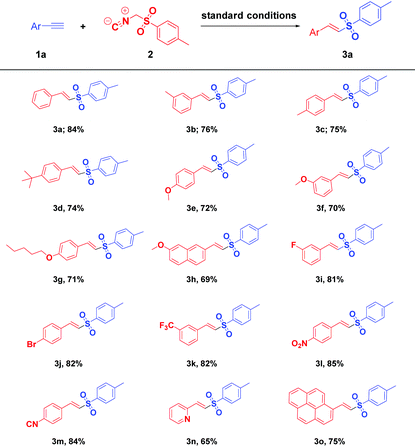
With the optimal conditions in hand (Table 1, entry 4), the substrate scope of the present study with respect to various aryl alkynes (1a–1o) was evaluated and the results are summarised in Table 2. Both electron donating (EDGs) and electron withdrawing groups (EWGs) (1b–1n) on phenyl rings were well tolerated and the corresponding desired E-vinyl sulfones (3b–3n) were obtained in moderate yields. A wide range of aryl acetylenes having tert-butyl (1d) alkoxy (1e–1h), fluoro (1i), bromo (1j), trifluoromethyl (1k), nitro (1l) and cyano (1m) groups afforded corresponding E-vinyl sulfones (3d–3m) in moderate to high yields. Moreover, bicyclic and tetracyclic aryl alkynes such as 6-methoxy-2-ethynylnaphthalene (1h) and pyrenes (1o) gave products in 69% and 75% yields respectively. Furthermore, heteroaryl alkyne211n effectively reacted under the optimised reaction conditions and furnished the corresponding vinyl sulfone 3n in 65% yield. Unambiguous proof for the stereochemical assignment of the vinyl sulfones was obtained by single crystal X-ray crystallography of 3e (Fig. 2).
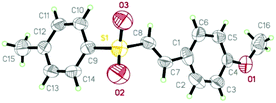 | ||
| Fig. 2 ORTEP diagram of 3e with the atom-numbering scheme. Displacement ellipsoids are drawn at the 30% probability level. CCDC No. 1053806. | ||
| Reaction conditions:a Acetylenes 1a–1o (1.0 mmol), TosMIC (1.0 mmol), K2CO3 (1.0 mmol) and Cu0/Fe3O4 (40 mg) at 85 °C, under an oxygen atmosphere, 2 h. |
|---|
|
In order to expand the synthetic competence of our strategy, a gram-scale synthesis of 3a was performed under the standard conditions. Pleasingly, E-vinyl sulfone 3a was obtained in a satisfactory yield (75%) when the reaction was conducted on a 10 mmol scale (Scheme 2). It indicates that there is a potential for industrial application too.
This initial success propelled us to investigate the application of the present method in one pot synthesis of cyclopropane. Thus, a mixture of phenyl acetylene (1.0 mmol), TosMIC (1.0 mmol) and t-BuOK was heated in DMSO (4.0 mL) at 85 °C. After 3 h of heating trimethyl sulfoxonium iodide (1.0 mmol) was slowly added to the above mixture and the reaction was stirred at the same temperature for another 10 h. Surprisingly, the expected cyclopropane 4 was not formed and the reaction gave an allylic sulfone 5 (60%). The spectral data of 5 were found in good agreement with the reported value (Scheme 3).22
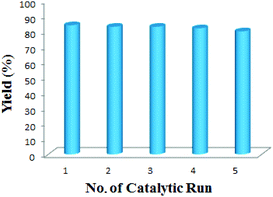
Next we have demonstrated the recovery and reusability of the catalyst. After completion of the reaction, the catalyst was separated magnetically and washed thoroughly with diethyl ether, dried at 100 °C overnight and used directly for the next round of reaction. The catalyst was reused five times without any significant loss of activity (Fig. 3).
The mechanism of nano-copper catalyzed synthesis of E-vinyl sulfone from alkynes and TosMIC can be rationalized as depicted in Scheme 4. The active methylene of ToSMIC gets oxidised to give intermediate 7. The intermediate 7 in the presence of copper gives a tosyl radical 8, which reacts with copper acetylide to form 9 which eventually takes a proton from the solvent to furnish the desired E-vinyl sulfone.
Conclusions
In summary, we have achieved a catalytic, mild and efficient method for the synthesis of E-vinyl sulfones from readily available starting materials in very good yields. Furthermore, the nano-copper catalyst was magnetically separated and reused five times without any significant loss of activity. In this newly developed protocol TosMIC acts as a source of the sulfonyl group. The ready availability of starting materials, broad substrate scope, and operational simplicity make the present method an attractive option for the synthesis of vinyl sulfones. Further study on the wider applications of the current methodology is under progress in our laboratory.Acknowledgements
The authors thank the Department of Science and Technology (DST) New Delhi, India, for financial support in the form of the INSPIRE Faculty and startup research grant for young researchers (GAP 0414 and 0512 respectively). MP thanks UGC, New Delhi for a research fellowship. The authors are grateful to the Director, CSIR-IICT for providing the necessary infrastructure.References
- (a) J. J. Reddick, J. Cheng and W. R. Roush, Org. Lett., 2003, 5, 1967 CrossRef CAS PubMed; (b) G. Wang, U. ahesh, G. Y. J. Chen and S. Q. Yao, Org. Lett., 2003, 5, 737 CrossRef CAS PubMed; (c) D. C. Meadows, T. Sanchez, N. Neamati, T. W. North and J. Gervay-Hague, Bioorg. Med. Chem., 2007, 15, 1127 CrossRef CAS PubMed; (d) M. Uttamchandani, K. Liu, R. C. Panicker and S. Q. Yao, Chem. Commun., 2007, 1518 RSC.
- (a) Q. Zhu and Y. Lu, Org. Lett., 2009, 11, 1721 CrossRef CAS PubMed; (b) O. Arjona, R. Menchaca and J. Plumet, Org. Lett., 2001, 3, 107 CrossRef CAS PubMed; (c) H. Kumamoto, K. Deguchi, T. Wagata, Y. Furuya, Y. Odanaka, Y. Kitade and H. Tanaka, Tetrahedron, 2009, 65, 8007 CrossRef CAS; (d) D. J. Wardrop and J. Fritz, Org. Lett., 2006, 8, 3659 CrossRef CAS PubMed; (e) M. N. Noshi, A. Elawa, E. Torres and P. L. Fuchs, J. Am. Chem. Soc., 2007, 129, 11242 CrossRef CAS PubMed; (f) A. López-Pérez, R. Robles-Machín, J. Adrio and J. C. Carretero, Angew. Chem., Int. Ed., 2007, 46, 9261 CrossRef PubMed.
- (a) P. Mauleon, I. Alonso, R. M. Rodriguez and J. C. Carretero, J. Org. Chem., 2007, 72, 9924 CrossRef CAS PubMed; (b) A. R. Usera and G. H. Posner, J. Org. Chem., 2007, 72, 2329 CrossRef CAS PubMed; (c) P. Mauleon and J. C. Carretero, Chem. Commun., 2005, 4961 RSC; (d) P. Mauleon and J. C. Carretero, Org. Lett., 2004, 6, 3195 CrossRef CAS PubMed; (e) V. Padmavathi, G. D. Reddy and G. S. Reddy, J. Heterocycl. Chem., 2008, 45, 1633 CrossRef CAS.
- (a) M. G. Edwards, R. J. Paxton, D. S. Pugh, A. C. Whitwood and R. J. K. Taylor, Synthesis, 2008, 3279 CAS; (b) W. Cao, H. Zhang, J. Chen, X. Zhou, M. Shao and M. C. McMills, Tetrahedron, 2007, 64, 163 CrossRef.
- (a) R. F. De la Pradilla, A. Castellanos, J. Fernandez, M. Lorenzo, P. Manzano, P. Mendez, J. Priego and A. Viso, J. Org. Chem., 2006, 71, 1569 CrossRef CAS PubMed; (b) J. M. Lopez-Pedrosa, M. R. Pitts, S. M. Roberts, S. Saminathan and J. Whittall, Tetrahedron Lett., 2004, 45, 5073 CrossRef CAS.
- C. Calata, E. Pfund and T. Lequeux, J. Org. Chem., 2009, 74, 9399 CrossRef CAS PubMed.
- (a) J. T. Palmer, D. Rasnick, J. L. Klaus and D. Brömme, J. Med. Chem., 1995, 38, 3193 CrossRef CAS PubMed; (b) M. M. M. Santos and R. Moreira, Mini-Rev. Med. Chem., 2007, 7, 1040 CrossRef CAS PubMed.
- L. Ni, X. S. Zheng, P. K. Somers, L. K. Hoong, R. R. Hill, E. M. Marino, K. L. Suen, U. Saxena and C. Q. Meng, Bioorg. Med. Chem. Lett., 2003, 13, 745 CrossRef CAS PubMed.
- (a) G. E. Korver, C. M. Kam, J. C. Powers and D. Hudig, Int. J. Immunopharmacol., 2001, 1, 21 CrossRef CAS; (b) C. M. Kam, M. G. Götz, G. Koot, M. McGuire, D. Thiele, D. Hudig and J. C. Powers, Arch. Biochem. Biophys., 2004, 427, 123 CrossRef CAS PubMed.
- B. A. Frankel, M. Bentley, R. G. Kruger and D. G. McCafferty, J. Am. Chem. Soc., 2004, 126, 3404 CrossRef CAS PubMed.
- S. Y. Woo, J. H. Kim, M. K. Moon, S.-H. Han, S. K. Yeon, J. W. Choi, B. K. Jang, H. J. Song, Y. G. Kang, J. W. Kim, J. Lee, D. J. Kim, O. Hwang and K. D. Park, J. Med. Chem., 2014, 57, 1473 CrossRef CAS PubMed.
- (a) D. C. Reeves, S. Rodriguez, H. Lee, N. Haddad, D. Krishnamurthy and C. H. Senanayake, Tetrahedron Lett., 2009, 50, 2870 CrossRef CAS; (b) R. Chawla, R. Kapoor, A. K. Singh and D. S. Yadav, Green Chem., 2012, 14, 1308 RSC; (c) J. L. G. Ruano, J. Alemán and C. G. Paredes, Org. Lett., 2006, 8, 2683 CrossRef CAS PubMed; (d) N. Kamigata, H. Sawada and M. Kobayashi, J. Org. Chem., 1983, 48, 3793 CrossRef CAS.
- J. Chen, M. Jincheng, Z. Yang, L. Defu, R. Guangwei, Y. Hong, Z. Cheng and D. Shi, Tetrahedron, 2015, 71, 5059 CrossRef CAS.
- G. Jian, L. Junyi and Y. Gaoqing, RSC Adv., 2015, 5, 66723 RSC.
- N. Taniguchi, Tetrahedron, 2014, 70, 1984 CrossRef CAS.
- G. W. Rong, J. C. Mao, H. Yan, Y. Zheng and G. Q. Zhang, J. Org. Chem., 2015, 80, 4697 CrossRef CAS PubMed.
- (a) T. Kaur, P. Wadhwa and A. Sharma, RSC Adv., 2015, 5, 52769 RSC; (b) A. V. Lygin and A. de Meijere, Angew. Chem., Int. Ed., 2010, 49, 9049 CrossRef PubMed.
- (a) H. Bounar, Z. Liu, L. Zhang, X. Guan, Z. Yang, P. Liao, X. Bi and X. Li, Org. Biomol. Chem., 2015, 13, 8723 RSC; (b) J. Liu, Z. Liu, P. Liao and X. Bi, Org. Lett., 2014, 16, 6204 CrossRef CAS PubMed.
- (a) D. K. Tiwari, R. A. Maurya and J. B. Nanubolu, Chem. – Eur. J., 2016, 22, 526 CrossRef CAS PubMed; (b) D. K. Tiwari, K. C. Bharadwaj, V. G. Puranik and D. K. Tiwari, Org. Biomol. Chem., 2014, 12, 7389 RSC; (c) K. C. bhardwaj and D. K. Tiwari, Tetrahedron, 2016, 72, 312 CrossRef; (d) R. M. Singh, K. C. Bhardwaj and D. K. Tiwari, Beilstein J. Org. Chem., 2014, 10, 2975 CrossRef PubMed.
- (a) D. K. Tiwari, J. Pogula, B. Sridhar, D. K. Tiwari and P. R. Likhar, Chem. Commun., 2015, 51, 13646 RSC; (b) D. K. Tiwari, J. Pogula and D. K. Tiwari, RSC Adv., 2015, 5, 53111 RSC.
- A. K. Morri, Y. thummala and V. R. Doddi, Org. Lett., 2015, 17, 4640 CrossRef CAS PubMed.
- X. Li, X. Xu and C. Zhou, Chem. Commun., 2012, 48, 12240 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1053806. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00063k |
| This journal is © the Partner Organisations 2016 |