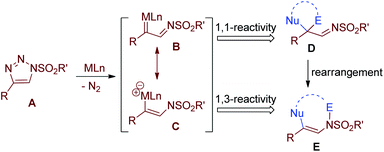
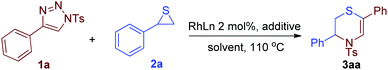Straightforward regioselective construction of 3,4-dihydro-2H-1,4-thiazine by rhodium catalysed [3 + 3] cycloaddition of thiirane with 1-sulfonyl-1,2,3-triazole: a pronounced acid additive effect†
Xiao-Long
Lu
,
Ya-Ting
Liu
,
Qing-Xia
Wang
,
Mei-Hua
Shen
* and
Hua-Dong
Xu
*
School of Pharmaceutical Engineering and Life Science, Changzhou University, Changzhou 213164, China. E-mail: shemmh@cczu.edu.cn; huadongxu@gmail.com
First published on 31st March 2016
Abstract
A rhodium catalyzed regioselective coupling of thiirane with 1-sulfonyl-1,2,3-triazole has been realized to give a formal [3 + 3] cycloaddition product 3,4-dihydro-2H-1,4-thiazine via the 1,3-insertion model of azavinyl carbene. An acetic acid additive is used to improve the reaction in terms of both yield and rate.
1-Sulfonyl-1,2,3-triazole as a convenient metal α-azavinyl carbene precursor has found broad application since the seminal discovery made by Fokin, Gevorgyan and Murakami.1–4 This carbenoid can (formally) take part in two distinguished bond forming fashions, namely 1,1-difunctionalization to give a product of type D and 1,3-difunctionalization to give a product of type E. Formally, D could be viewed as an 1,1-addition product of metal carbene B with a nucleophile–electrophile pair and E could be viewed as a 1,3-addition product of 1,3-dipole C with a nucleophile–electrophile pair,5–7 whereby various mechanism details have been proposed for different cases (Fig. 1). Reactions that give products of type D include cyclopropanation of alkene,8–14 C–H insertion,15–24 ylide formation/[2,3]-sigmatropic rearrangement,25–28etc. With the advantage of reservation of a sulfonyl amide moiety, the formation of structure E has also contributed to a number of elegant N-heterocyclic construction protocols.29–41
Recently, the formal 1,3-insertion of dipole C into C–O/C–S bonds has been reported. The Lacour research group realized 1,3-azavinyl carbene insertion into 1,3-dioxolane and 1,3-dioxane C–O bonds to form medium-sized dioxazocine and dioxazonine.42 Li and co-workers reported a 1,3-azavinyl carbene insertion into the C–S bond of β-(methylthio)-α,β-unsaturated ketone.43 Miura and Murakami also disclosed a 1,3-azavinyl carbene insertion into the thioester C–S bond.44 The Chen group achieved a regioselective synthesis of 3,4-dihydro-2H-1,4-oxazine through rhodium-catalyzed formal [3 + 3] cycloaddition of 1,2,3-triazoles and epoxides.45 Additionally, Tang and Shi took advantage of the strong nucleophilic thioether to accomplish interesting transformations using 1-sulfonyl-1,2,3-triazole substrates.46,47 Here we wish to report an efficient regioselective synthesis of N-sulfonyl-3,4-dihydro-2H-1,4-thiazines via 1,3-azavinyl carbene insertion into the thiirane C–S bond, which would be a significant advance to the synthesis of this class of heterocycles since there are limited methods available for this purpose.
Initially, a solution of 1-tosyl-1,2,3-triazole 1a and 2-phenylthiirane 2a in dichloroethane (DCE) was heated at 110 °C in the presence of 2 mol% Rh2(OAc)4 for 5 hours, 3aa was obtained in 53% isolated yield as the sole regioisomer, and its structure was established by NMR experiments (Table 1, entry 1). Using toluene as reaction medium, the same reaction afforded comparable results (entry 2). Replacement of the catalyst Rh2(OAc)4 with Rh2(esp)2 increased the yield of 3aa significantly to 69% (entry 3). During the course of optimization of conditions, it was found adventitiously that the introduction of a sub-stoichiometric amount of acetic acid (AcOH) can promote this reaction notably. With 5 mol% of AcOH in the reaction mixture, both acceleration of the reaction rate and rise in yield were observed (entry 4). When the amount of this additive was enhanced to 20 mol%, the reaction completed in less than 1.5 hours and afforded 3aa in a high yield of 85% (entries 5 and 6). To the best of our knowledge, only two examples have been noticed for the beneficial effects of (Lewis) acid additives on Rh-azavinyl carbene reactions.48,49
| Entry | Cat. | Solvent | Additive (equiv.) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.5 mmol, 1.0 equiv.), phenylthiirane 2a (1.0 mmol, 2 equiv.), AcOH (0–0.1 mmol, 0–0.2 equiv.), RhLn (0.01 mmol, 0.02 equiv.), solvent (2 mL), 110 °C. b Isolated yield based on 1a. | |||||
| 1 | Rh2(OAc)4 | DCE | — | 5 | 53% |
| 2 | Rh2(OAc)4 | Toluene | — | 4 | 56% |
| 3 | Rh2(esp)2 | Toluene | — | 4 | 69% |
| 4 | Rh2(esp)2 | Toluene | AcOH (0.05) | 3 | 75% |
| 5 | Rh2(esp)2 | Toluene | AcOH (0.1) | 2 | 84% |
| 6 | Rh2(esp)2 | Toluene | AcOH (0.2) | 1.5 | 85% |
With the optimal reaction conditions in hand, we started to explore the substrate scope for 1-sulfonyl triazole 1 using 2a as the reaction partner (Table 2). Firstly, it was found that the electronic properties of the sulfonyl group exert a great effect on the reaction yield. With an electron rich 4-methoxybenzenesulfonyl (Ans) group, 93% yield of 3ba was achieved (entry 2), while with an electron poor 4-nitrobenzenesulfonyl (Nos) group, the yield of 3ca dropped to as low as 31% (entry 3). Not surprisingly, in line with this trend, the reaction of mesylated triazole 1d gave 79% yield of 3da (entry 4). On the other hand, the electronic effect of substituent on the 4-phenyl group (R = Ph) on the reaction yield was not that prominent. Substrates 1e, 1g, 1h, 1i and 1j with divergent electronic properties all gave the corresponding dihydro-1,4-thiazines in yields ranging from 64–85% (entries 5 and 7–10). However, the steric effect is much more significant as demonstrated by the dramatic drop in yield of 3fa from the coupling of 1f and 2a (entry 6). Heterocyclic substituents on triazole can impact the reaction considerably relying on their own features. Thiophenyl triazole 1k reacted with 2a to deliver 3ak in 89% yield; another heterolytic triazole pyridinyl triazole 1l can also furnish related dihydrothiazine 3la, but in lower yield (entry 11 vs. 12). Alkyl triazoles were much inferior substrates for this reaction. Dihydrothiazine 3ma was produced from 1m in only 24% yield, and the reaction of benzyl triazole 1n with phenylthiirane 2a yielded a complex reaction mixture (entries 13 and 14). Without the AcOH additive, the reaction of 1e and 1h–1j with 2a proceeded much slower and resulted in lower yield in all cases (entries 5 and 8–10). These observations further confirmed the favourable influence of AcOH on this reaction.
| Entry | Triazole 1 | Product, yieldb | Entry | Triazole 1 | Product, yieldb |
|---|---|---|---|---|---|
| a Conditions: 1 (0.5 mmol, 1.0 equiv.), phenylthiirane 2a (1.0 mmol, 2 equiv.), AcOH (0.1 mmol, 0.2 equiv.), Rh2(esp)2 (0.01 mmol, 0.02 equiv.), toluene (2 mL), 110 °C, 1.5 hours. b Isolated yield based on 1. c Without the AcOH additive. | |||||
| 1 |
|
|
8 |
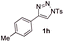
|
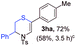
|
| 2 |
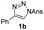
|
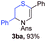
|
9 |
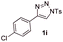
|
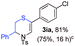
|
| 3 |
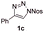
|
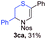
|
10 |
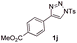
|
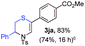
|
| 4 |
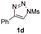
|
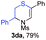
|
11 |
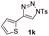
|
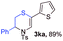
|
| 5 |
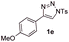
|
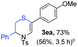
|
12 |
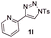
|
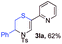
|
| 6 |
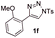
|
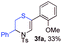
|
13 |
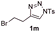
|
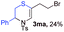
|
| 7 |
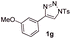
|
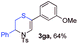
|
14 |
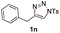
|
Complex |
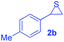
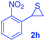
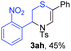
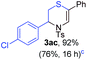
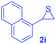
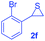
Next, the thiirane scope for this reaction was probed using phenyltriazole 1a as the reaction partner (Table 3). 4-Substituted phenylthiirane 2b–2d all reacted with 1a smoothly giving rise to the corresponding dihydro-1,4-thiazines 3ab–3ad in high yields (entries 1–3). The reaction of 2e carrying a meta-Br phenyl group produced 3ae in 76% yield, about 10% lower than its para-isomer 3ad (entry 4 vs. 3). The yields of 3af–3ah, obtained from 2-substituted phenylthiiranes 2f–2h, dropped drastically to 33%–48% which underlined again the steric effect on this reaction (entries 5–7). These data demonstrated that this reaction is not so sensitive to the electronic properties of aromatic substituents on phenylthiirane but is very susceptible to the steric effect. Interestingly, naphthalen-1-ylthiirane 2i can be efficiently converted to dihydro-1,4-thiazine 3ai in 83% (entry 8). 2,2-Disubstituted thiiranes 2j and 2k were also viable substrates for this reaction though giving the corresponding thiazines 3aj and 3ak with diminished yields (entries 9 and 10). 2-Alkylthiiranes 2f and 2g failed to deliver any desired dihydro-1,4-thiiranes (entries 11 and 12). Again, reactions of 2b, 2c and 2j with 1a in the absence of AcOH constantly delivered inferior outcomes in comparison with those obtained under optimal conditions (entries 1, 2 and 9). It is noteworthy that the other regioisomers were not detected in all cases.
| Entry | Thiirane | Product, yieldb | Entry | Thiirane | Product, yieldb |
|---|---|---|---|---|---|
| a Conditions: 4-phenyl-1-tosyl-1,2,3-triazole 1a (0.5 mmol, 1.0 equiv.), thiirane 2 (1.0 mmol, 2 equiv.), AcOH (0.1 mmol, 0.2 equiv.), Rh2(esp)2 (0.01 mmol, 0.02 equiv.), toluene (2 mL), 110 °C, 1.5 hours. b Isolated yield based on 1a. c Without the AcOH additive. | |||||
| 1 |
|
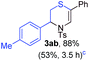
|
7 |
|
|
| 2 |
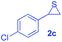
|
|
8 |
|
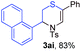
|
| 3 |

|
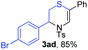
|
9 |
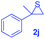
|
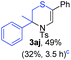
|
| 4 |

|
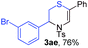
|
10 |
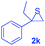
|
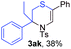
|
| 5 |
|
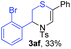
|
11 |

|
Complex |
| 6 |
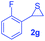
|
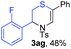
|
12 |
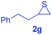
|
Complex |
Based on well-established azavinyl carbene chemistry, it is proposed that the in situ generated rhodium carbene B is attacked by aryl thiirane 2 to form strained sulfonium ylide complex F which would undergo ring opening/ring closing rearrangement to dihydro-1,4-thiazine 3. The electrophilic rhodium carbene B might be further activated by the formation of a hydrogen bond between the imino nitrogen and acetic acid as shown in intermediate B′. The more electrophilic metal carbene B′ would combine with thiirane 2 in an accelerated rate en route to the final product 3. The developing cationic character at the benzylic carbon in F and G was much more stabilized by an aryl group than an alkyl group. This accounted for the absolute regioselectivity and the incompetent alkyl triazoles as substrates (Fig. 2). It was reasoned that the bimolecular interaction of thiirane 2 with carbenoid B or B′ is the rate-determining step (RDS) for this reaction that serves as the origin of the accelerating effect of AcOH.
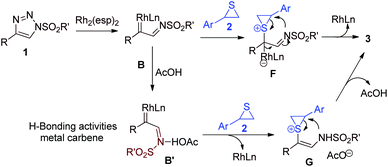 | ||
| Fig. 2 Proposed mechanism: H-bonding activation of azavinyl carbene, sulfonium ylide formation and ring opening/ring closing rearrangement. | ||
Conclusions
We have developed an efficient regioselective protocol to access 3,4-dihydro-2H-1,4-thiazine via the reaction of 1-sulfonyl triazole with aryl thiirane. This formal [3 + 3] cycloadduct can be viewed as a result of the 1,3-reaction model of azavinyl carbene. It is noticed that AcOH as an additive serves as an activator to promote the Rh-azavinyl carbene electrophilicity probably through hydrogen-bonding with the Rh-azavinyl intermediate. Considering the lack of straightforward means to construct 3,4-dihydro-2H-1,4-thiazine in the literature, the current advancement would hold great importance.Acknowledgements
The authors wish to thank the Natural Science Foundation of China (21402014 and 21272077), the Natural Science Foundation of Jiangsu Province (BK20131143), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PADA), and the Jiangsu Key Laboratory of Advanced Catalytic Materials and Technology (BM2012110).Notes and references
- H. M. L. Davies and J. S. Alford, Chem. Soc. Rev., 2014, 43, 5151–5162 RSC.
- S. C. Hockey and L. C. Henderson, Aust. J. Chem., 2015, 68, 1796–1800 CrossRef CAS.
- T. Horneff, S. Chuprakov, N. Chernyak, V. Gevorgyan and V. V. Fokin, J. Am. Chem. Soc., 2008, 130, 14972–14974 CrossRef CAS PubMed.
- T. Miura, M. Yamauchi and M. Murakami, Chem. Commun., 2009, 1470–1471 RSC.
- S. Chuprakov, B. T. Worrell, N. Selander, R. K. Sit and V. V. Fokin, J. Am. Chem. Soc., 2014, 136, 195–202 CrossRef CAS PubMed.
- S. Park, W.-S. Yong, S. Kim and P. H. Lee, Org. Lett., 2014, 16, 4468–4471 CrossRef CAS PubMed.
- S. Shin, Y. Park, C.-E. Kim, J.-Y. Son and P. H. Lee, J. Org. Chem., 2015, 80, 5859–5869 CrossRef CAS PubMed.
- J. S. Alford and H. M. L. Davies, Org. Lett., 2012, 14, 6020–6023 CrossRef CAS PubMed.
- S. Chuprakov, S. W. Kwok, L. Zhang, L. Lercher and V. V. Fokin, J. Am. Chem. Soc., 2009, 131, 18034–18035 CrossRef CAS PubMed.
- J. C. Culhane and V. V. Fokin, Org. Lett., 2011, 13, 4578–4580 CrossRef CAS PubMed.
- N. Grimster, L. Zhang and V. V. Fokin, J. Am. Chem. Soc., 2010, 132, 2510–2511 CrossRef CAS PubMed.
- X.-H. Pan, P. Jiang, Z.-H. Jia, K. Xu, J. Cao, C. Chen, M.-H. Shen and H.-D. Xu, Tetrahedron, 2015, 71, 5124–5129 CrossRef CAS.
- H.-D. Xu, K. Xu, Z.-H. Jia, H. Zhou, P. Jiang, X.-L. Lu, Y.-P. Pan, H. Wu, Y. Ding, M.-H. Shen and X.-H. Pan, Asian J. Org. Chem., 2014, 3, 1154–1158 CrossRef CAS.
- M. Zibinsky and V. V. Fokin, Org. Lett., 2011, 13, 4870–4872 CrossRef CAS PubMed.
- S. Chuprakov, J. A. Malik, M. Zibinsky and V. V. Fokin, J. Am. Chem. Soc., 2011, 133, 10352–10355 CrossRef CAS PubMed.
- L. Li, X.-H. Xia, Y. Wang, P. P. Bora and Q. Kang, Adv. Synth. Catal., 2015, 357, 2089–2097 CrossRef CAS.
- V. N. G. Lindsay, H. M. F. Viart and R. Sarpong, J. Am. Chem. Soc., 2015, 137, 8368–8371 CrossRef CAS PubMed.
- X. Ma, F. Wu, X. Yi, H. Wang and W. Chen, Chem. Commun., 2015, 51, 6862–6865 RSC.
- B. Rajagopal, C.-H. Chou, C.-C. Chung and P.-C. Lin, Org. Lett., 2014, 16, 3752–3755 CrossRef CAS PubMed.
- M. Senoo, A. Furukawa, T. Hata and H. Urabe, Chem. – Eur. J., 2016, 22, 890–895 CrossRef CAS PubMed.
- B. Seo, W. H. Jeon, J. Kim, S. Kim and P. H. Lee, J. Org. Chem., 2015, 80, 722–732 CrossRef CAS PubMed.
- M.-H. Shen, Y.-P. Pan, Z.-H. Jia, X.-T. Ren, P. Zhang and H.-D. Xu, Org. Biomol. Chem., 2015, 13, 4851–4854 CAS.
- H.-D. Xu, Y.-P. Pan, X.-T. Ren, P. Zhang and M.-H. Shen, Tetrahedron Lett., 2015, 56, 6734–6737 CrossRef CAS.
- J.-M. Yang, C.-Z. Zhu, X.-Y. Tang and M. Shi, Angew. Chem., Int. Ed., 2014, 53, 5142–5146 CAS.
- A. Boyer, Org. Lett., 2014, 16, 5878–5881 CrossRef CAS PubMed.
- A. Boyer, Org. Lett., 2014, 16, 1660–1663 CrossRef CAS PubMed.
- J. Fu, H. Shen, Y. Chang, C. Li, J. Gong and Z. Yang, Chem. – Eur. J., 2014, 20, 12881–12888 CrossRef CAS PubMed.
- H.-D. Xu, Z.-H. Jia, K. Xu, H. Zhou and M.-H. Shen, Org. Lett., 2015, 17, 66–69 CrossRef CAS PubMed.
- J. S. Alford, J. E. Spangler and H. M. L. Davies, J. Am. Chem. Soc., 2013, 135, 11712–11715 CrossRef CAS PubMed.
- S. Chuprakov, S. W. Kwok and V. V. Fokin, J. Am. Chem. Soc., 2013, 135, 4652–4655 CrossRef CAS PubMed.
- C.-E. Kim, S. Park, D. Eom, B. Seo and P. H. Lee, Org. Lett., 2014, 16, 1900–1903 CrossRef CAS PubMed.
- D. J. Lee, H. S. Han, J. Shin and E. J. Yoo, J. Am. Chem. Soc., 2014, 136, 11606–11609 CrossRef CAS PubMed.
- T. Miura, Y. Funakoshi and M. Murakami, J. Am. Chem. Soc., 2014, 136, 2272–2275 CrossRef CAS PubMed.
- T. Miura, K. Hiraga, T. Biyajima, T. Nakamuro and M. Murakami, Org. Lett., 2013, 15, 3298–3301 CrossRef CAS PubMed.
- T. Miura, T. Tanaka, K. Hiraga, S. G. Stewart and M. Murakami, J. Am. Chem. Soc., 2013, 135, 13652–13655 CrossRef CAS PubMed.
- R.-Q. Ran, J. He, S.-D. Xiu, K.-B. Wang and C.-Y. Li, Org. Lett., 2014, 16, 3704–3707 CrossRef CAS PubMed.
- E. E. Schultz, V. N. G. Lindsay and R. Sarpong, Angew. Chem., Int. Ed., 2014, 53, 9904–9908 CrossRef CAS PubMed.
- E. E. Schultz and R. Sarpong, J. Am. Chem. Soc., 2013, 135, 4696–4699 CrossRef CAS PubMed.
- H. Shang, Y. Wang, Y. Tian, J. Feng and Y. Tang, Angew. Chem., Int. Ed., 2014, 53, 5662–5666 CrossRef CAS PubMed.
- Y. Shi and V. Gevorgyan, Org. Lett., 2013, 15, 5394–5396 CrossRef CAS PubMed.
- J. E. Spangler and H. M. L. Davies, J. Am. Chem. Soc., 2013, 135, 6802–6805 CrossRef CAS PubMed.
- F. Medina, C. Besnard and J. Lacour, Org. Lett., 2014, 16, 3232–3235 CrossRef CAS PubMed.
- J. He, Z. Man, Y. Shi and C.-Y. Li, J. Org. Chem., 2015, 80, 4816–4823 CrossRef CAS PubMed.
- T. Miura, Y. Fujimoto, Y. Funakoshi and M. Murakami, Angew. Chem., Int. Ed., 2015, 54, 9967–9970 CrossRef CAS PubMed.
- X. Ma, S. Pan, H. Wang and W. Chen, Org. Lett., 2014, 16, 4554–4557 CrossRef CAS PubMed.
- Y. Jiang, X.-Y. Tang and M. Shi, Chem. Commun., 2015, 51, 2122–2125 RSC.
- Y. Jiang, R. Sun, Q. Wang, X.-Y. Tang and M. Shi, Chem. Commun., 2015, 51, 16968–16971 RSC.
- Y.-S. Zhang, X.-Y. Tang and M. Shi, Org. Chem. Front., 2015, 2, 1516–1520 RSC.
- Y. Wang, X. Lei and Y. Tang, Chem. Commun., 2015, 51, 4507–4510 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, characterization data and NMR spectra for all new compounds. See DOI: 10.1039/c6qo00084c |
| This journal is © the Partner Organisations 2016 |