A zinc-catalyzed oxidative reaction of ynamides with phenols and thiophenols: highly site-selective synthesis of versatile α-aryloxy amides and α-arylthio amides†
Peng-Peng
Ruan‡
a,
Cang-Hai
Shen‡
a,
Long
Li
a,
Chao-Yue
Liu
a and
Long-Wu
Ye
*ab
aState Key Laboratory of Physical Chemistry of Solid Surfaces & The Key Laboratory for Chemical Biology of Fujian Province, Department of Chemistry, Xiamen University, Xiamen 361005, China. E-mail: longwuye@xmu.edu.cn
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
First published on 22nd June 2016
Abstract
A zinc-catalyzed oxidative reaction of ynamides with phenols and thiophenols under mild reaction conditions has been developed, which provides various α-aryloxy amides and α-arylthio amides in moderate to good yields, respectively. Importantly, high chemoselectivity is achieved by such a non-noble metal-catalyzed alkyne oxidation.
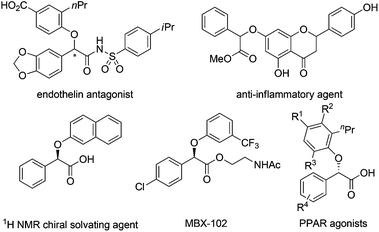
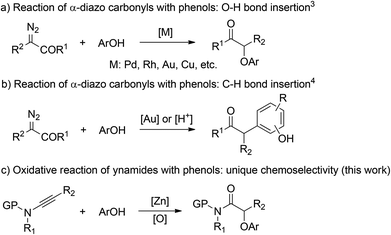
α-Aryloxy carbonyls are highly valuable compounds that can be found in a large number of biologically active molecules (Fig. 1).1 As a result, a range of synthetic methods have been developed mainly by transition metal-catalyzed insertion of α-diazo carbonyls into the O–H bonds of phenols.2,3 However, the use of hazardous, not easily accessible and potentially explosive α-diazo carbonyls has severely hampered the further synthetic applications of this methodology. Moreover, it remains challenging to control the chemoselectivity of phenols because of the competitive C–H bond insertion (Scheme 1).2,4 Therefore, the development of an alternative approach is still highly desirable.
In the last few years, gold-catalyzed intermolecular alkyne oxidation by an N–O bond oxidant, presumably involving an α-oxo gold carbenoid intermediate,5 has attracted considerable attention because this protocol makes alkynes surrogates of hazardous α-diazo carbonyls in accessing α-oxo metal carbenes.6 As a result, this strategy has evolved into a robust and reliable method for the construction of C–C and C–X bonds in a highly stereoselective manner.7 Despite these significant achievements, intermolecular alkyne oxidation with external nucleophiles is still rare8 and challenging mainly due to the competing background reaction of external nucleophiles with the activated alkynes and the overoxidation of the highly electrophilic carbene center by the very oxidant,9 thus generating the unwanted olefin and diketone byproducts, respectively. In our continuing effort on studies of ynamides in organic synthesis,10,11 we recently disclosed that zinc could also catalyze such an intermolecular alkyne oxidation and, importantly, the undesired over-oxidation could be substantially suppressed in such an oxidative zinc catalysis.12 Inspired by these results, we envisioned that the synthesis of α-aryloxy amides might be accessed directly through such a Lewis acid-catalyzed reaction of ynamides with phenols (Scheme 1). Herein, we describe the realization of the zinc-catalyzed oxidative reaction of ynamides with phenols with high chemoselectivity, which constitutes a very practical approach to the generation of various α-aryloxy amides. In addition, this zinc-catalyzed ynamide oxidation could also be extended to the highly site-selective synthesis of synthetically useful α-arylthio amides.
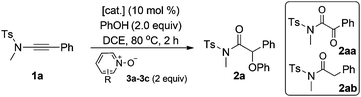
Ynamide 1a was employed as the model substrate for our initial study (Table 1). To our delight, the reaction of 1a with PhOH by the use of Zn(OTf)2 as the catalyst and 2,6-dibromopyridine N-oxide 3a as the oxidant in DCE at 80 °C could afford the desired α-phenoxy amide 2a in 61% yield albeit with a small amount of hydration by-product 2ab (entry 1). Importantly, neither C–H functionalization of phenol nor a direct background reaction of PhOH with 1a was observed. The subsequent screening of other N-oxides such as 2,6-lutidine N-oxide 3b and 3,5-dichloropyridine N-oxide 3c only led to diketone 2aa as the main product (entries 2 and 3). In addition, the influence of different Lewis acids was also investigated but it failed to improve the yield (entries 4–6). Finally, it was found that a slightly better result could be obtained at 40 °C (entries 7 and 8), and the desired 2a was formed in 69% yield in the presence of 20 mol% Zn(OTf)2 (entry 8). Notably, diketone 2aa was formed as the major product if catalyzed by typical gold catalysts such as Ph3PAuNTf2 and Cy-JohnPhosAuNTf2 (entries 9 and 10), and no 2a formation was observed in the absence of the catalyst (>95% of 1a remained unreacted). It should be mentioned that the use of 1.0 equiv. of PhOH led to significant formation of 2ab (entry 11).
| Entry | Catalyst | Oxidant (R) | Yieldb (%) | ||
|---|---|---|---|---|---|
| 2a | 2aa | 2ab | |||
| a Reaction conditions: [1a] = 0.10 M; DCE: 1,2-dichloroethane. b Estimated by 1H NMR using diethylphthalate as an internal reference. c DCM, 40 °C, 7 h. d 20 mol% Zn(OTf)2 was used, DCM, 40 °C, 4 h. e 5 mol% gold catalyst was used. f 1.0 equiv. of PhOH was used. | |||||
| 1 | Zn(OTf)2 | 3a (2,6-Br2) | 61 | <1 | 9 |
| 2 | Zn(OTf)2 | 3b (2,6-Me2) | 23 | 35 | 10 |
| 3 | Zn(OTf)2 | 3c (3,5-Cl2) | 20 | 75 | <1 |
| 4 | Yb(OTf)3 | 3a (2,6-Br2) | 56 | <1 | 12 |
| 5 | Sm(OTf)3 | 3a (2,6-Br2) | 55 | <1 | 14 |
| 6 | Y(OTf)3 | 3a (2,6-Br2) | 50 | <1 | 15 |
| 7c | Zn(OTf)2 | 3a (2,6-Br2) | 66 | <1 | <5 |
| 8d | Zn(OTf)2 | 3a (2,6-Br2) | 69 | <1 | <5 |
| 9e | Ph3PAuNTf2 | 3a (2,6-Br2) | <1 | 85 | 9 |
| 10e | CyJohnPhosAuNTf2 | 3a (2,6-Br2) | <1 | 75 | 13 |
| 11d,f | Zn(OTf)2 | 3a (2,6-Br2) | 50 | <1 | 15 |
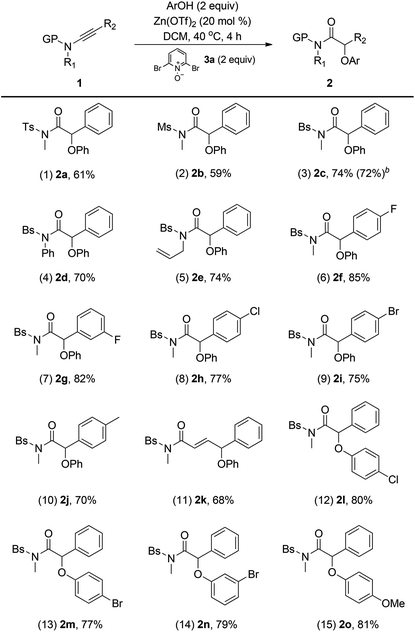
With the optimized reaction conditions in hand, the scope of this oxidative zinc catalysis was explored (Table 2). Ynamides with different protecting groups were first examined (entries 1–3), and the Bs-substituted ynamide gave the best result (entry 3). Meanwhile, different aryl-substituted ynamides (R2 = aryl) also worked well to yield the desired α-aryloxy amides 2f–2j smoothly (entries 6–10). In the case of styryl-substituted ynamide, the reaction afforded α,β-unsaturated amide 2k as a single isomer in 68% yield (entry 11). In addition, various substituted phenols with both electron-donating and -withdrawing substituents are applicable to the present reaction, thus delivering the corresponding 2l–2o in 77–81% yields (entries 12–15). Importantly, no diketone formation was detected in all the cases. Moreover, all these reactions are chemospecific, leading to the α-aryloxy amides as the sole product. Attempts to extend the reaction to aliphatic-substituted ynamides only resulted in the formation of α,β-unsaturated amides.13 It is to be noted that the reaction proceeded with a low efficiency (<10% yield) on employing ethyl phenylethynyl ether instead of ynamides under the optimal reaction conditions. To test the practicality of the current catalytic system, a gram-scale reaction of 1c and PhOH was carried out in the presence of 10 mol% Zn(OTf)2, and the desired 2c was obtained in 72% yield (entry 3).
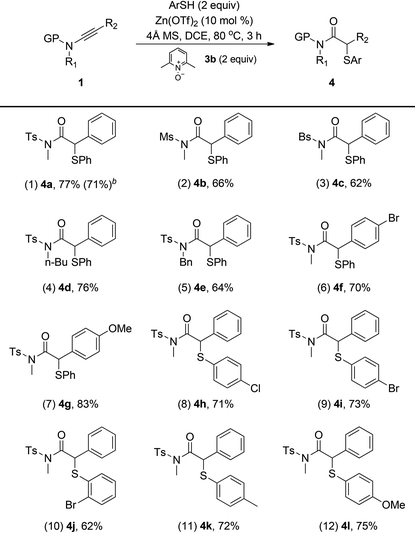
We then wondered whether this oxidative zinc catalysis is applicable to thiophenols. As outlined in Table 3, the reaction proceeded smoothly with various thiophenols and ynamides under the optimal reaction conditions (2 equiv. of ArSH, 2 equiv. of 3b, 10 mol% of Zn(OTf)2, 4 ÅMS, DCE, 80 °C, 3 h).14 Besides the tosyl group, Ms and Bs protected ynamides were also suitable substrates for this transformation, thus delivering the corresponding α-arylthio amides 4b (66%) and 4c (62%), respectively (entries 2 and 3). The reaction also worked satisfactorily with various substituted ynamides, providing the desired 4d–4g in good yields (entries 4–7). In addition, thiophenols bearing both electron-donating and -withdrawing substituents turned out to be suitable substrates as well, leading to the desired 4h–4l in moderate to good yields (entries 8–12). Finally, a gram scale synthesis was also feasible (entry 1). Again, neither C–H functionalization of thiophenols nor overoxidation of ynamides was observed in all the cases. Thus, this protocol provides a highly efficient and practical route for the construction of the valuable α-arylthio carbonyl compounds.15 Notably, the use of ethyl phenylethynyl ether instead of ynamides under the optimal reaction conditions only resulted in the formation of the hydration product (85% yield).
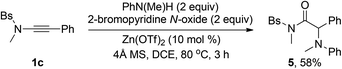
Attempts to expand this chemistry to aliphatic alcohols or thiols only led to the formation of the corresponding amides in low yield (<50% yield), and further studies in this direction are currently ongoing. Besides phenols and thiophenols, this oxidative zinc catalysis could also be extended to anilines by the employment of 2-bromopyridine N-oxide as the oxidant, leading to the corresponding α-amino amide 5 with serviceable yield (eqn (1)).
 | (1) |
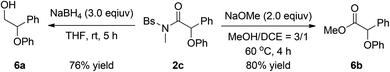
The synthetic utility of this protocol was demonstrated by the transformation of the above α-phenoxy amide 2c. As depicted in eqn (2), the amide 2c could be readily transformed into the corresponding β-phenoxy alcohol 6a in 76% yield by NaBH4 reduction. In addition, treatment of amide 2c with NaOMe could afford the synthetically useful α-phenoxy ester 6b in 80% yield.
 | (2) |
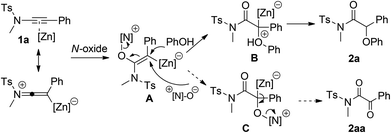
Based on the above results and our previous work12,13 on the oxidative zinc catalysis, a tentative mechanism is proposed in Scheme 2. The pyridine N-oxide first attacked the Zn-activated alkyne leading to the generation of the Zn-substituted alkene A, which could then be converted into B through an SN2′ pathway.7e,g Finally, the intermediate B would undergo protodemetalation to furnish the final product 2a along with the regeneration of the zinc catalyst. The formation of by-product 2aa might be rationalized by an intermolecular secondary oxidation through a similar SN2′ pathway.
In summary, we have developed a novel zinc-catalyzed oxidative reaction of ynamides with phenols and thiophenols, allowing the efficient synthesis of various α-aryloxy amides and α-arylthio amides, respectively. Importantly, high chemoselectivity is achieved by such a non-noble metal-catalyzed alkyne oxidation. Besides, the use of readily available substrates, a simple procedure, and mild reaction conditions makes this method potentially useful in organic synthesis. Further investigations into the asymmetric version of the current protocol are in progress in our laboratory.
We are grateful for financial support from the National Natural Science Foundation of China (21272191 and 21572186), the Natural Science Foundation of Fujian Province for Distinguished Young Scholars (2015J06003), the President Research Funds from Xiamen University (20720150045), NFFTBS (J1310024), and XMU Undergraduate Innovation and Entrepreneurship Training Programs (201510384008).
Notes and references
- For recent selected examples, see: (a) X.-L. Xie, S.-F. Zhu, J.-X. Guo, Y. Cai and Q.-L. Zhou, Angew. Chem., Int. Ed., 2014, 53, 2978 CrossRef CAS PubMed; (b) S. Wang, R. Beck, A. Burd, T. Blench, F. Marlin, T. Ayele, S. Buxton, C. Dagostin, M. Malic, R. Joshi, J. Barry, M. Sajad, C. Cheung, S. Shaikh, S. Chahwala, C. Chander, C. Baumgartner, H.-P. Holthoff, E. Murray, M. Blackney and A. Giddings, J. Med. Chem., 2010, 53, 1473 CrossRef CAS PubMed; (c) E. N. Koini, P. Papazafiri, A. Vassilopoulos, M. Koufaki, Z. Horváth, I. Koncz, L. Virág, G. J. Papp, A. Varró and T. Calogeropoulou, J. Med. Chem., 2009, 52, 2328 CrossRef CAS PubMed; (d) G. Q. Shi, J. F. Dropinski, B. M. McKeever, S. Xu, J. W. Becker, J. P. Berger, K. L. MacNaul, A. Elbrecht, G. Zhou, T. W. Doebber, P. Wang, Y.-S. Chao, M. Forrest, J. V. Heck, D. E. Moller and A. B. Jones, J. Med. Chem., 2005, 48, 4457 CrossRef CAS PubMed.
- For selected reviews, see: (a) L. Liu and J. Zhang, Chem. Soc. Rev., 2016, 45, 506 RSC; (b) A. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire and M. A. McKervey, Chem. Rev., 2015, 115, 9981 CrossRef CAS PubMed; (c) F. Wei, C. Song, Y. Ma, L. Zhou, C.-H. Tung and Z. Xu, Sci. Bull., 2015, 60, 1479 CrossRef CAS; (d) D. Gillingham and N. Fei, Chem. Soc. Rev., 2013, 42, 4918 RSC; (e) X. Guo and W. Hu, Acc. Chem. Res., 2013, 46, 2427 CrossRef CAS PubMed; (f) S.-F. Zhu and Q.-L. Zhou, Acc. Chem. Res., 2012, 45, 1365 CrossRef CAS PubMed; (g) Z. Zhang and J. Wang, Tetrahedron, 2008, 64, 6577 CrossRef CAS; (h) T. Ye and M. A. McKervey, Chem. Rev., 1994, 94, 1091 CrossRef CAS.
- For selected examples, see: (a) X. Gao, B. Wu, W.-X. Huang, M.-W. Chen and Y.-G. Zhou, Angew. Chem., Int. Ed., 2015, 54, 11956 CrossRef CAS PubMed; (b) T. Osako, D. Panichakul and Y. Uozumi, Org. Lett., 2012, 14, 194 CrossRef CAS PubMed; (c) K. J. Kilpin, U. S. D. Paul, A.-L. Lee and J. D. Crowley, Chem. Commun., 2011, 47, 328 RSC; (d) T. C. Maier and G. C. Fu, J. Am. Chem. Soc., 2006, 128, 4594 CrossRef CAS PubMed; (e) Z. Qu, W. Shi and J. Wang, J. Org. Chem., 2004, 69, 217 CrossRef CAS PubMed; (f) P. Yates, J. Am. Chem. Soc., 1952, 74, 5376 CrossRef CAS.
- For recent selected examples, see: (a) Y. Liu, Z. Yu, J. Z. Zhang, L. Liu, F. Xia and J. Zhang, Chem. Sci., 2016, 7, 1988 RSC; (b) Z. Yu, B. Ma, M. Chen, H.-H. Wu, L. Liu and J. Zhang, J. Am. Chem. Soc., 2014, 136, 6904 CrossRef CAS PubMed; (c) Y. Xi, Y. Su, Z. Yu, B. Dong, E. J. McClain, Y. Lan and X. Shi, Angew. Chem., Int. Ed., 2014, 53, 9817 CrossRef CAS PubMed; (d) C. Zhai, D. Xing, C. Jing, J. Zhou, C. Wang, D. Wang and W. Hu, Org. Lett., 2014, 16, 2934 CrossRef CAS PubMed.
- For recent selected reviews on the generation of gold carbenes, see: (a) R. Dorel and A. M. Echavarren, Chem. Rev., 2015, 115, 9028 CrossRef CAS PubMed; (b) D. Qian and J. Zhang, Chem. Soc. Rev., 2015, 44, 677 RSC; (c) Y. Wang, M. E. Muratore and A. M. Echavarren, Chem. – Eur. J., 2015, 21, 7332 CrossRef CAS PubMed; (d) L. Fensterbank and M. Malacria, Acc. Chem. Res., 2014, 47, 953 CrossRef CAS PubMed; (e) C. Obradors and A. M. Echavarren, Acc. Chem. Res., 2014, 47, 902 CrossRef CAS PubMed; (f) A. S. K. Hashmi, Acc. Chem. Res., 2014, 47, 864 CrossRef CAS PubMed; (g) C. Obradors and A. M. Echavarren, Chem. Commun., 2014, 50, 16 RSC; (h) A. S. K. Hashmi, Angew. Chem., Int. Ed., 2010, 49, 5232 CrossRef CAS PubMed.
- For reviews, see: (a) Z. Zheng, Z. Wang, Y. Wang and L. Zhang, Chem. Soc. Rev., 2016 10.1039/c5cs00887e; (b) H.-S. Yeom and S. Shin, Acc. Chem. Res., 2014, 47, 966 CrossRef CAS PubMed; (c) L. Zhang, Acc. Chem. Res., 2014, 47, 877 CrossRef CAS PubMed; (d) J. Xiao and X. Li, Angew. Chem., Int. Ed., 2011, 50, 7226 CrossRef CAS PubMed.
- For recent selected examples, see: (a) Y. Wang, Z. Zheng and L. Zhang, J. Am. Chem. Soc., 2015, 137, 5316 CrossRef CAS PubMed; (b) H. Chen and L. Zhang, Angew. Chem., Int. Ed., 2015, 54, 11775 CrossRef CAS PubMed; (c) K. Ji, Z. Zheng, Z. Wang and L. Zhang, Angew. Chem., Int. Ed., 2015, 54, 1245 CrossRef CAS PubMed; (d) Z. Zheng and L. Zhang, Org. Chem. Front., 2015, 2, 1556 RSC; (e) M. Chen, Y. Chen, N. Sun, J. Zhao, Y. Liu and Y. Li, Angew. Chem., Int. Ed., 2015, 54, 1200 CrossRef CAS PubMed; (f) J. Schulz, L. Jašíková, A. Škríba and J. Roithová, J. Am. Chem. Soc., 2014, 136, 11513 CrossRef CAS PubMed; (g) D. Qian, H. Hu, F. Liu, B. Tang, W. Ye, Y. Wang and J. Zhang, Angew. Chem., Int. Ed., 2014, 53, 13751 CrossRef CAS PubMed; (h) S. N. Karad and R.-S. Liu, Angew. Chem., Int. Ed., 2014, 53, 5444 CrossRef CAS PubMed; (i) T. Wang, S. Shi, M. M. Hansmann, E. Rettenmeier, M. Rudolph and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2014, 53, 3715 CrossRef CAS PubMed; (j) T. Wang, L. Huang, S. Shi, M. Rudolph and A. S. K. Hashmi, Chem. – Eur. J., 2014, 20, 14868 CrossRef CAS PubMed; (k) T. Wang, S. Shi, M. Rudolph and A. S. K. Hashmi, Adv. Synth. Catal., 2014, 356, 2337 CrossRef CAS; (l) C. Shu, L. Li, X.-Y. Xiao, Y.-F. Yu, Y.-F. Ping, J.-M. Zhou and L.-W. Ye, Chem. Commun., 2014, 50, 8689 RSC; (m) C. Shu, L. Li, Y.-F. Yu, S. Jiang and L.-W. Ye, Chem. Commun., 2014, 50, 2522 RSC; (n) K. Ji and L. Zhang, Org. Chem. Front., 2014, 1, 34 RSC.
- For examples on the strategy by employing P,N- or P,S-bidentate ligands and slow addition of oxidants via a syringe pump, see: (a) J. Li, K. Ji, R. Zheng, J. Nelson and L. Zhang, Chem. Commun., 2014, 50, 4130 RSC; (b) K. Ji, Y. Zhao and L. Zhang, Angew. Chem., Int. Ed., 2013, 52, 6508 CrossRef CAS PubMed; (c) Y. Luo, K. Ji, Y. Li and L. Zhang, J. Am. Chem. Soc., 2012, 134, 17412 CrossRef CAS PubMed. For other examples, see: (d) V. A. Rassadin, V. P. Boyarskiy and V. Y. Kukushkin, Org. Lett., 2015, 17, 3502 CrossRef CAS PubMed; (e) M. D. Santos and P. W. Davies, Chem. Commun., 2014, 50, 6001 RSC; (f) W. He, C. Li and L. Zhang, J. Am. Chem. Soc., 2011, 133, 8482 CrossRef CAS PubMed.
- (a) P. Nösel, L. N. dos Santos Comprido, T. Lauterbach, M. Rudolph, F. Rominger and A. S. K. Hashmi, J. Am. Chem. Soc., 2013, 135, 15662 CrossRef PubMed; (b) K.-B. Wang, R.-Q. Ran, S.-D. Xiu and C.-Y. Li, Org. Lett., 2013, 15, 2374 CrossRef CAS PubMed; (c) L.-Q. Yang, K.-B. Wang and C.-Y. Li, Eur. J. Org. Chem., 2013, 2775 CrossRef CAS; (d) R. B. Dateer, K. Pati and R.-S. Liu, Chem. Commun., 2012, 48, 7200 RSC; (e) A. Mukherjee, R. B. Dateer, R. Chaudhuri, S. Bhunia, S. N. Karad and R.-S. Liu, J. Am. Chem. Soc., 2011, 133, 15372 CrossRef CAS PubMed; (f) D. Vasu, H.-H. Hung, S. Bhunia, S. A. Gawade, A. Das and R.-S. Liu, Angew. Chem., Int. Ed., 2011, 50, 6911 CrossRef CAS PubMed.
- For recent reviews on the ynamide reactivity, see: (a) G. Evano, C. Theunissen and M. Lecomte, Aldrichimica Acta, 2015, 48, 59 CAS; (b) X.-N. Wang, H.-S. Yeom, L.-C. Fang, S. He, Z.-X. Ma, B. L. Kedrowski and R. P. Hsung, Acc. Chem. Res., 2014, 47, 560 CrossRef CAS PubMed; (c) K. A. DeKorver, H. Li, A. G. Lohse, R. Hayashi, Z. Lu, Y. Zhang and R. P. Hsung, Chem. Rev., 2010, 110, 5064 CrossRef CAS PubMed; (d) G. Evano, A. Coste and K. Jouvin, Angew. Chem., Int. Ed., 2010, 49, 2840 CrossRef CAS PubMed.
- For our recent study on the gold-catalyzed tandem reactions based on ynamides, see: (a) C. Shu, Y.-H. Wang, B. Zhou, X.-L. Li, Y.-F. Ping, X. Lu and L.-W. Ye, J. Am. Chem. Soc., 2015, 137, 9567 CrossRef CAS PubMed; (b) A.-H. Zhou, Q. He, C. Shu, Y.-F. Yu, S. Liu, T. Zhao, W. Zhang, X. Lu and L.-W. Ye, Chem. Sci., 2015, 6, 1265 RSC; (c) L. Li, C. Shu, B. Zhou, Y.-F. Yu, X.-Y. Xiao and L.-W. Ye, Chem. Sci., 2014, 5, 4057 RSC; (d) F. Pan, S. Liu, C. Shu, R.-K. Lin, Y.-F. Yu, J.-M. Zhou and L.-W. Ye, Chem. Commun., 2014, 50, 10726 RSC; (e) C.-H. Shen, L. Li, W. Zhang, S. Liu, C. Shu, Y.-E. Xie, Y.-F. Yu and L.-W. Ye, J. Org. Chem., 2014, 79, 9313 CrossRef CAS PubMed.
- (a) L. Li, B. Zhou, Y.-H. Wang, C. Shu, Y.-F. Pan, X. Lu and L.-W. Ye, Angew. Chem., Int. Ed., 2015, 54, 8245 CrossRef CAS PubMed; (b) B. Zhou, L. Li and L.-W. Ye, Synlett, 2016, 493 CAS.
- F. Pan, C. Shu, Y.-F. Ping, Y.-F. Pan, P.-P. Ruan, Q.-R. Fei and L.-W. Ye, J. Org. Chem., 2015, 80, 10009 CrossRef CAS PubMed.
- For details, please see the ESI.†.
- For recent selected examples, see: (a) A. Cadu, R. A. Watile, S. Biswas, A. Orthaber, P. J. R. Sjöberg and J. S. M. Samec, Org. Lett., 2014, 16, 5556 CrossRef CAS PubMed; (b) S. Biswas, R. A. Watile and J. S. M. Samec, Chem. – Eur. J., 2014, 20, 2159 CrossRef CAS PubMed; (c) S. Biswas, C. Dahlstrand, R. A. Watile, M. Kalek, F. Himo and J. S. M. Samec, Chem. – Eur. J., 2013, 19, 17939 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6qo00169f |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2016 |