Synthesis of trifluoromethylated 3,4-dihydroquinolin-2(1H)-ones via a photo-induced radical cyclization of benzene-tethered 1,7-enynes with Togni reagent†
Yuanyuan
An
a,
Yunyan
Kuang
*a and
Jie
Wu
*ab
aDepartment of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433, China. E-mail: jie_wu@fudan.edu.cn
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China
First published on 22nd June 2016
Abstract
A photoinduced radical cyclization of benzene-tethered 1,7-enynes with Togni reagent in the presence of sodium iodide is developed. Under ultraviolet irradiation, (Z)-4-(iodomethylene)-3-(2,2,2-trifluoroethyl)-3,4-dihydroquinolin-2(1H)-ones are generated in moderate to good yields. The transformation works well without any metals or photo-redox catalysts at room temperature. During the reaction process, the reaction proceeds through a trifluoromethyl radical-triggered α,β-conjugated addition/intramolecular 6-exo cyclization/iodination, with the formation of multiple bonds. Good functional group tolerance is observed under the reaction conditions.
Introduction
Continuous efforts have been made to develop methodologies for the rapid generation of nitrogen-containing heterocycles.1 Meanwhile, there is growing interest in the incorporation of fluorinated substituents into privileged scaffolds, with the expectation of improving their biological properties.2 In particular, much attention has been paid to methods for the introduction of a trifluoromethyl group into privileged scaffolds,3 due to the unique properties of trifluoromethylated molecules. So far, many strategies have been developed and various trifluoromethyl reagents such as the Togni reagent, Umemoto's reagent, TMSCF3, or CF3SO2Na are involved in this system.4 Among the protocols for fluorination, radical processes under photoredox conditions are attractive for access to fluoro-containing molecules. Usually, the reaction affords a trifluoromethyl radical with high reactivity in the process.5,6 As part of a program for the generation of natural product-like compounds,7 we are interested in the synthesis of trifluoromethylated nitrogen-containing heterocycles and their applications in various biological evaluations. Very recently, we described an efficient synthesis of fluorinated 3,3-disubstituted 2-oxindoles through a photoinduced radical cyclization of N-arylacrylamides under ultraviolet irradiation.5d During the reaction process, no oxidant or photoredox catalyst were needed, and the trifluoromethyl radical could be generated efficiently under the conditions.Recently, 1,n-enynes have been used as convenient building blocks for the construction of diverse fused polycyclic skeletons via [2 + 2 + m] annulation reactions.8,9 The unsaturated bonds of 1,n-enynes are reactive for functionalization. Different metal catalysts have been utilized in the cyclization reactions of 1,n-enynes. For example, Li and co-workers developed an efficient and selective route to fused [6.6.6] pyran derivatives via an iron-catalyzed radical [2 + 2 + 2] annulation of benzene-linked 1,7-enynes with aldehydes.8b Additionally, 1,n-enyne cyclization reactions could also proceed well in a photocatalytic system. For instance, Li's group9a and Xia's group9b both disclosed a visible light initiated 1,5-hydride radical shift strategy starting from 1,n-enynes. Metal-free radical cyclization reactions of 1,n-enynes were also established and diverse fused polycyclic compounds could be assembled in efficient ways. Jiang and Tu reported a metal-free radical haloazidation of benzene-tethered 1,7-enynes using PhI(OAc)2 as the oxidant in the presence of NBS (or NCS, NIS).9e
Prompted by the advancement of 1,n-enyne chemistry and the photoinduced trifluoromethylation, we postulated that trifluoromethylated 3,4-dihydroquinolin-2(1H)-ones could also be generated through a photo-induced radical cyclization of benzene-tethered 1,7-enynes with the Togni reagent. We envisioned that under ultraviolet irradiation, this reaction might proceed in the absence of metal catalyst and oxidant. Therefore, we started to explore the feasibility of this proposed photoinduced trifluoromethylation of benzene-tethered 1,7-enynes.
Results and discussion
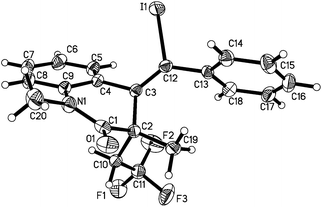
Initially, our studies focused on the model reaction of benzene-tethered 1,7-enyne 1a with Togni reagent 2 under ultraviolet irradiation at room temperature. The reaction was performed under ultraviolet irradiation (mercury lamp, 0.67 W cm−2 using a standard photo-reactor) at room temperature (see ESI†). It was found that no reaction took place when the transformation occurred in MeCN or 1,4-dioxane (Table 1, entries 1 and 2). To our delight, the desired trifluoromethylated 3,4-dihydroquinolin-2(1H)-one 3a was isolated and obtained in 26% yield when the solvent was changed to 1,2-dichloroethane (Table 1, entry 3). The structure of compound 3a was confirmed by X-ray diffraction analysis (Fig. 1). A lower yield was observed when the solvent was replaced by toluene (Table 1, entry 4). From the structure of compound 3a, it was found that iodide anions would be involved in the reaction. Thus, tetrabutylammonium iodide (TBAI) was added to the reaction and the corresponding product 3a was generated in 35% yield (Table 1, entry 5). The yield reached up to 43% when the amount of Togni reagent was increased to 1.5 equivalents (Table 1, entry 6). We further examined other iodide sources. The desired product 3a was obtained in 45% yield when the reaction occurred in the presence of 1.0 equivalent of sodium iodide (Table 1, entry 7). We assumed that 2-iodobenzoic acid would be formed in situ, which might promote the reaction. We therefore tested the reaction with the addition of 2-Iodobenzoic acid (Table 1, entry 8). Gratifyingly, the expected product 3a was obtained in 54% yield. The yield was increased to 60% when benzoic acid was used instead (Table 1, entry 9). The conversion was retarded when the reaction was performed in the dark (Table 1, entry 10). These results were promising and attractive, since the reaction occurred in the absence of any metals or photo-redox catalysts under mild conditions at room temperature.| Entry | Solvent | Additive | Yieldb (%) |
|---|---|---|---|
| a N-Methyl-N-(2-(phenylethynyl)phenyl)methacrylamide 1a (0.2 mmol), Togni reagent 2 (1.2 equiv.), solvent (4.0 mL), irradiation supplied by 600 W Hg light, rt, 12 h. b Isolated yield based on N-methyl-N-(2-(phenylethynyl)phenyl)methacrylamide 1a. c In the presence of 1.5 equiv. of Togni reagent. d The reaction was performed in the dark. | |||
| 1 | MeCN | — | nr |
| 2 | 1,4-Dioxane | — | nr |
| 3 | DCE | — | 26 |
| 4 | Toluene | — | 22 |
| 5 | DCE | TBAI | 35 |
| 6c | DCE | TBAI | 43 |
| 7c | DCE | NaI | 46 |
| 8c | DCE | 2-Iodobenzoic acid/NaI | 54 |
| 9c | DCE | Benzoic acid/NaI | 60 |
| 10c,d | DCE | Benzoic acid/NaI | Trace |
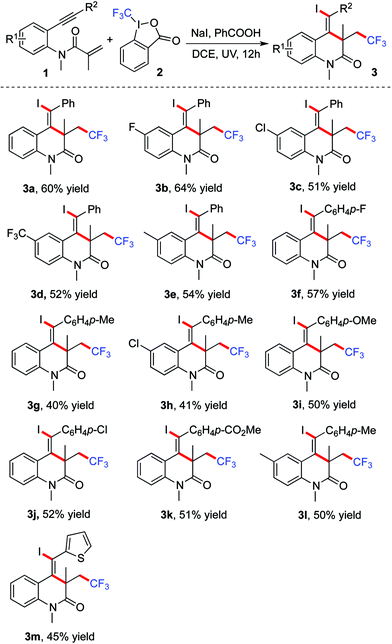
Encouraged by the above results, we next explored the scope of the reaction of benzene-tethered 1,7-enynes 1 with Togni reagent 2 in the presence of sodium iodide. The results are summarized in Table 2. Various N-(2-(alkynyl)phenyl)methacrylamides 1 were treated with Togni reagent 2 under the optimized conditions, resulting in the corresponding trifluoromethylated 3,4-dihydroquinolin-2(1H)-ones 3 in moderate yields. Both electron-rich or electron-poor benzene-tethered 1,7-enynes 1 were compatible under the standard conditions. Different functional groups were tolerated, including methyl, methoxy, chloro, fluoro, acetoxyl, and trifluoromethyl groups. For instance, N-(4-fluoro-2-(phenylethynyl)phenyl)-N-methylmethacrylamide reacted with Togni reagent 2 gave rise to the product 3b in 64% yield. Additionally, this photoinduced CF3-containing 3,4-dihydroquinolin-2(1H)-one formation was found feasible when thiophenyl-substituted alkyne 1m was utilized, providing the corresponding product 3m in 45% yield.
| a Reaction conditions: N-(2-(alkynyl)phenyl)methacrylamide 1 (0.2 mmol), Togni reagent 2 (0.3 mmol), NaI (0.2 mmol), PhCOOH (0.2 mmol), DCE (4.0 mL), N2 atmosphere, irradiation supplied by 600 W Hg light, rt, 12 h. Isolated yield is based on benzene-tethered 1,7-enyne 1. |
|---|
|
On the basis of the present results and the previous reports,5,6 we proposed a possible mechanism for the photoinduced radical trifluoromethyliodation of benzene-tethered 1,7-enynes (Scheme 1). We reasoned that the trifluoromethyl radical would be formed in the presence of the Togni reagent 2 under ultraviolet irradiation. Subsequently, the addition of the trifluoromethyl radical to the double bond of N-methyl-N-(2-(phenylethynyl)phenyl)methacrylamide 1 would afford intermediate A. Then an intramolecular radical cyclization would occur, resulting in the formation of the vinyl radical intermediate B. The following electron transfer would provide the cation intermediate C, which would combine with iodide to furnish the corresponding product 3. However, since the corresponging product 3a could also be obtained in the absence of sodium iodide (Table 1, entries 3 and 4), we postulated that another possible route could not be excluded. We reasoned that under ultraviolet irradiation, the Togni reagent 2 would provide a trifluoromethyl radical and 2-iodobenzoic acid concurrently. Recently, we and others reported that a halo radical could be generated from aryl halide via photo-induced Ar–X bond dissociation under metal-free conditions.10 Thus, an iodo radical would be formed subsequently from 2-iodobenzoic acid, which might react with intermediate B to furnish the final product 3.
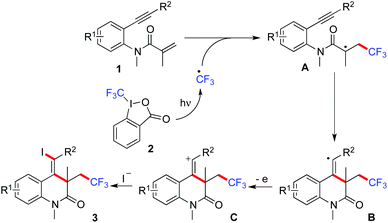 | ||
| Scheme 1 A plausible mechanism for the photoinduced radical trifluoromethyliodation of benzene-tethered 1,7-enyne 1. | ||
Conclusions
In conclusion, we have described a photoinduced radical cyclization of benzene-tethered 1,7-enynes with Togni reagent in the presence of sodium iodide. Under ultraviolet irradiation, (Z)-4-(iodomethylene)-3-(2,2,2-trifluoroethyl)-3,4-dihydroquinolin-2(1H)-ones are generated in moderate yields. The transformation works well without any metals or photo-redox catalysts at room temperature. During the reaction process, the reaction proceeds through the trifluoromethyl radical-triggered α,β-conjugated addition/intramolecular 6-exo cyclization/iodination, with the formation of multiple bonds. Good functional group tolerance is observed under the reaction conditions. Further elaboration of (Z)-4-(iodomethylene)-3-(2,2,2-trifluoroethyl)-3,4-dihydroquinolin-2(1H)-ones could be expected via cross-coupling reactions. Further trifluoromethylation of other nitrogen-containing heterocycles under ultraviolet irradiation is under exploration in our laboratory.Acknowledgements
Financial support from National Natural Science Foundation of China (no. 21372046 and 21532001) is gratefully acknowledged.Notes and references
- Selected reviews for the synthesis of heterocycles: (a) A. Armstrong and J. C. Collins, Angew. Chem., Int. Ed., 2010, 49, 2282 CrossRef CAS PubMed; (b) A. R. Katritzky and S. Rachwal, Chem. Rev., 2010, 110, 1564 CrossRef CAS PubMed; (c) M. A. P. Martins, C. P. Frizzo, D. N. Moreira, L. Buriol and P. Machado, Chem. Rev., 2009, 109, 4140 CrossRef CAS PubMed; (d) M. Alvarez-Corral, M. Munoz-Dorado and I. Rodrıguez-Garcıa, Chem. Rev., 2008, 108, 3174 CrossRef CAS PubMed; (e) A. Minatti and K. Muniz, Chem. Soc. Rev., 2007, 36, 1142 RSC; (f) G. Zeni and R. C. Larock, Chem. Rev., 2006, 106, 4644 CrossRef CAS PubMed; (g) E. Ruijter, R. Scheffelaar and R. V. A. Orru, Angew. Chem., Int. Ed., 2011, 50, 6234 CrossRef CAS PubMed.
- For recent reviews, see: (a) J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2013, 114, 2432 CrossRef PubMed; (b) T. Liang, C. N. Neumann and T. Ritter, Angew. Chem., Int. Ed., 2013, 52, 8214 CrossRef CAS PubMed; (c) C. Hollingworth and V. Gouverneur, Chem. Commun., 2012, 48, 929 RSC; (d) O. A. Tomashenko and V. V. Grushin, Chem. Rev., 2011, 111, 4475 CrossRef CAS PubMed; (e) J. Hu, W. Zhang and F. Wang, Chem. Commun., 2009, 7465 RSC; (f) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881 CrossRef PubMed; (g) J.-A. Ma and D. Cahard, Chem. Rev., 2004, 104, 6119 CrossRef CAS PubMed; (h) Organofluorine Chemistry: Principles and Commercial Applications, ed. R. E. Banks, B. E. Smart and J. C. Tatlow, Plenum Press, New York, 1994 Search PubMed; (i) Fluorine in Bioorganic Chemistry, ed. J. T. Welch and S. Eswarakrishman, Wiley, New York, 1991 Search PubMed.
- For selected examples, see: (a) T. Besset, T. Poisson and X. Pannecoucke, Chem. – Eur. J., 2014, 20, 16830 CrossRef CAS PubMed; (b) H. Egami and M. Sodeoka, Angew. Chem., Int. Ed., 2014, 53, 8294 CrossRef CAS PubMed; (c) T. Nishida, H. Ida, Y. Kuninobu and M. Kanai, Nat. Commun., 2014, 5, 3387 Search PubMed; (d) H. Liu, Z. Gu and X. Jiang, Adv. Synth. Catal., 2013, 355, 617 CrossRef CAS; (e) P. Chen and G. Liu, Synthesis, 2013, 2919 CAS; (f) T. Liu, X. Shao, Y. Wu and Q. Shen, Angew. Chem., Int. Ed., 2012, 51, 540 CrossRef CAS PubMed; (g) Y. Ye and M. S. Sanford, J. Am. Chem. Soc., 2012, 134, 9034 CrossRef CAS PubMed; (h) J. Xu, Y. Fu, D.-F. Luo, Y.-Y. Jiang, B. Xiao, Z.-J. Liu, T.-J. Gong and L. Liu, J. Am. Chem. Soc., 2011, 133, 15300 CrossRef CAS PubMed; (i) T. Furuya, A. S. Kamlet and T. Ritter, Nature, 2011, 473, 470 CrossRef CAS PubMed; (j) J. Nie, H. Guo, D. Cahard and J. Ma, Chem. Rev., 2011, 111, 455 CrossRef CAS PubMed.
- For selected examples, see: (a) C. Ni, M. Hu and J. Hu, Chem. Rev., 2015, 115, 765 CrossRef CAS PubMed; (b) X. Liu, C. Xu, M. Wang and Q. Liu, Chem. Rev., 2015, 115, 683 CrossRef CAS PubMed; (c) C. Alonso, E. M. de Marigorta, G. Rubiales and F. Palacios, Chem. Rev., 2015, 115, 1847 CrossRef CAS PubMed; (d) E. Merino and C. Nevado, Chem. Soc. Rev., 2014, 43, 6598 RSC; (e) P. Yu, J.-S. Lin, L. Li, S.-C. Zheng, Y.-P. Xiong, L.-J. Zhao, B. Tan and X.-Y. Liu, Angew. Chem., Int. Ed., 2014, 53, 11890 CrossRef CAS PubMed; (f) C. Yu, N. Iqbal, S. Park and E. J. Cho, Chem. Commun., 2014, 50, 12884 RSC; (g) X.-L. Jiang, Z.-H. Chen, X.-H. Xu and F.-L. Qing, Org. Chem. Front., 2014, 1, 774 RSC; (h) C. Matheis, K. Jouvin and L. J. Goossen, Org. Lett., 2014, 16, 5984 CrossRef CAS PubMed; (i) D. J. Wilger, N. J. Gesmundo and D. A. Nicewicz, Chem. Sci., 2013, 4, 3160 RSC; (j) G. Landelle, A. Panossian, S. Pazenok, J. Vors and F. R. Leroux, Beilstein J. Org. Chem., 2013, 9, 2476 CrossRef PubMed; (k) T. Xu and G. Liu, Org. Lett., 2012, 14, 5416 CrossRef CAS PubMed; (l) X. Yang, T. Wu, R. J. Phipps and F. D. Toste, Chem. Rev., 2015, 115, 826 CrossRef CAS PubMed; (m) X.-H. Xu, K. Matsuzaki and N. Shibata, Chem. Rev., 2015, 115, 731 CrossRef CAS PubMed; (n) L. Chu and F.-L. Qing, Acc. Chem. Res., 2014, 47, 1513 CrossRef CAS PubMed; (o) Y. A. Serguchev, M. V. Ponomarenko and N. V. Ignaťev, J. Fluorine Chem., 2016, 185, 1 CrossRef CAS; (p) T. H. Rehm, Chem. Eng. Technol., 2016, 39, 66 CrossRef; (q) Y. Zeng, C. Ni and J. Hu, Chem. – Eur. J., 2016, 22, 3210 CrossRef CAS PubMed; (r) X. Xu and F. Qing, Curr. Org. Chem., 2015, 19, 1566 CrossRef CAS; (s) C. Ni, M. Hu and J. Hu, Chem. Rev., 2015, 115, 765 CrossRef CAS PubMed; (t) Y. Lu, C. Liu and Q. Chen, Curr. Org. Chem., 2015, 19, 1638 CrossRef CAS; (u) G. K. S. Prakash and J. Hu, Acc. Chem. Res., 2007, 40, 921 CrossRef CAS PubMed; (v) C. Ni and J. Hu, Synthesis, 2014, 842 Search PubMed; (w) X. Shen and J. Hu, Eur. J. Org. Chem., 2014, 4437 CrossRef CAS; (x) Z. Hang, Z. Li and Z.-Q. Liu, Org. Lett., 2014, 16, 3648 CrossRef CAS PubMed.
- For selected examples, see: (a) A. Carboni, G. Dagousset, E. Magnier and G. Masson, Org. Lett., 2014, 16, 1240 CrossRef CAS PubMed; (b) S. P. Pitre, C. D. McTiernan, H. Ismaili and J. C. Scaiano, ACS Catal., 2014, 4, 2530 CrossRef CAS; (c) J. Xie, X. Yuan, A. Abdukader, C. Zhu and J. Ma, Org. Lett., 2014, 16, 1768 CrossRef CAS PubMed; (d) Y. An, Y. Li and J. Wu, Org. Chem. Front., 2016, 3, 570 RSC; (e) F. Gao, C. Yang, G. Gao, L. Zheng and W. Xia, Org. Lett., 2015, 17, 3478 CrossRef CAS PubMed; (f) R. Tomita, Y. Yasu, T. Koike and M. Akita, Angew. Chem., Int. Ed., 2014, 53, 7144 CrossRef CAS PubMed; (g) L. Li, Q. Chen and Y. Guo, J. Fluorine Chem., 2014, 167, 79 CrossRef CAS; (h) Y. Yasu, T. Koike and M. Akita, Chem. Commun., 2013, 49, 2037 RSC; (i) P. Xu, A. Abdukader, K. Hu, Y. Cheng and C. Zhu, Chem. Commun., 2014, 50, 2308 RSC; (j) S. Mizuta, K. M. Engle, S. Verhoog, O. Galicia-López, M. O'Duill, M. Médebielle, K. Wheelhouse, G. Rassias, A. L. Thompson and V. Gouverneur, Org. Lett., 2013, 15, 1250 CrossRef CAS PubMed; (k) R. Beniazza, F. Molton, C. Duboc, A. Tron, N. D. McClenaghan, D. Lastécouèresa and J.-M. Vincent, Chem. Commun., 2015, 51, 9571 RSC; (l) T. Koike, Y. Yasu and M. Akita, Angew. Chem., Int. Ed., 2012, 51, 9567 CrossRef PubMed; (m) S. Mizuta, S. Verhoog, K. M. Engle, T. Khotavivattana, M. O'Duill, K. Wheelhouse, G. Rassias, M. Medebielle and V. Gouverneur, J. Am. Chem. Soc., 2013, 135, 2505 CrossRef CAS PubMed; (n) Y. Yasu, T. Koike and M. Akita, Org. Lett., 2013, 15, 2136 CrossRef CAS PubMed; (o) A. Carboni, G. Dagousset, E. Magnier and G. Masson, Chem. Commun., 2014, 50, 14197 RSC; (p) Q. Deng, J. Chen, Q. Wei, Q. Zhao, L. Lu and W. Xiao, Chem. Commun., 2015, 51, 3537 RSC; (q) N. Noto, K. Miyazawa, T. Koike and M. Akita, Org. Lett., 2015, 17, 3710 CrossRef CAS PubMed; (r) Y. Yasu, Y. Arai, R. Tomita, T. Koike and M. Akita, Org. Lett., 2014, 16, 780 CrossRef CAS PubMed; (s) Q. Wei, J. Chen, X. Hu, X. Yang, B. Lu and W. Xiao, Org. Lett., 2015, 17, 4464 CrossRef CAS PubMed; (t) Q. Lin, X. Xu and F. Qing, J. Org. Chem., 2014, 79, 10434 CrossRef CAS PubMed; (u) R. Tomita, Y. Yasu, T. Koike and M. Akita, Beilstein J. Org. Chem., 2014, 10, 1099 CrossRef PubMed; (v) M. Asano, R. Tomita, T. Koike and M. Akita, J. Fluorine Chem., 2015, 185, 83 CrossRef; (w) A. Sahoo, J. Li and F. Glorius, Angew. Chem., Int. Ed., 2015, 54, 11577 CrossRef PubMed; (x) S. B. Woo and D. Y. Kim, J. Fluorine Chem., 2015, 178, 214 CrossRef CAS.
- For selected examples, see: (a) Y. Cheng, X. Yuan, H. Jiang, R. Wang, J. Ma, Y. Zhang and S. Yu, Adv. Synth. Catal., 2014, 356, 2859 CrossRef CAS; (b) H. Jiang, C. Huang, J. Guo, C. Zeng, Y. Zhang and S. Yu, Chem. – Eur. J., 2012, 18, 15158 CrossRef CAS PubMed; (c) S. H. Oh, Y. R. Malpani, N. Ha, Y. Jung and S. B. Han, Org. Lett., 2014, 16, 1310 CrossRef CAS PubMed; (d) X. Tang and W. R. Dolbier Jr., Angew. Chem., Int. Ed., 2015, 54, 4246 CrossRef CAS PubMed; (e) D. B. Bagal, G. Kachkovskyi, M. Knorn, T. Rawner, B. M. Bhanage and O. Reiser, Angew. Chem., Int. Ed., 2015, 54, 6999 CrossRef CAS PubMed; (f) L. Zhu, L. Wang, B. Li, B. Fu, C. Zhang and W. Li, Chem. Commun., 2016, 52, 6371 RSC; (g) X. Tang, C. S. Thomoson and W. R. Dolbier Jr., Org. Lett., 2014, 16, 4594 CrossRef CAS PubMed; (h) L. Zheng, C. Yang, Z. Xu, F. Gao and W. Xia, J. Org. Chem., 2015, 80, 5730 CrossRef CAS PubMed; (i) H. Jiang, Y. Cheng, Y. Zhang and S. Yu, Eur. J. Org. Chem., 2013, 5485 CrossRef CAS; (j) L. Cui, Y. Matusaki, N. Tada, T. Miura, B. Uno and A. Itoha, Adv. Synth. Catal., 2013, 355, 2203 CrossRef CAS; (k) L. Li, X. Mu, W. Liu, Y. Wang, Z. Mi and C. Li, J. Am. Chem. Soc., 2016, 138, 5809 CrossRef CAS PubMed; (l) M. Baar and S. Blechert, Chem. – Eur. J., 2015, 21, 526 CrossRef CAS PubMed; (m) Q. Lefebvre, N. Hoffmann and M. Rueping, Chem. Commun., 2016, 52, 2493 RSC; (n) L. Li, M. Huang, C. Liu, J. Xiao, Q. Chen, Y. Guo and Z. Zhao, Org. Lett., 2015, 17, 4714 CrossRef CAS PubMed; (o) N. Iqbal, J. Jung, S. Park and E. J. Cho, Angew. Chem., Int. Ed., 2014, 53, 539 CrossRef CAS PubMed; (p) S. Park, J. M. Joo and E. J. Cho, Eur. J. Org. Chem., 2015, 4093 CrossRef CAS; (q) N. J. W. Straathof, B. J. P. Tegelbeckers, V. Hessel, X. Wang and T. Noël, Chem. Sci., 2014, 5, 4768 RSC; (r) J. Sun, X. Peng and H. Guo, Tetrahedron Lett., 2015, 56, 797 CrossRef CAS.
- For selected reviews, see: (a) Y. Luo, X. Pan, X. Yu and J. Wu, Chem. Soc. Rev., 2014, 43, 834 RSC; (b) G. Qiu, Y. Kuang and J. Wu, Adv. Synth. Catal., 2014, 356, 3483 CrossRef CAS; (c) L. He, H. Nie, G. Qiu, Y. Gao and J. Wu, Org. Biomol. Chem., 2014, 12, 9045 RSC; (d) G. Qiu, Q. Ding and J. Wu, Chem. Soc. Rev., 2013, 42, 5257 RSC; (e) G. Qiu and J. Wu, Chem. Rec., 2016, 16, 19 CrossRef CAS PubMed.
- For selected examples, see: (a) X.-H. Ouyang, R.-J. Song, Y. Liu, M. Hu and J.-H. Li, Org. Lett., 2015, 17, 6038 CrossRef CAS PubMed; (b) L. Lv and Z. Li, Org. Lett., 2016, 18, 2264 CrossRef CAS PubMed; (c) Y. Liu, J.-L. Zhang, M.-B. Zhou, R.-J. Song and J.-H. Li, Chem. Commun., 2014, 50, 14412 RSC; (d) M. Hu, R.-J. Song, X.-H. Ouyang, F.-L. Tan, W.-T. Wei and J.-H. Li, Chem. Commun., 2016, 52, 3328 RSC; (e) J.-K. Qiu, B. Jiang, Y.-L. Zhu, W.-J. Hao, D.-C. Wang, J. Sun, P. Wei, S.-J. Tu and G. Li, J. Am. Chem. Soc., 2015, 137, 8928 CrossRef CAS PubMed; (f) Y. Liu, J.-L. Zhang, R.-J. Song and J.-H. Li, Org. Lett., 2014, 16, 5838 CrossRef CAS PubMed; (g) L.-Z. Yu, Q. Xu, X.-Y. Tang and M. Shi, ACS Catal., 2016, 6, 526 CrossRef CAS.
- For selected examples, see: (a) Y. Li, B. Liu, R.-J. Song, Q.-A. Wang and J.-H. Li, Adv. Synth. Catal., 2016, 358, 1219 CrossRef CAS; (b) F. Gao, C. Yang, N. Ma, G.-L. Gao, D. Li and W. Xia, Org. Lett., 2016, 18, 600 CrossRef CAS PubMed; (c) Y. Liu, J.-L. Zhang, R.-J. Song, P.-C. Qian and J.-H. Li, Angew. Chem., Int. Ed., 2014, 53, 9017 CrossRef CAS PubMed; (d) M. Hu, J.-H. Fan, Y. Liu, X.-H. Ouyang, R.-J. Song and J.-H. Li, Angew. Chem., Int. Ed., 2015, 54, 9577 CrossRef CAS PubMed; (e) A.-F. Wang, Y.-L. Zhu, S.-L. Wang, W.-J. Hao, G. Li, S.-J. Tu and B. Jiang, J. Org. Chem., 2016, 81, 1099 CrossRef CAS PubMed; (f) Y. Li, M. Sun, H. Wang, Q. Tian and S. Yang, Angew. Chem., Int. Ed., 2013, 52, 3972 CrossRef CAS PubMed; (g) M. Zhou, R. Song, X. Ouyang, Y. Liu, W. Wei, G. Deng and J. Li, Chem. Sci., 2013, 4, 2690 RSC; (h) W. Wei, M. Zhou, J. Fan, W. Liu, R. Song, Y. Liu, M. Hu, P. Xie and J. Li, Angew. Chem., Int. Ed., 2013, 52, 3638 CrossRef CAS PubMed; (i) L. Zhang, Z. Li and Z.-Q. Liu, Org. Lett., 2014, 16, 3688 CrossRef CAS PubMed.
- For selected examples, see: (a) W. Liu, L. Li and C.-J. Li, Nat. Commun., 2015, 6, 6526 CrossRef CAS PubMed; (b) L. Li, W. Liu, H. Zeng, X. Mu, G. Cosa, Z. Mi and C.-J. Li, J. Am. Chem. Soc., 2015, 137, 8328 CrossRef CAS PubMed; (c) J. Ruch, A. Aubin, G. Erbland, A. Fortunato and J.-P. Goddard, Chem. Commun., 2016, 52, 2326 RSC; (d) Y. Li, D. Zheng, Z. Li and J. Wu, Org. Chem. Front., 2016, 3, 574 RSC; (e) Y. An, Y. Li and J. Wu, Org. Chem. Front., 2016, 3, 570 RSC; (f) G. Qiu, Y. Li and J. Wu, Org. Chem. Front., 2016, 3 10.1039/C6QO00103C.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure, characterization data, 1H & 13C NMR spectra of compound 3. CCDC 1477485. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00267f |
| This journal is © the Partner Organisations 2016 |