A new method to access triazole-fused spiro-guanidines from the reaction of isothiocyanates tethered N-sulfonyl-1,2,3-triazoles and amines†
Hou-Lu
Liu
a,
Yu
Jiang
b,
Jian
Hao
*a,
Xiang-Ying
Tang
*b and
Min
Shi
*b
aDepartment of Chemistry, Shanghai University, 99 Shangda Road, Shanghai 200444, China. E-mail: jhao@shu.edu.cn
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 354 Fenglin Road, Shanghai 200032, China. E-mail: siocxiangying@mail.sioc.ac.cn; mshi@mail.sioc.ac.cn; Fax: +86-21-64166128
First published on 22nd August 2016
Abstract
A novel method to access triazole-fused spiro-guanidine from the reaction of isothiocyanate tethered N-sulfonyl-1,2,3-triazoles and amine has been developed. The reactions proceeded through a two-component tandem reaction, including nucleophilic addition, base-assisted intramolecular (or intermolecular) nucleophilic substitution and subsequent intramolecular nucleophilic addition–elimination process. A wide range of protected oxindole skeletons and amines are well-tolerated.
Spiro-oxindole skeletons often show a broad range of biological activities in a large number of natural products and clinical pharmaceutical ingredients1 and have attracted considerable research attention in the recent decades. On the other hand, guanidine-containing moiety is a ubiquitous structure in numerous functional materials and compounds. The guanidinium ions in GPNA (Guanidine-Based Peptide Nucleic Acids) play an important role in binding to RNA with high affinity and sequence selectivity.2 In a family of guanidinium toxins-saxitoxin, neosaxitoxin, and gonyautoxins, bis-guanidinium structures are unique in both their forms and functions.3 Moreover, guanidine-containing compounds also can be found in drugs,4 organic catalysts,5 surfactants,6 ionic liquids7 and other domains8 due to their basicity and hydrogen-bonding properties. Thus, developing a new method to synthesize guanidines with high efficiency under mild conditions to meet different needs of the pharmaceutical, agrochemical, and chemical industry is of tremendous significance.
1,2,3-Triazoles are very important structural motifs found in a broad range of applications in medicinal chemistry,9 biochemistry,10 as well as materials science,11 and synthetic organic chemistry.12 During the last ten years, triazole chemistry has been widely explored based on electron-deficient N-sulfonyl-1,2,3-triazoles for the fact that such molecules can generate aza-vinyl carbenes towards the synthesis of numerous N-containing heterocycles.13 In sharp contrast, reactions based on modification of triazole core itself are less studied. This is because of the fact that most researchers have focused on building triazoles using click chemistry that have been reported in the literature hitherto.
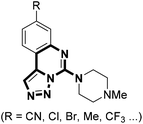
It is proposed that triazole can serve as a bioisostere due to the similarities in both electronic and spacial characteristics of the amide bond; therefore, as a bioisosteric replacement for the amide moiety, triazole is frequently introduced into those amide-containing drugs to facilitate SAR analysis.14 Moreover, triazoles are metabolically stable to hydrolysis and have different hydrogen bonding capabilities as compared to their amide counterparts.15 Despite the fact that the construction of triazole is convenient by click chemistry, sometimes difficulties stem from the synthesis of the azide part bearing special functional groups of interest. To this regard, there is an increasing need for novel modifications based on the triazole core itself. Previously, we developed a base-induced reaction of N-sulfonyl-1,2,3-triazoles with protected methanediamines, affording facile access to N-dialkylaminomethyl-2H-1,2,3-triazoles.16 To continue our research interest, we envisaged that the combination of guanidine and triazole might offer a chance to build structurally novel scaffold possessing potential biological activities. Herein, we wish to report a novel reaction of isothiocyanate tethered N-sulfonyl-1,2,3-triazoles and amines to rapidly construct triazole-fused spiro-guanidine derivatives. To our delight, the triazole-fused guanidine motif is also found in biologically active compounds such as inhibitors of histamine receptors (Fig. 1).17
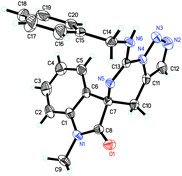
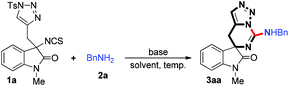
We initially utilized isothiocyanate tethered N-sulfonyl-1,2,3-triazoles 1a (0.10 mmol, 1.0 equiv.) and benzylamine 2a (0.10 mmol, 1.0 equiv.) as the substrates and carried out the reaction in toluene at 120 °C to evaluate the reaction progress (Table 1, entry 1). However, it is noteworthy that only trace amount of product was detected. Interestingly, upon addition of K2CO3 (0.20 mmol, 2.0 equiv.), the reaction could furnish the corresponding product 3aa in 42% yield (entry 2), and its structure has been unambiguously determined by X-ray diffraction (Fig. 2).21 Then, the solvent effect was tested, and it was found that the reactions went on smoothly in 1,4-dioxane, 1,3-xylene and acetonitrile; however, acetonitrile is more suitable for this reaction (entries 3–5). Next, based on the understanding of the importance of a base in this reaction, the amount of base to be employed was investigated. Notably, when the amount of base was decreased to 1.0 equivalent, the yield of 3aa increased to 74% (entry 6). Subsequently, we examined different bases such as Na2CO3, Cs2CO3 and CsOAc, and identified that K2CO3 gave the best result (entries 7–10). Moreover, when the temperature of the reaction was lowered, the yield of 3aa decreased slightly (entries 11 and 12). When the concentration of the reaction mixture was doubled or reduced by half, inferior results were obtained (entries 13 and 14). Finally, it was determined that the best reaction condition is entry 6 in Table 1, giving 3aa in 74% isolated yield (see ESI† for more details).
| Entrya | 1a (equiv.) | 2a (equiv.) | Base (equiv.) | Temp. (°C) | Solvent | 3aa |
|---|---|---|---|---|---|---|
| Yieldb,c (%) | ||||||
| a Reaction conditions: 1a (0.10 mmol), 2a (0.10 mmol), base, in solvent (1.0 mL) at the indicated temperature for 24 h under an Ar atmosphere. b Crude yields were determined by 1H NMR spectroscopy (internal standard: 1, 3, 5-trimethoxybenzene). c Isolated yields are shown in parentheses. d The solvent is 0.5 mL. e The solvent is 2.0 mL. | ||||||
| 1 | 1.0 | 1.0 | — | 120 | Toluene | Trace |
| 2 | 1.0 | 1.0 | K2CO3 (2.0) | 120 | Toluene | 42 |
| 3 | 1.0 | 1.0 | K2CO3 (2.0) | 120 | 1,4-Dioxane | 45 |
| 4 | 1.0 | 1.0 | K2CO3 (2.0) | 120 | 1,3-Xylene | 39 |
| 5 | 1.0 | 1.0 | K2CO3 (2.0) | 120 | CH3CN | 47 |
| 6 | 1.0 | 1.0 | K 2 CO 3 (1.0) | 120 | CH3CN | 74 (74) |
| 7 | 1.0 | 1.0 | Na2CO3 (1.0) | 120 | CH3CN | 14 |
| 8 | 1.0 | 1.0 | Cs2CO3 (1.0) | 120 | CH3CN | 34 |
| 9 | 1.0 | 1.0 | CsOAc (2.0) | 120 | CH3CN | Trace |
| 10 | 1.0 | 1.0 | K3PO4 (0.67) | 120 | CH3CN | 65 (63) |
| 11 | 1.0 | 1.0 | K2CO3 (1.0) | 100 | CH3CN | 67 (65) |
| 12 | 1.0 | 1.0 | K2CO3 (1.0) | 80 | CH3CN | 64 (63) |
| 13d | 1.0 | 1.0 | K2CO3 (1.0) | 120 | CH3CN | 67 (65) |
| 14e | 1.0 | 1.0 | K2CO3 (1.0) | 120 | CH3CN | 60 (56) |
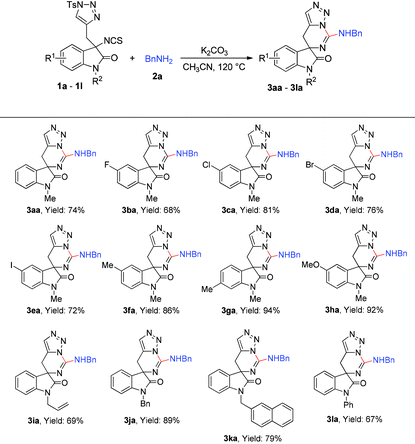
With the optimized condition in hand, the scope of the reaction with regard to substrates 1 and substrates 2 was investigated. As shown in Table 2, when 5- or 6-substituted 1 was used to react with 2a under standardised conditions, the corresponding spiro-guanidines were afforded in good to excellent yields (3ba–3ha). Next, we paid our attention to investigate the N-protecting group of oxindole by changing the protecting group from methyl to allyl, benzyl, naphthalen-2-ylmethyl and phenyl. To our delight, these reactions went on smoothly to afford the desired products 3ia–3la in good yields.
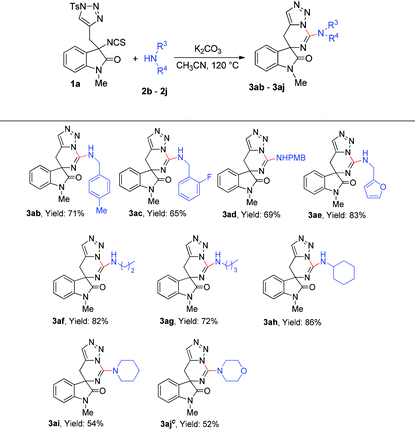
We next turned our interest to determine the scope of substrate 2 (Table 3). First, benzyl amines with para-methyl, para-methoxy and ortho-fluoro substituents on the benzene ring were employed and the reactions gave the corresponding products 3ab–3ad in moderate yields. Notably, furan-2-ylmethanamine was also compatible in the reaction and furnished the desired product 3ae in 83% yield. Then, several aliphatic amines were also examined in this reaction, and we found that the reactions of all the primary amines went through very well to give the corresponding products 3af–3ah in good yields. While secondary amines were treated under the standard reaction conditions, the desired products 3ai–3aj were afforded in relatively low yields, which could probably be reasoned for their steric hindrance.
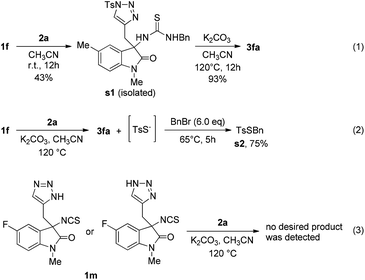
To clarify the possible pathway of this reaction, several controlled experiments were conducted (Scheme 1). When 1f reacted with 2a in acetonitrile at room temperature, a thiourea intermediate s1 was obtained, and it could be converted into the desired product 3fa under the optimised conditions (Scheme 1, eqn (1)). In addition, the formation of s2 revealed that the proposed intermediate TsSNa is formed in the reaction (Scheme 1, eqn (2)). Moreover, when we used unprotected triazole 1m to react with 2a, no desired product was detected, indicating that the N-Ts group is necessary for the reaction to be successful.
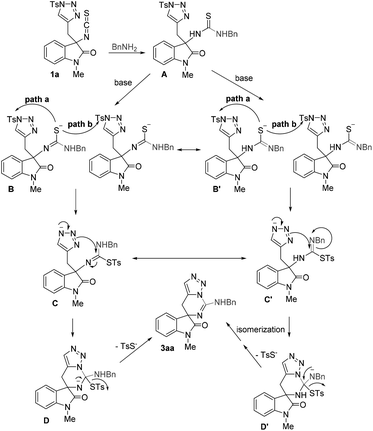
Based on the abovementioned results and the previous works,18–20 a possible mechanism has been outlined in Scheme 2. At the beginning, the nucleophilic attack of amine to the isothiocyanate moiety affords the thiourea intermediate A. Then, the intermediate A undergoes deprotonation and isomerization initiated by a base to give intermediate B (or B′), which subsequently proceeds through an intramolecular (path a) or intermolecular (path b) nucleophilic attack to the tosyl group of triazole to give intermediate C (or C′). Finally, the release of 4-methylbenzenesulfonothioate through an intramolecular nucleophilic addition–elimination process via intermediate D (or D′) accompanied with the ring closure affords the target molecule 3. The release of TsS– may provide a driving force for this tandem process.
In conclusion, we developed a novel method to access triazole-fused guanidine derivatives from the reaction of isothiocyanate tethered N-sulfonyl-1,2,3-triazoles and amines. The reactions proceeded through a two-component tandem reaction, including nucleophilic addition, base-assisted intramolecular (or intermolecular) nucleophilic substitution and subsequent intramolecular nucleophilic addition–elimination process. A wide range of protected oxindole skeletons and amines are well-tolerated in the reaction to afford the desired products in good to excellent yields. Further investigations to extend the substrate scope as well as more intensive mechanistic studies are currently in progress.
Acknowledgements
This study was supported by the Joint NSFC-ISF Research Program and jointly funded by the National Natural Science Foundation of China and the Israel Science Foundation. We are also grateful for the financial support from the National Basic Research Program of China (973)-2015CB856603 and the National Natural Science Foundation of China (20472096, 21372241, 21361140350, 20672127, 21421091, 21372250, 21121062, 21302203, 20732008, 21572052 and 21172141).Notes and references
- (a) H. B. Yang, Y. Z. Zhao, R. Sang, Y. Wei and M. Shi, Adv. Synth. Catal., 2014, 356, 3799 CrossRef CAS; (b) H. B. Yang, X. Y. Guan, Y. Wei and M. Shi, Eur. J. Org. Chem., 2012, 2792 Search PubMed; (c) Z. H. Li, N. Sharma, U. K. Sharma, J. Jacobs, L. V. Meerveltb and E. V. V. Eycken, Chem. Commun., 2016, 52, 5516 RSC; (d) B. Tan, G. Hernández-Torres and C. F. Barbas(III), J. Am. Chem. Soc., 2011, 133, 12354 CrossRef CAS PubMed; (e) J. Xu, L. D. Shao, D. S. Li, X. Deng, Y. C. Liu, Q. S. Zhao and C. F. Xia, J. Am. Chem. Soc., 2014, 136, 17962 CrossRef CAS PubMed; (f) S. Diethelm and E. M. Carreira, J. Am. Chem. Soc., 2015, 137, 6084 CrossRef CAS PubMed; (g) E. V. Mercado-Marin and R. Sarpong, Chem. Sci., 2015, 6, 5048 RSC; (h) Q. Wang, Q. Xu and M. Shi, Org. Chem. Front., 2015, 2, 211 RSC; (i) Q. Wang, Z. Lian, Q. Xu and M. Shi, Adv. Synth. Catal., 2013, 355, 3344 CrossRef CAS.
- A. Dragulescu-Andrasi, P. Zhou, G. F. He and D. H. Ly, Chem. Commun., 2005, 244 RSC.
- (a) M. V. Laycock, P. Thibault, S. W. Ayer and J. A. Walter, Nat. Toxins, 1994, 2, 175 CrossRef CAS PubMed; (b) L. E. Llewellyn, Nat. Prod. Rep., 2006, 23, 200 RSC; (c) A. Hoehne, D. Behera, W. H. Parsons, M. L. James, B. Shen, P. Borgohain, D. Bodapati, A. Prabhakar, S. S. Gambhir, D. C. Yeomans, S. Biswal, F. T. Chin and J. D. Bois, J. Am. Chem. Soc., 2013, 135, 18012 CrossRef CAS PubMed; (d) Y. Shimizu, C. P. Hsu, W. E. Fallon, Y. Oshima, I. Miura and K. Nakanishi, J. Am. Chem. Soc., 1978, 100, 6791 CrossRef CAS; (e) J. V. Mulcahy and J. D. Bois, J. Am. Chem. Soc., 2008, 130, 12630 CrossRef CAS PubMed.
- (a) Y. Yang, Z. J. Xu, Z. Y. Zhang, Z. Yang, Y. T. Liu, J. Wang, T. T. Cai, S. J. Li, K. X. Chen, J. Y. Shi and W. L. Zhu, J. Phys. Chem. B, 2015, 119, 11988 CrossRef CAS PubMed; (b) H. Gunaydin, ACS Med. Chem. Lett., 2016, 7, 89 CrossRef CAS PubMed; (c) T. Vlaar, R. C. Cioc, P. Mampuys, B. U. W. Maes, R. V. A. Orru and E. Ruijter, Angew. Chem., Int. Ed., 2012, 51, 13058 CrossRef CAS PubMed; (d) S. M. Derayea, M. A. Omar, I. M. Mostafa and M. A. Hammad, RSC Adv., 2015, 5, 78920 RSC.
- (a) Y. C. Zhang, Q. N. Zhu, X. Yang, L. J. Zhou and F. Shi, J. Org. Chem., 2016, 81, 1681 CrossRef CAS PubMed; (b) A. Noble and J. C. Anderson, Chem. Rev., 2013, 113, 2887 CrossRef CAS PubMed; (c) T. Ma, X. Fu, C. W. Kee, L. L. Zong, Y. H. Pan, K. W. Huang and C. H. Tan, J. Am. Chem. Soc., 2011, 133, 2828 CrossRef CAS PubMed; (d) A. Kondoh, M. Oishi, T. Takeda and M. Terada, Angew. Chem., Int. Ed., 2015, 54, 15836 CrossRef CAS PubMed; (e) Y. Zhu, X. H. Liu, S. X. Dong, Y. H. Zhou, W. Li, L. L. Lin and X. M. Feng, Angew. Chem., Int. Ed., 2014, 53, 1636 CrossRef CAS PubMed; (f) Y. Tang, Q. G. Chen, X. H. Liu, G. Wang, L. L. Lin and X. M. Feng, Angew. Chem., Int. Ed., 2015, 54, 9512 CrossRef CAS PubMed.
- (a) A. Hirano, T. Tanaka, H. Kataura and T. Kameda, Chem. – Eur. J., 2014, 20, 4922 CrossRef CAS PubMed; (b) K. A. Schug and W. Lindner, Chem. Rev., 2005, 105, 67 CrossRef CAS PubMed; (c) M. Miyake, K. Yamada and N. Oyama, Langmuir, 2008, 24, 8527 CrossRef CAS PubMed.
- (a) X. X. Lu, J. Yu, J. Z. Wu, Y. S. Guo, H. J. Xie and W. J. Fang, J. Phys. Chem. B, 2015, 119, 8054 CrossRef CAS PubMed; (b) P. A. Hunt, C. R. AshwoYrth and R. P. Matthews, Chem. Soc. Rev., 2015, 44, 1257 RSC; (c) R. Bouchal, B. Prelot and P. Hesemann, RSC Adv., 2016, 6, 39125 RSC; (d) K. Goossens, K. Lava, C. W. Bielawski and K. Binnemans, Chem. Rev., 2016, 116, 4643 CrossRef CAS PubMed.
- (a) J. He, F. W. Kimani and J. C. Jewett, J. Am. Chem. Soc., 2015, 137, 9764 CrossRef CAS PubMed; (b) C. McKeever, M. Kaiser and I. Rozas, J. Med. Chem., 2013, 56, 700 CrossRef CAS PubMed.
- (a) H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004 CrossRef CAS; (b) P. Norris, Curr. Top. Med. Chem., 2008, 8, 101 CrossRef CAS PubMed; (c) B. L. Wilkinson, L. F. Bornaghi, T. A. Houston, A. Innocenti, D. Vullo, C. T. Supuran and S. A. Poulsen, J. Med. Chem., 2007, 50, 1651 CrossRef CAS PubMed; (d) S. K. De, J. L. Stebbins, L. H. Chen, M. Riel-Mehan, T. Machleidt, R. Dahl, H. Yuan, A. Emdadi, E. Barile, V. Chen, R. Murphy and M. Pellecchia, J. Med. Chem., 2009, 52, 1943 CrossRef CAS PubMed; (e) A. D. Moorhouse, A. M. Santos, M. Gunaratnam, M. Moore, S. Neidle and J. E. Moses, J. Am. Chem. Soc., 2006, 128, 15972 CrossRef CAS PubMed; (f) L. V. Lee, M. L. Mitchell, S. J. Huang, V. V. Fokin, K. B. Sharpless and C. H. Wong, J. Am. Chem. Soc., 2003, 125, 9588 CrossRef CAS PubMed.
- (a) D. S. Pedersen and A. Abell, Eur. J. Org. Chem., 2011, 2399 CrossRef CAS; (b) M. Zanda, New J. Chem., 2004, 28, 1401 RSC; (c) A. Volonterio, S. Bellosta, F. Bravin, M. C. Bellucci, L. Bruche, G. Colombo, L. Malpezzi, S. Mazzini, S. V. Meille, M. Meli, C. Ramirez de Arellano and M. Zanda, Chem. – Eur. J., 2003, 9, 4510 CrossRef CAS PubMed; (d) C. W. Tornoe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057 CrossRef CAS PubMed; (e) N. G. Angelo and P. S. Arora, J. Am. Chem. Soc., 2005, 127, 17134 CrossRef CAS PubMed; (f) Y. Angell and K. Burgess, J. Org. Chem., 2005, 70, 9595 CrossRef CAS PubMed; (g) K. Oh and Z. Guan, Chem. Commun., 2006, 3069 RSC.
- (a) B. Helms, J. L. Mynar, C. J. Hawker and J. M. J. Frechet, J. Am. Chem. Soc., 2004, 126, 15020 CrossRef CAS PubMed; (b) P. Wu, A. K. Feldman, A. K. Nugent, C. J. Hawker, A. Scheel, B. Voit, J. Pyun, M. J. Frechet, K. B. Sharpless and V. V. Fokin, Angew. Chem., Int. Ed., 2004, 43, 3928 CrossRef CAS PubMed.
- For selected reviews: (a) B. Chattopadhyay and V. Gevorgyan, Angew. Chem., Int. Ed., 2012, 51, 862 CrossRef CAS PubMed; (b) A. V. Gulevich and V. Gevorgyan, Angew. Chem., Int. Ed., 2013, 52, 1371 CrossRef CAS PubMed; (c) H. M. L. Davie and J. S. Alford, Chem. Soc. Rev., 2014, 43, 5151 RSC; (d) Y. Wang, X. Lei and Y. Tang, Synlett, 2015, 2051 Search PubMed; (e) P. Anbarasan, D. Yadagiri and S. Rajasekar, Synthesis, 2014, 3004 CrossRef CAS; (f) S. C. Hockey and L. C. Henderson, Aust. J. Chem., 2015, 68, 1796 CrossRef CAS.
- (a) R. Sun, Y. Jiang, X. Y. Tang and M. Shi, Chem. – Eur. J., 2016, 22, 5727 CrossRef CAS PubMed; (b) X. Cheng, Y. H. Yu, Z. F. Mao, J. X. Chen and X. L. Huang, Org. Biomol. Chem., 2016, 14, 3878 RSC; (c) Y. Jiang, X. Y. Tang and M. Shi, Chem. Commun., 2015, 51, 2122 RSC; (d) T. Miura, Y. Funakoshi and M. Murakami, J. Am. Chem. Soc., 2014, 136, 2272 CrossRef CAS PubMed; (e) T. Horneff, S. Chuprakov, N. Chernyak, V. Gevorgyan and V. V. Fokin, J. Am. Chem. Soc., 2008, 130, 14972 CrossRef CAS PubMed; (f) Y. S. Zhang, X. Y. Tang and M. Shi, Chem. Commun., 2014, 50, 15971 RSC; (g) Y. S. Zhang, X. Y. Tang and M. Shi, Chem. Commun., 2014, 50, 15971 RSC.
- (a) S. Kun, É. Bokor, G. Varga, B. Szocs, A. Páhi, K. Czifrák, M. Tóth, L. Juhász, T. Docsa, P. Gergely and L. Somsák, Eur. J. Med. Chem., 2014, 76, 567 CrossRef CAS PubMed; (b) M. Chemama, M. Fonvielle, M. Arthur, J.-M. Valéry and M. Etheve-Quelquejeu, Chem. – Eur. J., 2009, 15, 1929 CrossRef CAS PubMed; (c) É. Bokor, T. Docsa, P. Gergely and L. Somsák, ACS Med. Chem. Lett., 2013, 4, 612 CrossRef PubMed; (d) D. J. Lee, H. S. Han, J. Shin and E. J. Yoo, J. Am. Chem. Soc., 2014, 136, 11606 CrossRef CAS PubMed.
- (a) J. M. Holub and K. Kirshenbaum, Chem. Soc. Rev., 2010, 39, 1325 RSC; (b) C. L. Charron, J. L. Hickey, T. K. Nsiama, D. R. Cruickshank, W. L. Turnbull and L. G. Luyt, Nat. Prod. Rep., 2016, 33, 761 RSC.
- Y. Jiang, Q. Wang, R. Sun, X. Y. Tang and M. Shi, Org. Chem. Front., 2016, 3, 744 RSC.
- B. Allen, D. Robert, B. Clay, B. Daniel, G. Daniel, N. A. Stewart, H. R. Mark, K. G. Peter, Z. H. Qui, P. E. Joseph and Y. John, WO2011112731, 2011 Search PubMed.
- (a) A. V. Bogolubsky, A. Grishchenko, S. E. Pipko, A. Konovets, A. Chuprina, A. Tolmachev, A. N. Boyko, A. Chekotylo and O. Lukin, Mol. Diversity, 2007, 17, 471 CrossRef PubMed; (b) Y. Wang, D. R. Sauer and S. W. Djuric, Tetrahedron Lett., 2009, 50, 5145 CrossRef CAS; (c) K. B. Jensen, T. M. Braxmeier, M. Demarcus, J. G. Frey and J. D. Kilburn, Chem. – Eur. J., 2002, 8, 1300 CrossRef CAS PubMed.
- T. Maki, T. Tsuritani and T. Yasukata, Org. Lett., 2014, 16, 1868 CrossRef CAS PubMed.
- (a) X. J. Ma, S. F. Pan, H. X. Wang and W. Z. Chen, Org. Lett., 2014, 16, 4554 CrossRef CAS PubMed; (b) E. J. Yoo, M. Ahlquist, S. H. Kim, I. Bae, V. V. Fokin, K. B. Sharpless and S. Chang, Angew. Chem., Int. Ed., 2007, 46, 1730 CrossRef CAS PubMed.
- The crystal data of 3aa have been given in CCDC 949993.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data of new compounds. CCDC 949993. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00304d |
| This journal is © the Partner Organisations 2016 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)