Capture of CO2 in air for 4,5-disubstituted furan-2(5H)-ones†
Fei-Hu
Cui‡
ab,
Shi-Xia
Su‡
a,
Yan-li
Xu
c,
Ying
Liang
*b,
Heng-shan
Wang
a and
Ying-Ming
Pan
*a
aState Key Laboratory for the Chemistry and Molecular Engineering of Medicinal Resources, School of Chemistry and Pharmaceutical Sciences of Guangxi Normal University, Guilin 541004, People's Republic of China. E-mail: panym2013@hotmail.com
bSchool of Life and Environmental Sciences, Guilin University of Electronic Technology, Guilin, 541004, People's Republic of China. E-mail: yingl@aliyun.com
cCollege of Pharmacy, Guilin Medical University, Guilin, 541004, People's Republic of China
First published on 5th August 2016
Abstract
A highly efficient strategy for carbon dioxide capture and utilization (CCU) was applied to the synthesis of 4,5-disubstituted furan-2(5H)-ones by a Cu(I)/base catalytic system. An aqueous morpholine solution as a superior adsorbent for the capture of carbon dioxide directly from air was recycled continuously via adsorption/desorption cycles.
The increasing consumption of fossil fuels by human society has resulted in carbon dioxide concentration rises in the atmosphere and led to global warming. Carbon capture and utilization (CCU) is a desirable pathway, in which CO2 is recycled back to functional chemicals, to tackling this problem.1–3 Although CO2 capture technologies based on ionic liquids and metal–organic framework materials have achieved significant advances,4–6 the capture of carbon dioxide directly from gas mixtures, such as exhaust gases, of burning fossil fuels with amines has attracted a large amount of attention and has been reported in recent publications.7 In the process of amine scrubbing technology, amines are selectively reacted with CO2 in the flue gas to form carbamates, which not only function as a reactant material but also act as a storage medium for carbon dioxide for furnishing hyper-pure CO2 gas to a reaction system through adsorption/desorption cycles.8,9 For example, carbamates have been used to synthesis polycarbonates and methanol.1b,10 More recently, propiolic acids, cyclic carbonates and carboxylic acids were also prepared by Hong and co-worker via a CCU strategy.8e Nevertheless, the transformation of the captured CO2 into functionalization commodity chemicals has emerged as an attractive and challenging goal.
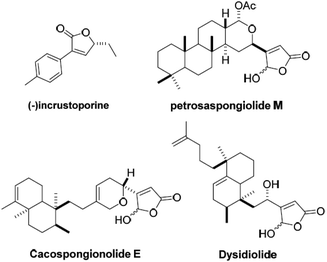
Compounds containing a furan-2(5H)-ones scaffold, such as (−)incrustoporine petrosaspongiolide M, cacospongionolide E, dysidiolide (Fig. 1),12 have been of interest for many years due to their biological activities, including antimicrobial, cytotoxic and anti-inflammatory activities.11–14 In particular, the compounds with halogen substituents on the phenyl ring, which were synthesized based on (−)incrustoporine as the lead structures, have displayed a superb antifungal effect.12a Due to the importance of furan-2(5H)-ones, a series of methods have been reported for the preparation of them, including the classical transition-metal-catalyzed cyclocarbonylation of alkynyl compounds,15 base-mediated cyclization reations16 and other elegant methods.17 In spite of these methods making a great contribution to furan-2(5H)-ones chemistry, an environmentally friendly protocol towards furan-2(5H)-ones from readily available starting materials is still highly desirable. Inspired by the overall CCU concept and based on our previous research on the reactions of unsaturated compounds18a–f and the development of carbon dioxide into valuable chemicals,18g we wish to report a strategy for using CO2 captured by aqueous morpholine solution from air to synthesis 4,5-disubstituted furan-2(5H)-ones.
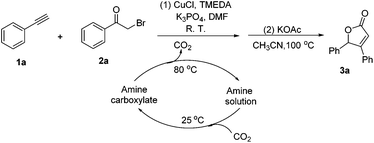
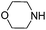
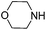
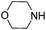
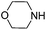
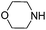
To optimize the reaction conditions, the copper(I)-catalyzed reaction of phenylacetylene, 2-bromoacetophenone and CO2 to form 4,5-disubstituted furan-2(5H)-ones was chosen as a model reaction to confirm the amount and purity of CO2 desorbing from the capture materials to convert into the target compound.16e,19 Initially, the reaction of phenylacetylene, 2-bromoacetophenone and hyper-pure CO2 (>99.999%) was directly carried out in DMF and monitored periodically by TLC. Upon completion, KOAc was added to the reaction mixture at 100 °C with stirring for another 10 h under a nitrogen atmosphere to afford the desired product in a 72% yield (Table 1, entry 1). To our delight, when the hyper-pure CO2 gas was introduced into 0.4 mol L−1 molarity of aqueous morpholine solution (100 mL) for 25 min, as a CO2 source the saturated CO2 solution worked rapidly and formed the desired product in a 70% yield (Table 1, entry 2). The investigation of various amine solutions (2,6-dimethylmorpholine, 3-methylmorpholine and ethanolamine) revealed that the aqueous morpholine solution was optimal (Table 1, entries 3–5). Further experiments demonstrated that decreasing the molarity of aqueous morpholine solution reduced the yield of 3a, but no improvement in the yield could be obtained when the molarity of the aqueous morpholine solution was increased to 0.6 mol L−1 (Table 1, entries 6–8). Moreover, even when the aqueous morpholine solution (0.4 mol L−1) was recycled 40 times through successive capture/release cycles, the isolated yield did not reduce significantly (65% yield, Table 1, entry 9). Furthermore, CO2 from ethanol combustion and air, despite its low CO2 concentration, could also be efficiently captured by morpholine. After bubbling the gas from ethanol combustion and air into the aqueous morpholine solution for 3 h 58% and 50% yields of the desired product were observed, respectively (Table 1, entries 10 and 11). Additionally, when the time of bubbling air was increased to 5 h, the yield was enhanced to 69% (Table 1, entry 12). Unfortunately, when the air was directly bubbled into the reaction mixture, no desired product was detected (Table 1, entry 13). This indicates the importance of passing through the aqueous morpholine solution to increase the CO2 concentration and purity of air. Thus, the aqueous morpholine solution (0.4 mol L−1) was chosen as the optimal CO2 capturing solution. Next, a variety of bases and solvents were screened for realization of the one-pot one-step procedure. Unfortunately, some bases and solvents gave low yields, while others did not promote the reaction to occur. However, to our delight, the reaction under the one-pot two-step procedure generated the desired product in a 69% yield. Hence, a one-pot two-step procedure was chosen as the optimized reaction condition (see ESI†).
| Entry | Amineb | Molarityc (mol L−1) | CO2 source | Yieldd |
|---|---|---|---|---|
| a Reaction conditions: 1a (1.0 mmol), 2a (1.2 mmol), CuCl (2.0 mol%), TMEDA (N,N,N′,N′-tetramethylethylenediamine) (1.5 mol%), K3PO4 (1.2 mmol), CO2 (ambient pressure) from an aqueous capturing solution, DMF (5 mL), room temperature (about 25 °C) in a Schlenk tube for 16 h. Then, KOAc (4 mmol) was added and stirred at 100 °C in a Schlenk tube for 10 h under a nitrogen atmosphere in the second step. b Pure CO2 (99.999%) was captured by the aqueous capturing solution for 25 min. c Volume of aqueous capturing solution: 100 mL. d Isolated yield (based on 1a). e Hyper-pure CO2 gas. f After recycling the aqueous morpholine solution 40 times. g Pure CO2 was captured by the aqueous morpholine solution for 3 h. h Pure CO2 was captured by the aqueous morpholine solution for 5 h. | ||||
| 1e | — | — | Pure CO2 | 72% |
| 2 |
|
0.4 | Pure CO2 | 70% |
| 3 |
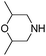
|
0.4 | Pure CO2 | 51% |
| 4 |
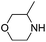
|
0.4 | Pure CO2 | 48% |
| 5 |
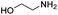
|
0.4 | Pure CO2 | 50% |
| 6 |
|
0.1 | Pure CO2 | 49% |
| 7 |
|
0.2 | Pure CO2 | 53% |
| 8 |
|
0.6 | Pure CO2 | 70% |
| 9f |
|
0.4 | Pure CO2 | 65% |
| 10g |
|
0.4 | Combustion ethanol | 58% |
| 11g |
|
0.4 | Air | 50% |
| 12 |
|
0.4 | Air | 69% |
| 13 | — | — | Air | 0% |
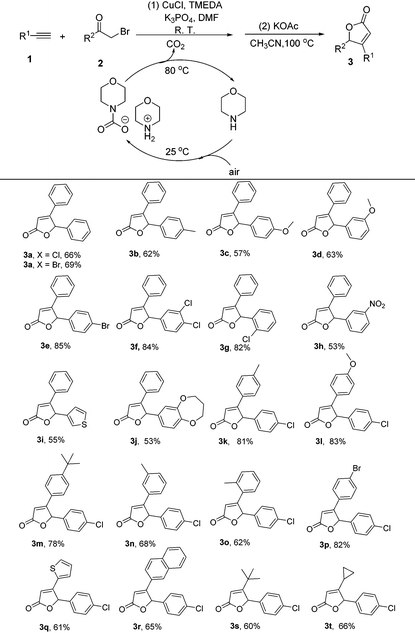
With the optimal reaction conditions in hand, we examined the substrate scope (Table 2). Gratifyingly, the desired products were obtained in good yields from the reactions of CO2 with various substitutes. Both α-chloroketone and α-bromoketone worked smoothly and formed the corresponding products in moderate to good yields (Table 2, 3a). Aromatic α-bromoketones were found to be suitable substrates for this reaction (Table 2, 3b–3j), while aliphatic α-bromoketone could not work smoothly under the standard conditions. The structure of 3b was further confirmed by X-ray diffraction analysis as a representative product (ESI†). On the other hand, it was observed that a wide range of ethynylbenzenes were well tolerated, providing the corresponding 4,5-disubstiuted furan-(5H)-ones in moderate to good yields (Table 2, 3k–3p). Aliphatic alkynes participated well and furnished the corresponding products 3s and 3t in 60% and 66% yields. Obviously, terminal alkynes or α-haloketones bearing halogens (Br or Cl) substituents on the aryl ring were found to be suitable substrates for this reaction with higher yields (Table 2, 3e–3g and 3k–3t). To our delight, the ethynylbenzenes or α-haloketones possessing groups at the meta-position or ortho-position of the phenyl ring reacted readily to afford the desired products in good yields (Table 2, 3d, 3f, 3g, 3h, 3n and 3o). Notably, heteroaryl-substituted ethynylbenzenes or α-haloketones were also converted into the corresponding 4,5-disubstiuted furan-2(5H)-ones 3i, 3j and 3q in 53%–61% yields. Unfortunately, the alkynes with hydroxyl groups, such as 3-ethynylphenol and 3-methylpent-1-yn-3-ol, failed to afford the desired products but gave products 4w and 4x in high yields (see ESI†).
| a Reaction conditions: 1a (1.0 mmol), 2a (1.2 mmol), CuCl (2.0 mol%), TMEDA (N,N,N′,N′-tetramethylethylenediamine) (1.5 mol%), K3PO4 (1.2 mmol), CO2 (ambient pressure) from an aqueous capturing solution, DMF (5 mL), room temperature (about 25 °C) in a Schlenk tube for 16 h. Then, KOAc (4 mmol) was added and stirred at 100 °C in a Schlenk tube for 10 h under a nitrogen atmosphere in the second step. Isolated yield (based on 1). |
|---|
|
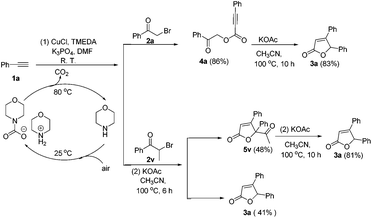
In order to explore the possible reaction pathway, control experiments were carried out and the results are presented in Scheme 1. The reaction of phenylacetylene 1a, 2-bromoacetophenone 2a and CO2 (CO2 captured by aqueous morpholine solution) in the presence of CuCl, TMEDA and K3PO4 in DMF at room temperature (about 25 °C) for 16 h afforded 4a in an 86% yield. Subsequently, an intramolecular cyclization/decarbonylation/protonation process of 4a provided 3a in an 83% yield. To our surprise, 2-bromo-1-phenylpropan-1-one 2v afforded the desired product 3a (41%) along with an unexpected product 5v (48%). Notably, 5v underwent the decarbonylation/protonation reaction to produce 3a in an 81% yield.
On the basis of the above results and literature,16e,19 a plausible mechanism for this tandem reaction is shown in Scheme 2. Primarily, phenylacetylene 1a reacted with CuCl catalyzed by K3PO4 and TMEDA, followed by incorporation with CO2, which was generated through the aqueous morpholine solution and air, to create the propynyl acid salt B. The subsequent reaction of the propynyl acid salt B with 2-bromoacetophenone 2a afforded the intermediate 4a. Deprotonation of the intermediate 4a generated species C, and then an acyl shift process afforded the intermediate D, followed by an intramolecular cyclization to give the intermediate 5a. Finally, the intermediate 5a was converted to the final product 3a and released the by-product 6a16e (confirmed by HPLC and HRMS, see ESI†) via a decarbonylation/protonation reaction.
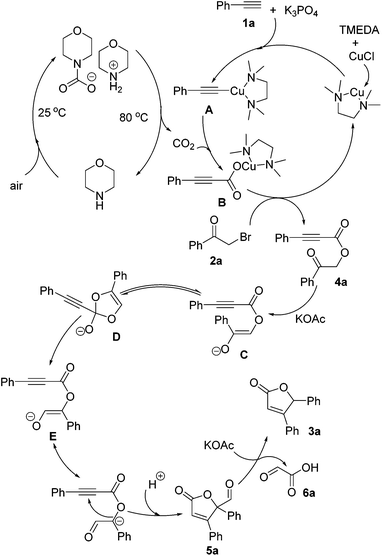 | ||
| Scheme 2 A plausible mechanism for the cascade reaction of terminal alkynes, α-haloketones and carbon dioxide. | ||
In conclusion, we developed a sustainable strategy for the assembly of furan-2(5H)-ones directly from carbon dioxide from air. By using this carboxylation/acyl shift/annulations/decarbonylation route, 4,5-disubstituted furan-2(5H)-ones with various functional groups were achieved in moderate to good yields. This approach represents an environmentally friendly example of the conversion of carbon dioxide in air into value-added chemicals. Further studies on this strategy for the construction of other valuable chemicals are underway in our laboratory.
Acknowledgements
We would like to thank the National Natural Science Foundation of China (21362002, 41465009 and 81260472), Guangxi Natural Science Foundation of China (2014GXNSFDA118007), State Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources (CMEMR2014-A02 and CMEMR2012-A20), Guangxi's Medicine Talented Persons Small Highland Foundation (1306), and The Fund of Guangxi Key Laboratory of Functional Phytochemicals Research and Utilization (FPRU2015-2) for financial support.Notes and references
- (a) S. D. Kenarsari, D. L. Yang, G. D. Jiang, S. J. Zhang, J. J. Wang, A. G. Russell, Q. Wei and M. H. Fan, RSC Adv., 2013, 3, 22739–22773 RSC; (b) M. North and P. Styring, Faraday Discuss., 2015, 183, 489–502 RSC; (c) O. Aschenbrenner and P. Styring, Energy Environ. Sci., 2010, 3, 1106–1113 RSC.
- (a) B. A. Vara, T. J. Struble, W. W. Wang, M. C. Dobish, J. N. Johnston, R. Ranjan, J. Olson, P. Singh, E. D. Lorance, D. A. Buttry and I. R. Gould, J. Phys. Chem. Lett., 2015, 6, 4943–4946 CrossRef PubMed; (b) Y. M. Liu, S. Chen, X. Quan and H. T. Yu, J. Am. Chem. Soc., 2015, 137, 11631–11636 CrossRef CAS PubMed.
- (a) B. A. Vara, T. J. Struble, W. W. Wang, M. C. Dobish and J. N. Johnston, J. Am. Chem. Soc., 2015, 137, 7302–7305 CrossRef CAS PubMed; (b) K. Huang, C. L. Sun and Z. J. Shi, Chem. Soc. Rev., 2011, 40, 2435–2452 RSC.
- (a) D. J. Heldebrant, P. K. Koech, M. T. C. Ang, C. Liang, J. E. Rainbolt, C. R. Yonker and P. G. Jessop, Green Chem., 2010, 12, 713–721 RSC; (b) C. Wang, S. M. Mahurin, H. Luo, G. A. Baker, H. Li and S. Dai, Green Chem., 2010, 12, 870–874 RSC; (c) C. M. Wang, H. M. Luo, X. Y. Luo, H. R. Li and S. Dai, Green Chem., 2010, 12, 2019–2023 RSC; (d) T. Yu and R. G. Weiss, Green Chem., 2012, 14, 209–216 RSC; (e) B. C. M. Martindale and R. G. Compton, Chem. Commun., 2012, 48, 6487–6489 RSC; (f) Z. Z. Yang, Y. Zhao, L. N. He, J. Gao and Z. S. Yin, Green Chem., 2012, 14, 519–527 RSC; (g) S. M. Sadeghzadeh, Green Chem., 2015, 17, 3059–3066 RSC.
- K. Anderson, M. P. Atkins, J. Estager, Y. Kuah, S. Ng, A. A. Oliferenko, N. V. Plechkova, A. V. Puga, K. R. Seddon and D. F. Wassell, Green Chem., 2015, 17, 4340–4354 RSC.
- (a) D. Ko, H. A. Patel and C. T. Yavuz, Chem. Commun., 2015, 51, 2915–2917 RSC; (b) J. J. Qian, F. L. Jiang, D. G. Yuan, M. Y. Wu, S. Q. Zhang, L. J. Zhang and M. C. Hong, Chem. Commun., 2012, 48, 9696–9698 RSC; (c) P. Li, C. Xing, S. Qu, B. Li and W. Z. Shen, ACS Sustainable Chem. Eng., 2015, 3, 1434–1442 CrossRef CAS; (d) Z. Z. Yang, Y. F. Zhao, H. G. Zhang, B. Yu, Z. H. Ma, G. P. Ji and Z. M. Liu, Chem. Commun., 2014, 50, 13910–13913 RSC.
- (a) A. Dibenedetto, M. Aresta, C. Fragale and M. Narracci, Green Chem., 2002, 4, 439–443 RSC; (b) J. Hu, J. Ma, Z. F. Zhang, Q. G. Zhu, H. C. Zhou, W. J. Lu and B. X. Han, Green Chem., 2015, 17, 1219–1225 RSC; (c) S. Sharma, A. K. Singh, D. Singh and D. P. Kim, Green Chem., 2015, 17, 1404–1407 RSC; (d) Y. H. Takeda, S. Okumura, S. Tone, I. Sasaki and S. Minakata, Org. Lett., 2012, 14, 4874–4877 CrossRef CAS PubMed; (e) G. T. Rochelle, Science, 2009, 325, 1652–1654 CrossRef CAS PubMed; (f) J. Su, M. Lu and H. F. Lin, Green Chem., 2015, 17, 2769–2773 RSC.
- (a) F. Barzagli, F. Mani and M. Peruzzin, Green Chem., 2011, 13, 1267–1274 RSC; (b) J. Su, M. Lu and H. F. Lin, Green Chem., 2015, 17, 2769–2773 RSC; (c) B. Yu, B. B. Cheng, W. Q. Liu, W. Li, S. S. Wang, J. Cao and C. W. Hu, Adv. Synth. Catal., 2016, 358, 90–97 CrossRef CAS; (d) A. Goeppert, M. S. Czaun, R. B. May, G. K. S. Prakash, G. A. Olah and S. R. Narayanan, J. Am. Chem. Soc., 2011, 133, 20164–20167 CrossRef CAS PubMed; (e) S. H. Kim, K. H. Kim and S. H. Hong, Angew. Chem., Int. Ed., 2014, 126, 790–793 CrossRef; (f) L. Baldini, M. Melegari, V. Bagnacani, A. Casnati, E. Dalcanale, F. Sansone and R. Ungaro, J. Org. Chem., 2011, 76, 3720–3732 CrossRef CAS PubMed.
- L. J. Murphy, K. N. Robertson, R. A. Kemp, H. M. Tuononen and J. A. C. Clyburne, Chem. Commun., 2015, 51, 3942–3956 RSC.
- J. K. Alain, M. Czaun, G. A. Olah and G. K. S. Prakash, J. Am. Chem. Soc., 2016, 138, 778–781 CrossRef PubMed.
- (a) R. J. Worthington, J. J. Richards and C. Melander, Org. Biomol. Chem., 2012, 10, 7457–7474 RSC; (b) N. B. Carter, A. E. Nadany and J. B. Sweeney, Chem. Soc., 2002, 1, 2324–2342 Search PubMed; (c) C. A. Lowery, T. J. Dickerson and K. D. Janda, Chem. Soc. Rev., 2008, 37, 1337–1346 RSC.
- (a) M. Pour, M. Spulak, V. Buchta, P. Kubanova, M. Voprsalova, V. Wsol, H. Fakova, P. Koudelka, H. Pourova and R. Schiller, J. Med. Chem., 2001, 44, 2701–2706 CrossRef CAS PubMed; (b) M. Aquino, I. Bruno, R. Riccio and L. Gomez-Paloma, Org. Lett., 2006, 8, 4831–4834 CrossRef CAS PubMed; (c) M. D. Guerrero, M. Aquino, I. Bruno, M. C. Terencio, M. Paya, R. Riccio and G. P. Luigi, J. Med. Chem., 2007, 50, 2176–2184 CrossRef CAS PubMed.
- (a) N. Singh, X. Gao, D. B. A. X. Yan and Y. Y. Luk, Med. Chem. Commun., 2013, 4, 1079–1084 RSC; (b) M. D. Guerrero, M. Aquino, I. Bruno, M. C. T. M. Paya, R. Riccio and L. Paloma, J. Med. Chem., 2007, 50, 2176–2184 CrossRef CAS PubMed; (c) M. J. Uddin, A. V. Elleman, K. Ghebreselasie, C. K. Daniel, B. C. Crews, D. Nance, T. Huda and L. J. Marnett, ACS Med. Chem. Lett., 2014, 5, 1254–1258 CrossRef CAS PubMed.
- A. K. Cheung, R. Murelli and M. L. Snapper, J. Org. Chem., 2004, 69, 5712–5719 CrossRef CAS PubMed.
- (a) J. Adrio and J. C. Carretero, J. Am. Chem. Soc., 2007, 129, 778–779 CrossRef CAS PubMed; (b) D. S. Müller, N. L. Untiedt, A. P. Dieskau, G. L. Lackner and L. E. Overman, J. Am. Chem. Soc., 2015, 137, 660–663 CrossRef PubMed; (c) A. Arcadi, A. Burini, S. Cacchi, M. Delmastro, F. Marinelli and B. R. Pietronit, J. Org. Chem., 1992, 57, 976–982 CrossRef CAS; (d) B. G. V. Hoven, B. E. Ali and H. Alper, J. Org. Chem., 2000, 65, 4131–4137 CrossRef; (e) M. Alfonsi, A. Arcadi, M. Chiarini and F. Marinelli, J. Org. Chem., 2007, 72, 9510–9517 CrossRef CAS PubMed; (f) R. R. L. J. Allen, T. Le, C. D. Taylor and A. R. Howell, Org. Lett., 2007, 9, 1699–1701 CrossRef PubMed.
- (a) B. A. Trofimov, A. V. Stepanov, A. G. Mal, O. G. Volostnykh, O. A. Shemyakina and I. A. Ushakov, Synth. Commun., 2015, 45, 2718–2729 CrossRef CAS; (b) M. Aquino, I. Bruno, R. Riccio and L. G. Paloma, Org. Lett., 2006, 8, 4831–4834 CrossRef CAS PubMed; (c) B. K. Bassora, C. E. D. Costa, R. A. Gariani, J. V. Comasseto and A. A. D. Santos, Tetrahedron Lett., 2007, 48, 1485–1487 CrossRef CAS; (d) H. He, C. Qi, X. H. Hu, L. Ouyang, W. F. Xiong and H. F. Jiang, J. Org. Chem., 2015, 80, 4957–4965 CrossRef CAS PubMed; (e) Y. Lei, Z. Q. Wang, Y. X. Xie, S. C. Yu, B. X. Tang and J. H. Li, Adv. Synth. Catal., 2011, 353, 31–35 CrossRef CAS.
- (a) V. K. Rai, R. Sharma and A. Kumar, Tetrahedron Lett., 2013, 54, 1071–1075 CrossRef CAS; (b) S. N. Patil and F. Liu, Org. Lett., 2007, 9, 195–198 CrossRef CAS PubMed; (c) S. P. Chavan, M. Thakkar and U. R. Kalkote, Tetrahedron Lett., 2007, 48, 643–646 CrossRef CAS; (d) A. Picado, S. J. Li and R. K. Dieter, J. Org. Chem., 2016, 81, 1391–1400 CrossRef CAS PubMed; (e) M. A. Ganiek, M. R. Becker, M. Ketels and P. Knochel, Org. Lett., 2016, 18, 828–831 CrossRef CAS PubMed; (f) X. f. Wu, S. Shirakawa and K. Maruoka, Org. Biomol. Chem., 2014, 12, 5388–5392 RSC.
- (a) P. Liu, H. S. Wang, Y. M. Pan, W. L. Dai, H. Liang and Z. F. Chen, Chem. Commun., 2013, 49, 5295–5297 RSC; (b) P. Liu, J. L. Liu, H. S. Wang, Y. M. Pan, H. Liang and Z. F. Chen, Chem. Commun., 2014, 50, 4795–4798 RSC; (c) H. Z. Xie, Q. Gao, Y. Liang, H. S. Wang and Y. M. Pan, Green Chem., 2014, 16, 2132–2135 RSC; (d) Y. L. Xu, Y. M. Pan, P. Liu, H. S. Wang, X. Y. Tian and G. F. Su, J. Org. Chem., 2012, 77, 3557–3562 CrossRef CAS PubMed; (e) G. B. Huang, X. Wang, Y. M. Pan, H. S. Wang, G. Y. Yao and Y. Zhang, J. Org. Chem., 2013, 78, 2742–2745 CrossRef CAS PubMed; (f) X. Wang, S. Y. Li, Y. M. Pan, H. S. Wang, H. Liang, Z. F. Chen and X. H. Qin, Org. Lett., 2014, 16, 580–583 CrossRef CAS PubMed; (g) Q. Gao, X. C. Tan, Y. M. Pan, H. S. Wang and Y. Liang, Chem. Commun., 2012, 48, 12080–12081 RSC.
- D. Y. Yu and Y. G. Zhang, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 20184–20189 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1457861. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00328a |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2016 |