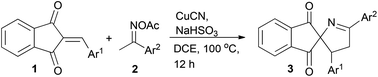
Synthesis of spiro[indane-1,3-dione-1-pyrrolines] via copper-catalyzed heteroannulation of ketoxime acetates with 2-arylideneindane-1,3-diones†
Jindian
Duan
,
Yuyu
Cheng
,
Rou
Li
and
Pengfei
Li
*
Department of Chemistry, South University of Science and Technology of China, Shenzhen, Guangdong 518055, China. E-mail: lipf@sustc.edu.cn; flyli1980@gmail.com; Fax: (+86) 755-88018304
First published on 27th September 2016
Abstract
A cuprous cyanide-catalyzed heteroannulation reaction of 2-arylideneindane-1,3-dione with ketoxime acetates has been developed for the synthesis of novel spiro[indane-1,3-dione-1-pyrrolines] through the cleavage of N–O and C–H bonds and formation of C–C and C–N bonds. The synthetic strategy shows broad substrate scope and furnishes spiro[indane-1,3-dione-1-pyrrolines] in high yields.
Among various nitrogen heterocyclic molecules, 1-pyrrolines have attracted much attention because of their great applications, such as semiochemicals,1 natural products,2 natural alkaloids,3 high-affinity radioligands,4 flavouring agents for various food products,5 and therapeutic compounds.6 More significantly, 1-pyrrolines were also used as building blocks for the synthesis of compounds possessing diversified pharmaceutical activities (Scheme 1, top).7 On the other hand, spiro[indane-1,3-diones] are privileged scaffolds in medicinal chemistry and also serve as versatile building blocks for the construction of many bioactive natural products, drugs, and therapeutic leads (Scheme 1, middle).8 Considering the relevance of 1-pyrrolines and spiro[indane-1,3-diones], it was anticipated that incorporation of both structural motifs into one molecule might lead to the creation of a new group of spiro[indane-1,3-dione-1-pyrrolines] with potentially interesting biological properties (Scheme 1, bottom). Although numerous methods have been developed for the synthesis of 1-pyrrolines9 and spiro[indane-1,3-diones],10 the general protocol leading to such nature-inspired synthetic targets has not been reported. Therefore, the development of novel and efficient methods for the straight forward synthesis of spiro[indane-1,3-dione-1-pyrrolines] from readily available starting materials remains desirable.
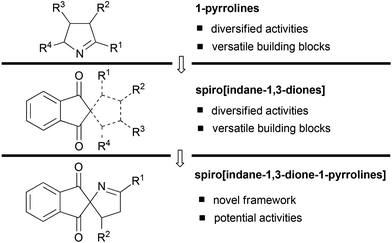 | ||
| Scheme 1 Relevance of 1-pyrroline and spiro[indane-1,3-dione] scaffolds and the synthetic objectives of this work. | ||
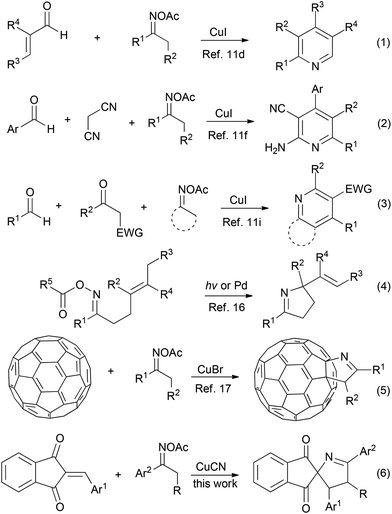
Recently, abundant reports on using ketoximes and their derivatives as substrates have emerged for diversified synthesis. Owing to the N–O bond cleavage of the ketoxime carboxylates acting as an internal oxidant to make the reactions proceed under mild redox-neutral conditions, transition metal catalyzed reactions of ketoxime carboxylates with a series of electrophiles were explored for efficient construction of aza-heterocycles, such as pyridines,11 phenanthridines,12 pyrroles,13 isoquinolines,14 and so on.15 Particularly, pyridine motifs were overwhelmingly obtained via a [3 + 3] fashion when ketoxime carboxylates reacted with linear electron deficient olefins in the presence of a copper catalyst. For example, Yoshikai and Wei have reported the modular pyridine synthesis from ketoxime carboxylates and enals through synergistic copper/iminium catalysis (Scheme 2, eqn (1)).11d Cui et al. revealed that 2-aminonicotinonitriles could be obtained from copper-catalyzed cyclization between ketoxime carboxylate and electron deficient olefin generated in situ from malononitrile and aldehydes (Scheme 2, eqn (2)).11f Jiang et al. demonstrated that the copper-catalyzed annulation reaction between ketoxime carboxylate and enone in situ from ketones and aldehydes resulted in the formation of pyridines (Scheme 2, eqn (3)).11i The intramolecular cyclization of γ,δ-unsaturated ketoxime carboxylates was well developed towards polysubstituted 1-pyrrolines (Scheme 2, eqn (4)).16 However, the report on the synthesis of polysubstituted 1-pyrrolines from the intermolecular reactions between ketoxime carboxylates and olefin is very limited. To our best knowledge, only one example of the intermolecular cyclization of ketoxime carboxylate was reported by Wang et al. for the construction of 1-pyrrolines. They realized the synthesis of 1-fulleropyrrolines via Cu-catalyzed heteroannulation of [60]fullerene with ketoxime acetates (Scheme 2, eqn (5)).17
Based on our work on reactions of electron deficient olefins18 and as a part of our research interest in the synthesis of multicyclic spiro[indane-1,3-diones],10 the heteroannulation between rigid cyclic electron deficient olefin and ketoxime carboxylate was surveyed. Here, we disclose a facile and efficient protocol to construct spiro[indane-1,3-dione-1-pyrrolines] from cuprous cyanide-catalyzed heteroannulation of 2-arylideneindane-1,3-diones with ketoxime acetates (Scheme 2, eqn (6)).
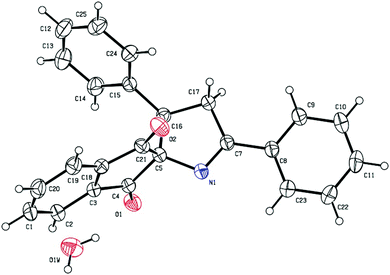
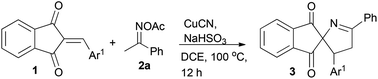
We carried out our investigation with the model reaction between 2-benzylidene indane-1,3-dione 1a and ketoxime acetate 2a in dichloroethane (DCE) at 100 °C. Without the copper catalyst, the reaction did not occur (Table 1, entry 1). In the presence of CuI without an additive, spiro[indane-1,3-dione-1-pyrroline] 3a was obtained in 42% yield, as confirmed by 1H NMR spectroscopic analysis (Table 1, entry 2). Furthermore, the structure of 3a was unambiguously confirmed by single-crystal X-ray analysis (Fig. 1).19 Different from the results of the reactions between ketoxime carboxylates and linear electron deficient olefins, cyclic 2-arylideneindane-1,3-dione reacted with ketoxime carboxylate via a [3 + 2] fashion to afford 1-pyrroline rather than pyridine. These encouraging results indicated that this methodology might be a very efficient tool for the preparation of spiro[indane-1,3-dione-1-pyrrolines].
| Entry | Catalyst | Additive | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: in a sealed tube, 1a (0.2 mmol), 2a (0.26 mmol), catalyst (10 mol%), and additive (0.4 mmol) were mixed in the solvent (3.0 mL) and stirred at 100 °C for 12 h. b Isolated yield. | ||||
| 1 | — | NaHSO3 | DCE | 0 |
| 2 | CuI | — | DCE | 42 |
| 3 | CuI | NaHSO3 | DCE | 61 |
| 4 | CuCl | NaHSO3 | DCE | 48 |
| 5 | CuBr | NaHSO3 | DCE | 65 |
| 6 | CuOAc | NaHSO3 | DCE | 55 |
| 7 | CuCN | NaHSO3 | DCE | 95 |
| 8 | CuCl2 | NaHSO3 | DCE | 52 |
| 9 | Cu(OAc)2 | NaHSO3 | DCE | 83 |
| 10 | Cu(OTf)2 | NaHSO3 | DCE | 60 |
| 11 | Cu | NaHSO3 | DCE | 50 |
| 12 | CuCN | NaHSO3 | EtOH | Trace |
| 13 | CuCN | NaHSO3 | Toluene | 31 |
| 14 | CuCN | NaHSO3 | DMF | 72 |
| 15 | CuCN | K2CO3 | DCE | 45 |
| 16 | CuCN | NaOAc | DCE | 21 |
| 17 | CuCN | K3PO4 | DCE | 30 |
Optimal reaction conditions were investigated. To our delight, the combination of CuI and NaHSO3 dramatically improved the yield and product 3a was isolated in 61% yield (Table 1, entry 3).11b,13a,b After careful screening of various copper catalysts, CuCN was found to be more suitable for the transformation, giving the desired product 3a in 95% yield (Table 1, entry 7). Both CuII and Cu0 could catalyse the reaction to yield the desired product 3a (Table 1, entries 8–11). Subsequently, different solvents, such as EtOH, toluene and DMF were tested (Table 1, entries 12–14). Using EtOH resulted in complex products and a trace of the desired product was isolated. After screening of reaction media, DCE was the first choice for the transformation. Other inorganic bases were also investigated as additives, and NaHSO3 furnished the best result (Table 1, entries 15–17).
With the optimized reaction conditions in hand, we next investigated the scope and limitation of the CuCN-catalyzed heteroannulation of a variety of 2-arylideneindane-1,3-diones 1 with ketoxime acetate 2a, and the results are presented in Table 2. Various 2-arylideneindane-1,3-diones, regardless of the electronic properties, steric hindrances, and substitution positions on the aromatic ring, were broadly tolerated, affording the corresponding 1-pyrrolines 3 in high to excellent yields (Table 2, entries 2–8). 2-(4-Methoxybenzylidene)-2H-indene-1,3-dione 1g was found to furnish the desired product 3g in 61% yield (Table 2, entry 7). It should be noted that 2-((naphthalen-1-yl)methylene)-2H-indene-1,3-dione 1i readily participated in this reaction, furnishing 3i in 51% yield (Table 2, entry 9). Moreover, heteroaromatic indane-1,3-diones were also readily accommodated under the standard reaction conditions, leading to the formation of the desired products 3j in 56% yield and 3k in 54% yield, respectively (Table 2, entries 10 and 11). The reaction between 2-(2-methylpropylidene)-2H-indene-1,3-dione and 2a proceeded slowly to furnish the desired product 3l in 21% yield (Table 2, entry 12). To explore the potential of the current catalyst system, the reaction was scaled up to 1.0 mmol of the starting material without optimization. 2-Benzylideneindane-1,3-dione 1a reacted with ketoxime acetate 2a to furnish the target product 3a in 81% yield (Table 2, entry 13). And the heteroannulation of 2-(3-bromobenzylidene)-2H-indene-1,3-dione with ketoxime acetate 2a afforded the product 3h in 85% yield (Table 2, entry 14).
| Entry | Ar | Product | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: in a sealed tube, 1 (0.2 mmol), 2a (0.26 mmol), CuCN (10 mol%), and NaHSO3 (0.4 mmol) were mixed in DCE (3.0 mL) and stirred at 100 °C for 12 h. b Isolated yield. c 1 (1.0 mmol), 2a (1.3 mmol), CuCN (10 mol%), and NaHSO3 (2.0 mmol) were mixed in DCE (10.0 mL) and stirred at 100 °C for 12 h. | |||
| 1 | Ph | 3a | 95 |
| 2 | 2-BrPh | 3b | 92 |
| 3 | 2-NO2Ph | 3c | 78 |
| 4 | 4-FPh | 3d | 85 |
| 5 | 4-CF3Ph | 3e | 88 |
| 6 | 4-MePh | 3f | 81 |
| 7 | 4-MeOPh | 3g | 61 |
| 8 | 3-BrPh | 3h | 95 |
| 9 | 1-Naphthyl | 3i | 51 |
| 10 | 2-Furyl | 3j | 56 |
| 11 | 2-Thienyl | 3k | 54 |
| 12 | i-Pr | 3l | 21 |
| 13c | Ph | 3a | 81 |
| 14c | 3-BrPh | 3h | 85 |
To explore the scope of the reaction, we then investigated the CuCN-catalyzed heteroannulation reactions between 2-benzylidene indane-1,3-dione 1a and a series of ketoxime acetates 2, which are shown in Table 3. Under the standard conditions, ketoxime acetates bearing different substituents on the aromatic ring were generally well tolerated, reacting with 2-benzylideneindane-1,3-dione 1a smoothly to provide the targeted products 3m–p in 82–86% yields (Table 3, entries 1–4). To further explore the scope of the reaction, both functionalized 2-arylideneindane-1,3-diones 1 and ketoxime acetates 2 were investigated. It was found that 2-arylideneindane-1,3-diones also reacted with ketoxime acetates from substituted aromatic ketone smoothly to afford the desired products in 84–93% yield (Table 3, entries 5–9). Importantly, valuable functional groups such as fluoro, chloro, bromo, methyl, methoxy, and nitro groups could survive the transformation safe and sound. A heteroaromatic ketoxime acetate afforded 3v with 77% yield (Table 3, entry 10). However, alkyl ketoxime acetates (cyclohexanone O-acetyl oxime, pentan-2-one O-acetyl oxime, 3-methylbutan-2-one O-acetyl oxime) were not applicable to this system (not given in Table 3).
| Entry | Ar1 | Ar2 | Product | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: in a sealed tube, 1 (0.2 mmol), 2 (0.26 mmol), CuCN (10 mol%), and NaHSO3 (0.4 mmol) were mixed in DCE (3.0 mL) and stirred at 100 °C for 12 h. b Isolated yield. | ||||
| 1 | Ph | 2-BrPh | 3m | 82 |
| 2 | Ph | 4-MePh | 3n | 85 |
| 3 | Ph | 4-MeOPh | 3o | 86 |
| 4 | Ph | 4-NO2Ph | 3p | 86 |
| 5 | 4-FPh | 3-BrPh | 3q | 93 |
| 6 | 3-BrPh | 4-ClPh | 3r | 84 |
| 7 | 4-BrPh | 4-MePh | 3s | 87 |
| 8 | 3-NO2Ph | 4-MeOPh | 3t | 84 |
| 9 | 2-ClPh | 4-MeOPh | 3u | 92 |
| 10 | Ph | 2-Thienyl | 3v | 77 |
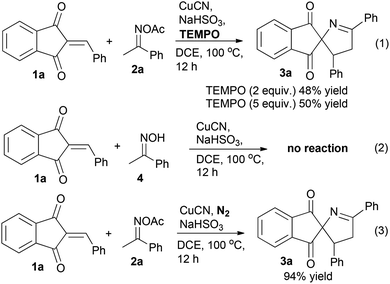
Cleavage of the N–O bond in oxime carboxylates by a low valent transition metal involves two pathways: oxidative addition and a radical process. To gain mechanistic insight into this reaction, some control experiments were performed (Scheme 3). When a free radical scavenger TEMPO with a loading of two equivalents was added to the reaction system, 1-pyrroline 3a was still obtained in 48% yield. Increasing the loading of TEMPO to five equivalents, product 3a was still obtained in 50% yield, which indicated that the reaction might not proceed through a radical pathway (Scheme 3, eqn (1)). Replacing ketoxime acetate 2a with ketoxime 4, no 1-pyrroline 3a was formed (Scheme 3, eqn (2)). When the CuCN-catalyzed heteroannulation between 2-benzylideneindane-1,3-dione 1a and ketoxime acetate 2a was carried out under N2, 1-pyrroline 3a was still obtained in 94% yield, which indicated that ketoxime acetate could serve as an internal oxidant to promote this transformation (Scheme 3, eqn (3)).
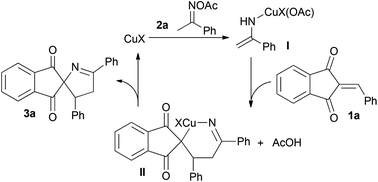
Based on the experimental results and previous studies,10–16 the tentative mechanism for this transformation is proposed in Scheme 4. In the presence of a copper catalyst, ketoxime acetate 2a was easily converted to copper enamide I which could react with 1a to give intermediate II. Then, the intramolecular heteroannulation of II occurred to deliver the desired product 3a and regenerated the catalyst via an intramolecular redox reaction.
Conclusions
In summary, we have developed a facile and efficient copper-catalyzed heteroannulation of 2-arylideneindane-1,3-diones with ketoxime acetates for the synthesis of spiro[indane-1,3-dione-1-pyrrolines] in good to excellent yields. This novel method tolerates a wide range of functionalities and is a reliable method for the rapid construction of valuable 1-pyrrolines. Further investigations involving the application of this methodology and novel spiro heterocycles are currently underway in our laboratory.Acknowledgements
We gratefully thank the South University of Science and Technology of China (Startup Fund, FRG-SUSTC1501A-57) and the National Natural Science Foundation of China (NSFC 21302089) for financial support. Peking university Shenzhen graduate school is gratefully acknowledged for the crystallographic data analysis of the annulation product.Notes and references
- D. C. Robacker, A. B. Demilo and D. J. Voaden, J. Chem. Ecol., 1997, 23, 1263 CrossRef CAS
.
- D. Tsukamoto, M. Shibano, R. Okamoto and G. Kusano, Chem. Pharm. Bull., 2001, 49, 492 CrossRef CAS PubMed
.
- S. Tyroller, W. Zwickenpflug and E. Richter, J. Agric. Food Chem., 2002, 50, 4909 CrossRef CAS PubMed
.
- D. Urosevic, S. Schann, J. D. Ehrhardt, P. Bousquet and H. Greney, Br. J. Pharmacol., 2004, 142, 609 CrossRef PubMed
. Corrigendum, Br. J. Pharmacol., 2004, 142, 1368.
- A. Adams and N. D. Kimpe, Chem. Rev., 2006, 106, 2299 CrossRef CAS PubMed
.
-
(a) C. A. Leach, T. H. Brown, R. J. Ife, D. J. Keeling, S. M. Laing, M. E. Parsons, C. A. Price and K. J. Wiggall, J. Med. Chem., 1992, 35, 1845 CrossRef CAS PubMed
; (b) M. Hadden and P. J. Stevenson, Tetrahedron Lett., 1999, 40, 1215 CrossRef CAS
; (c) S. Schann, V. Bruban, K. Pompermayer, J. Feldman, B. Pfeiffer, P. Renard, E. Scalbert, P. Bousquet and J.-D. Ehrhardt, J. Med. Chem., 2001, 44, 1588 CrossRef CAS PubMed
; (d) J.-B. Behr, M. S. M. Pearson, C. Bello, P. Vogel and R. Plantier-Royon, Tetrahedron: Asymmetry, 2008, 19, 1829 CrossRef CAS
.
- G. Dannhardt and W. Kiefer, Arch. Pharm. Pharm. Med. Chem., 2001, 334, 183 CrossRef CAS PubMed
.
- For selected examples, see:
(a) D. Leblois, S. Piessard, G. Le Baut, P. Kumar, J. D. Brion, L. Sparfel, R. Y. Sanchez, M. Juge, J. Y. Petit and L. Welin, Eur. J. Med. Chem., 1987, 22, 229 CrossRef CAS
; (b) P. A. Evans and T. A. Brandt, Tetrahedron Lett., 1996, 37, 1367 CrossRef CAS
; (c) D. L. J. Clive, X. Kong and C. C. Paul, Tetrahedron, 1996, 52, 6085 CrossRef CAS
; (d) D. Pizzirani, M. Roberti, S. Grimaudo, A. D. Cristina, R. M. Pipitone, M. Tolomeo and M. Recanatini, J. Med. Chem., 2009, 52, 6936 CrossRef CAS PubMed
. For a relevant review, see: G. S. Singh and Z. Y. Desta, Chem. Rev., 2012, 112, 6104 Search PubMed
.
-
(a) S. Peddibhotla and J. J. Tepe, J. Am. Chem. Soc., 2004, 126, 12776 CrossRef CAS PubMed
; (b) A. Soldevilla, D. Sampedro, P. J. Campos and M. A. Rodríguez, J. Org. Chem., 2005, 70, 6976 CrossRef CAS PubMed
; (c) S. J. Pastine, D. V. Gribkov and D. Sames, J. Am. Chem. Soc., 2006, 128, 14220 CrossRef CAS PubMed
; (d) J. D. White and D. C. Ihle, Org. Lett., 2006, 8, 1081 CrossRef CAS PubMed
; (e) J. M. Carney, P. J. Donoghue, W. M. Wuest, O. Wiest and P. Helquist, Org. Lett., 2008, 10, 3903 CrossRef CAS PubMed
; (f) M. P. Sibi, T. Soeta and C. P. Jasperse, Org. Lett., 2009, 11, 5366 CrossRef CAS PubMed
; (g) K. K. Toh, Y. Wang, E. P. J. Ng and S. Chiba, J. Am. Chem. Soc., 2011, 133, 13942 CrossRef CAS PubMed
; (h) P. Pandit, N. Chatterjee and D. K. Maiti, Chem. Commun., 2011, 47, 1285 RSC
; (i) X. Zhu, Y. Wang, W. Ren, F. Zhang and S. Chiba, Org. Lett., 2013, 15, 3214 CrossRef CAS PubMed
; (j) K. Goutham, N. S. V. M. Mangina, S. Suresh, P. Raghavaiah and G. V. Karunakar, Org. Biomol. Chem., 2014, 12, 2869 RSC
.
-
(a) J. Duan, J. Cheng and P. Li, Org. Chem. Front., 2015, 2, 1048 RSC
; and references cited therein. (b) J. Duan, J. Cheng, B. Li, F. Qi and P. Li, Eur. J. Org. Chem., 2015, 6130 CrossRef CAS
; (c) J. Duan, J. Cheng and P. Li, Curr. Organocatal., 2016, 3, 216 CrossRef CAS
; (d) J. Duan, J. Cheng, Y. Cheng and P. Li, Asian J. Org. Chem., 2016, 5, 477 CrossRef CAS
.
-
(a) S. Liu and L. S. Liebeskind, J. Am. Chem. Soc., 2008, 130, 6918 CrossRef CAS PubMed
; (b) Z. Ren, Z. Zhang, B. Yang, Y. Wang and Z. Guan, Org. Lett., 2011, 13, 5394 CrossRef CAS PubMed
; (c) J. M. Neely and T. Rovis, J. Am. Chem. Soc., 2013, 135, 66 CrossRef CAS PubMed
; (d) Y. Wei and N. Yoshikai, J. Am. Chem. Soc., 2013, 135, 3756 CrossRef CAS PubMed
; (e) J. M. Neely and T. Rovis, J. Am. Chem. Soc., 2014, 136, 2735 CrossRef CAS PubMed
; (f) Q. Wu, Y. Zhang and S. Cui, Org. Lett., 2014, 16, 1350 CrossRef CAS PubMed
; (g) Mi. Zhao, R. Hui, Z. Ren, Y. Wang and Z. Guan, Org. Lett., 2014, 16, 3082 CrossRef CAS PubMed
; (h) Z. Cai, X. Lu, S. Wang and S. Ji, Acta Chim. Sin., 2014, 72, 914 CrossRef CAS
; (i) H. Jiang, J. Yang, X. Tang, J. Li and W. Wu, J. Org. Chem., 2015, 80, 8763 CrossRef CAS PubMed
.
-
(a) T. Gerfaud, L. Neuville and J. Zhu, Angew. Chem., Int. Ed., 2009, 48, 572 CrossRef CAS PubMed
; (b) I. Deb and N. Yoshikai, Org. Lett., 2013, 15, 4254 CrossRef CAS PubMed
; (c) C. Tang, X. Wu, F. Sha, F. Zhang and H. Li, Tetrahedron Lett., 2014, 55, 1036 CrossRef CAS
.
-
(a) X. Tang, L. Huang, C. Qi, W. Wu and H. Jiang, Chem. Commun., 2013, 49, 9597 RSC
; (b) L. Ran, Z. Ren, Y. Wang and Z. Guan, Green Chem., 2014, 16, 112 RSC
; (c) W. Du, M.-N. Zhao, Z.-H. Ren, Y. Y. Wang and Z.-H. Guan, Chem. Commun., 2014, 50, 7437 RSC
.
-
(a) K. Parthasarathy and C.-H. Cheng, J. Org. Chem., 2009, 74, 9359 CrossRef CAS PubMed
; (b) P. C. Too, Y.-F. Wang and S. Chiba, Org. Lett., 2010, 12, 5688 CrossRef CAS PubMed
; (c) X. Zhang, D. Chen, M. Zhao, J. Zhao, A. Jia and X. Li, Adv. Synth. Catal., 2011, 353, 719 CrossRef CAS
; (d) P. C. Too, S. H. Chua, S. H. Wong and S. Chiba, J. Org. Chem., 2011, 76, 6159 CrossRef CAS PubMed
; (e) Z. Shi, D. C. Koester, M. Boultadakis-Arapinis and F. Glorius, J. Am. Chem. Soc., 2013, 135, 12204 CrossRef CAS PubMed
; (f) D. Zhao, F. Lied and F. Glorius, Chem. Sci., 2014, 5, 2869 RSC
; (g) Z. Zhang, A. Lin and J. Yang, J. Org. Chem., 2014, 79, 7041 CrossRef CAS PubMed
; (h) W. Han, G. Zhang, G. Li and H. Huang, Org. Lett., 2014, 16, 3532 CrossRef CAS PubMed
.
-
(a) Y. Tan and J. F. Hartwig, J. Am. Chem. Soc., 2010, 132, 3676 CrossRef CAS PubMed
; (b) X. Tang, L. Huang, J. Yang, Y. Xu, W. Wu and H. Jiang, Chem. Commun., 2014, 50, 14793 RSC
; (c) X. Tang, H. Gao, J. Yang, W. Wu and H. Jiang, Org. Chem. Front., 2014, 1, 1295 RSC
; (d) X. Tang, Z. Zhu, C. Qi, W. Wu and H. Jiang, Org. Lett., 2016, 18, 180 CrossRef CAS PubMed
.
- For reviews, see:
(a) K. Narasaka and M. Kitamura, Eur. J. Org. Chem., 2005, 4505–4519 CrossRef CAS
; (b) M. Kitamura and K. Narasaka, Bull. Chem. Soc. Jpn., 2008, 81, 539 CrossRef CAS
. For selected recent examples, see: (c) A. Faulkner and J. F. Bower, Angew. Chem., Int. Ed., 2012, 51, 1675 CrossRef CAS PubMed
; (d) N. J. Race and J. F. Bower, Org. Lett., 2013, 15, 4616 CrossRef CAS PubMed
; (e) A. Faulkner, J. S. Scott and J. F. Bower, Chem. Commun., 2013, 49, 1521 RSC
; (f) A. Faulkner, N. J. Race, J. S. Scott and J. F. Bower, Chem. Sci., 2014, 5, 2416 RSC
.
- S.-P. Jiang, Y.-T. Su, K.-Q. Liu, Q.-H. Wu and G.-W. Wang, Chem. Commun., 2015, 51, 6548 RSC
.
-
(a) L. Yu and P. Li, Tetrahedron Lett., 2014, 55, 3697 CrossRef CAS
; (b) P. Li, F. Fang, J. Chen and J. Wang, Tetrahedron: Asymmetry, 2014, 25, 98 CrossRef CAS
; (c) L. Yu, Q. Yang and P. Li, Eur. J. Org. Chem., 2014, 7499 CrossRef CAS
; (d) L. Yu, Z. Yang, J. Peng and P. Li, Eur. J. Org. Chem., 2016, 535 CrossRef CAS
; (e) L. Yu, Y. Cheng, R. Li, Y. Jiao and P. Li, Chin. J. Org. Chem., 2016, 36, 1572 CrossRef CAS
.
- CCDC 1444563 contains the supplementary crystallographic data for this paper.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1444563. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00454g |
| This journal is © the Partner Organisations 2016 |