A biomimetic Setaria viridis-inspired imprinted nanoadsorbent: green synthesis and application to the highly selective and fast removal of sulfamethazine
Ping Maa,
Zhiping Zhou*a,
Jiangdong Daiab,
Ling Qina,
Xubo Yea,
Xiang Chenb,
Jinsong Heb,
Atian Xieb,
Yongsheng Yanb and
Chunxiang Li*b
aSchool of Material Science and Engineering, Jiangsu University, Zhenjiang 212013, China. E-mail: Zhouzp@ujs.edu.cn; Fax: +86-511-88791800; Tel: +86-511-88790683
bSchool of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang 212013, China. E-mail: ujs2013txh@163.com
First published on 8th January 2016
Abstract
Nowadays, it is very necessary to develop high-efficiency nanoadsorbents to remove drug contaminants from wastewater. Inspired by a biomimetic Setaria viridis-like structure, we provide a simple and general approach for the preparation of hydrophilic magnetic surface molecularly imprinted core–shell nanorods (HMMINs) via a two-step surface-initiated atom transfer radical polymerization in a green alcohol/water solvent mixture at room temperature, with magnetic halloysite nanotubes (HNTs, a hollow tubular structured natural clay mineral) used as nano-cores. HMMINs showed a well-defined core–shell structure with an ultra-thin imprinted film (12 nm) and hydrophilic polymer brushes (2–4 nm), where magnetic nanoparticles (11 nm) were uniformly dispersed onto the surface of halloysite nanotubes. HMMINs possess good magnetic properties and thermal stability. Surface grafting of the hydrophilic polymer brushes enhanced the adsorption selectivity and kinetics. HMMINs exhibited a large adsorption capacity (37.64 ± 1.36 μmol g−1) and fast kinetics (within 45 min) towards a typical antibiotic drug sulfamethazine (SMZ) from pure water. Adsorption isotherm and kinetics data were well described by the Freundlich isotherm model and pseudo-second-order kinetic equation, respectively. HMMINs displayed good selectivity towards SMZ as compared with other antibiotics, as well as good regeneration performance, providing a potentially practical application in the highly efficient and selective removal of antibiotic contaminants from wastewater.
1. Introduction
The widespread occurrence of veterinary antibiotics detected in food and eco-environments, particularly in aquatic systems, has remarkably attracted more and more international concerns.1,2 Sulfonamides (SAs), as a class of bacteriostatic agents, are most commonly used to treat humans and animals and/or to promote growth in animals for more than sixty years, due to their broad-spectrum activity against infections caused by Gram-positive and Gram-negative bacteria and relatively low cost. However, a high proportion of unmetabolized SAs is excreted in the parent form and is finally transported from manured fields to water environments. The intake of SAs (particularly sulfamethazine, SMZ) through food chains and drinking water into the body is dangerous for human health, which can cause adverse effects, including allergic reactions, dysbacteriosis and even carcinogenic effects.3,4 Conventional water treatment methods, such as coagulation, flocculation, sedimentation and ion exchange, are relatively ineffective in removing antibiotic pollutions from wastewater. Due to its relatively low cost and high efficiency, adsorption is usually considered as an effective method to eliminate many organic pollutants.5–7 Adsorption performance of the adsorbents used is key to remove the targeted substances. Thus, it is very important to synthesize novel and high-performance adsorbents to remove SAs from water environments.Molecular imprinting, as an advanced and powerful technique, can obtain artificial receptors with a strong ability to recognize and rebind predetermined/targeted molecules.8 Due to their excellent characteristics, such as easy preparation, specific recognition, low cost and high stability, molecularly imprinted polymers (MIPs) have attracted more and more attention in a wide range of application fields including solid phase extraction, chromatography, sensors, and catalysis.9–12 However, conventional bulk MIPs prepared using traditional techniques, owing to their highly cross-linked nature, suffer from many limitations such as time-consuming crushing, heterogeneous particle size, difficult template removal and low adsorption/desorption kinetics, which thereby decrease their rebinding efficiency. Thus, the development of high-efficient imprinting techniques to solve these problems is notably important. The establishment of a nanoimprinted system, which can control the template molecules to be located on the surface or approximate surface, is an effective approach, for example, the surface imprinting technique.13–15 The preparation of core–shell MIPs has recently provided a general method for obtaining surface imprinted polymers with various solids as core materials.
Halloysite nanotubes (HNTs, Al2Si2O5(OH)4·nH2O) are 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 layer silicate clay minerals with an obvious hollow tubular structure, where the outer surface and the inner surface consist of a tetrahedral (Si–O) and an octahedral (Al–OH) sheet, respectively.16,17 The chemical properties of the HNTs are similar to those of pure SiO2 and Al2O3. Generally, the size range of the inner and outer diameters for HNTs is 10–30 nm and 50–100 nm, respectively. Owing to their superior characteristics including high porosity, large surface area and tunable surface chemistry, HNTs have attracted great interest in chemical, material and environmental science, especially for catalysis, drug delivery, template and separation.18–21 Compared with artificial synthetic materials (e.g. carbon nanotubes, silica-based materials and polymer spheres), HNTs are cheaper and surface hydrophilic, and thus can be used as an ideal core material for loading imprinted polymers. In our previous research, magnetic nanoparticles were first loaded onto HNTs and the imprinted polymer nanoshell with a tunable thickness was coated onto the HNTs surface via a simple precipitation polymerization process.22 When magnetic properties are introduced into molecularly imprinted polymers, the obtained imprinted composites can be easily collected in the presence of an external magnetic field, which facilitates the separation process.
1 layer silicate clay minerals with an obvious hollow tubular structure, where the outer surface and the inner surface consist of a tetrahedral (Si–O) and an octahedral (Al–OH) sheet, respectively.16,17 The chemical properties of the HNTs are similar to those of pure SiO2 and Al2O3. Generally, the size range of the inner and outer diameters for HNTs is 10–30 nm and 50–100 nm, respectively. Owing to their superior characteristics including high porosity, large surface area and tunable surface chemistry, HNTs have attracted great interest in chemical, material and environmental science, especially for catalysis, drug delivery, template and separation.18–21 Compared with artificial synthetic materials (e.g. carbon nanotubes, silica-based materials and polymer spheres), HNTs are cheaper and surface hydrophilic, and thus can be used as an ideal core material for loading imprinted polymers. In our previous research, magnetic nanoparticles were first loaded onto HNTs and the imprinted polymer nanoshell with a tunable thickness was coated onto the HNTs surface via a simple precipitation polymerization process.22 When magnetic properties are introduced into molecularly imprinted polymers, the obtained imprinted composites can be easily collected in the presence of an external magnetic field, which facilitates the separation process.
Usually, the preparation and application of MIPs are mostly restricted in various organic solvents; however, the natural recognition system and practical applications of MIPs are mainly in aqueous environments. Obviously, it is necessary to develop novel hydrophilic MIPs, which can be applied in water-phase systems. Two strategies have been developed to improve the MIPs' surface hydrophilicity. One involves the copolymerization of a hydrophilic monomer, functional monomer and cross-linking agent in the preparation process.23–25 Although the process is very simple, the optimization of the synthesis parameters is complicated and time-consuming, which can affect MIPs' properties due to the extra addition of hydrophilic monomers. Another way is post-modification of the already obtained imprinted materials,26–28 which is relatively simple and incurs low cost. Moreover, the persistent pursuit of general and facile methodologies is highly desirable towards the development of hydrophilic MIPs.
Atom transfer radical polymerization (ATRP), a new class of controllable radical techniques, has been recently used for the generation of MIPs to improve their properties in the field of molecular imprinting.29–32 ATRP is tolerant to a wide range of monomers and can be carried out using mild reaction conditions such as low temperature (especially at room temperature) and water-phase environments.33,34 Previously, we have reported that the narrowly dispersed imprinted microspheres were first prepared using reverse ATRP and then used as macro-active initiator for further surface-modification of hydrophilic polymer brushes.35 This proved that ATRP is a powerful technique that can be used to synthesize “living” polymers with well-defined structures, which can be used as a macro-initiator for further surface multi-functionalization.
To the best of our knowledge, the preparation of hydrophilic magnetic organic–inorganic selective nanoadsorbents and their application in the selective recognition and removal of sulfamethazine antibiotic in pure water is reported herein for the first time. Biomimetically inspired by the unique appearance of a common green plant, Setaria viridis, which has a rod-like tassel with abundant bristle structure along the plant stem (as shown in Fig. 1),36 the aim of this study was to prepare hydrophilic magnetic surface molecularly imprinting core–shell nanorods (HMMINs) based on magnetic halloysite nanotubes via a facile two-step ATRP in a green alcohol/water solvent mixture at room temperature. The obtained HMMINs were used in the adsorption and removal of the SMZ antibiotic from a water environment. The physical and chemical properties, adsorption isotherms, adsorption kinetics, selectivity and regeneration performance of the HMMINs towards SMZ were investigated in detail using a batch adsorption technique.
2. Experimental
2.1. Materials
HNTs were purchased from Zhengzhou Jinyangguang Chinaware Co., Ltd, Henan, China, and purified via repeated sedimentation processes to remove the quartz impurities, followed by drying at 80 °C for 12 h and grinding. 4-Vinylpyridine (4-VP, 96%), hydroxyethyl methylacrylate (HEMA, 96%), 2-bromoisobutyryl bromide (2-BiBr, 98%), 3-aminopropyltriethoxysilane (APTES, 99%), N,N,N′,N′′,N′′-pentamethyldiethylenetriamine (PMDETA, 98%), SMZ (98%), tetracycline (TC, 98%), ethylene glycol dimethacrylate (EGDMA, 98%) and ciprofloxacin (CIP, 98%) were of analytical grade and purchased from Aladdin Reagent Co., Ltd. (Shanghai, China). Cuprous bromide (CuBr), anhydrous toluene, acetone, acetic acid, ethanol, methanol, ethylene glycol (EG, 98%), iron(III) nitrate nonahydrate (Fe(NO3)3·9H2O, 98%), dichloromethane and triethylamine were of analytical grade and purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). Deionized ultrapure water was obtained from a Purelab Ultra system (Organo, Tokyo, Japan).2.2. Preparation of magnetic halloysite nanotubes (MHNTs)
5.0 g of HNTs was added to 150 ml of HNO3 aqueous solution (2.0 mol L−1) in a three-necked flask and uniformly dispersed under ultrasonication for 30 min. The mixtures were vigorously stirred to remove the impurities at 80 °C for 12 h. The resultant product was collected by vacuum filtration, repeatedly washed with deionized water and dried in an oven. Subsequently, 1.0 g of HNTs and 0.6 g of Fe(NO3)3·9H2O were added to 20 ml of ethanol under ultrasonication for complete dispersion and dissolution. After stirring at room temperature overnight, the mixture was dried at 60 °C until ethanol was completely evaporated. The yellow solid was impregnated with 2.0 ml of EG and heated at 400 °C for 2 h at a heating rate of 5.0 °C min−1 under the protection of a nitrogen atmosphere in a tube furnace. The black product was repeatedly washed with ethanol and water, and dried to obtain MHNTs.2.3. Preparation of NH2-modified MHNTs (MHNTs@NH2)
1.0 g of MHNTs was added to 100 ml of anhydrous toluene in a three-necked flask (250 ml) and uniformly dispersed under ultrasonication. 1.5 ml of APTES was added and the mixture was vigorously stirred and reacted at 90 °C for 24 h. After the reaction, the product was collected with the help of an external magnet and was washed several times with ethanol and deionized water, followed by drying to a constant weight. The APTES-modified product was obtained and denoted as MHNTs@NH2.2.4. Preparation of “living” Br-modified MHNTs (MHNTs@Br)
0.5 g of MHNTs@NH2 and 50 ml of dichloromethane were added into a round bottom flask and were uniformly dispersed under ultrasonication. Then, 1.0 ml of triethylamine and 1.0 ml of 2-bromoisobutyryl bromide were added to the mixture. Under the protection of an nitrogen atmosphere, the reaction was carried out at 0 °C for 2 h and at 25 °C for 12 h with vigorous agitation. After the reaction, the resultant product was washed with water and ethanol and was dried to a constant weight. The “living” Br-modified MHNTs were denoted as MHNTs@Br.2.5. Preparation of magnetic surface molecularly imprinting core–shell nanorods (MMINs)
In the synthesis process for the imprinted nanoadsorbent, SMZ was used as the template molecule, 4-VP as the functional monomer, and EGDMA as the cross-linking agent. 16 ml of methanol and 4.0 ml of water were added into a flask. 27.8 mg of SMZ, 0.26 ml of 4-VP and 2.30 ml of EGDMA were added to the solvent and self-assembled for 12 h at room temperature in the dark. Under the protection of an nitrogen atmosphere, 14.3 mg of CuBr and 42.3 μl of PMDETA were added, and then 0.2 g of MHNTs@Br was finally added to the mixture. The reaction was performed at 25 °C for 24 h with vigorous stirring. After the reaction, the product was washed six times with ethanol and acetone and was dried at 60 °C for 12 h. The template molecules were removed from the obtained product through Soxhlet extraction using a mixture of methanol and acetic acid (9.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0, v/v) as the eluent until SMZ could no longer be detected. Thus, the magnetic surface molecularly imprinting core–shell nanorods (MMINs) were obtained. For comparison, using the same method, magnetic surface non-imprinting core–shell nanorods (MNINs) were also prepared without the addition of the template molecules in the polymerization process.
1.0, v/v) as the eluent until SMZ could no longer be detected. Thus, the magnetic surface molecularly imprinting core–shell nanorods (MMINs) were obtained. For comparison, using the same method, magnetic surface non-imprinting core–shell nanorods (MNINs) were also prepared without the addition of the template molecules in the polymerization process.
2.6. Preparation of hydrophilic magnetic surface molecularly imprinting core–shell nanorods (HMMINs)
In brief, 2.0 ml of deionized water and 2.0 ml of methanol were added into a 25 ml flask. 10.47 mg of CuBr, 45.8 μl of PMEDTA and 1.5 ml of HEMA were added to the flask to form a homogeneous solution. Then, the solution was degassed under ultrasonication and exchanged with N2 to remove any oxygen. 100 mg of MMINs was added to the flask and the reaction was carried out at 25 °C for 24 h. The product was washed six times with acetone, ethanol and water, and then dried in an oven to obtain the HMMINs. Hydrophilic magnetic surface molecularly non-imprinting core–shell nanorods (HMNINs) were prepared using the same method with the addition of MNINs as a macro-initiator.2.7. Batch adsorption experiments
Adsorption isotherm experiments were performed to study the adsorption equilibrium properties of the nanoadsorbents. 5.0 mg of MMINs, HMMINs, MNINs and HMNINs were added to SMZ aqueous solutions at different initial concentrations between 5.0 and 120 μmol L−1. The adsorption experiments were carried out at 25 °C for 12 h to reach equilibrium. The adsorbents were quickly separated with the help of an external magnet. The free SMZ concentration in the solutions was measured using a UV-vis spectrophotometer (UV-2450, Shimadzu, Japan) at 249 nm. The equilibrium adsorption capacity Qe (μmol g−1) of SMZ was calculated according to the following equation:
 | (1) |
To investigate the adsorption kinetic properties of the nanoadsorbents, 5.0 mg of MMINs, HMMINs, MNINs and HMNINs were added into 10 ml of SMZ solution at an initial concentration of 100 μmol L−1. The adsorption experiments were conducted at 25 °C for different contact times, including 10, 20, 30, 45, 60, 90 and 120 min. The supernatant was quickly obtained with the help of an external magnet and then tested. The adsorption amount of SMZ (Qt, μmol g−1) at time t (min) was calculated according to the following equation:
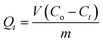 | (2) |
The selective recognition properties of HMMINs were studied by adsorption experiments with TC and CIP as reference antibiotics. 5.0 mg of HMMINs and HMNINs were added to each antibiotic solution with an initial concentration of 100 μmol L−1. After the adsorption reached equilibrium at 25 °C, the nanoadsorbents were separated using an external magnetic field, and the free antibiotic concentrations of TC and CIP were tested using a UV-vis spectrophotometer at 357 nm and 276 nm, respectively.
All the adsorption experiments were carried out six times and the experimental data was stable and reproducible, the error of which was much less than 5.0%.
2.8. Instrumentation
Fourier transform infrared spectroscopy was recorded on a Nicolet NEXUS-470 FTIR apparatus (U.S.A.) using KBr pellets. Scanning electron microscopy (SEM, S-4800, Hitachi, Japan) and transmission electron microscopy (TEM, JEOL IEM-200CX, U.S.A) were used to describe the morphology of the samples. Magnetic measurements were carried out using a VSM (7300, Lakeshore, U.S.A) under a magnetic field up to 10 kOe. Thermogravimetric analysis (TGA) of the samples was carried out using a Diamond TG/DTA instruments (STA 449C Jupiter, Netzsch, Germany) under N2 up to 800 °C at a heating rate of 10 °C min−1. The samples were dispersed in ethanol under ultrasonication (20 mg ml−1) and dropped onto a clean glass. After the ethanol was allowed to evaporate at ambient temperature, the static water contact angle was tested using a contact angle instrument (KSV CM200, Finland).3. Results and discussion
3.1. Preparation method for HMMINs
Fig. 1 shows a schematic diagram of the synthetic route used to prepare the HMMINs. First, iron ions were introduced into the lumen and surface of HNTs through a wetness impregnation step. MHNTs were obtained via the reduction of the iron ions using EG at high temperature under the protection of a nitrogen atmosphere. Second, amino and bromine groups were successively modified onto the surface of the MHNTs via a silanization and amidation reactions to obtain the MHNTs@NH2 and MHNTs@Br, respectively. Third, the magnetic surface molecularly imprinting core–shell nanorods (MMINs) were prepared via a surface-initiated ATRP performed at room temperature using a mixture of methanol and water as the reaction solvent, SMZ as the template molecule, 4-VP as the functional monomer, EGDMA as the cross-linking agent, and PMDETA/CuBr as the catalyst system. Finally, poly(HEMA) (PHEMA) brushes were grafted from the surface of MMINs via a surface-initiated ATRP method again to obtain HMMINs with selective recognition ability. This imprinted polymerization technique was non-toxic and required low energy consumption, as compared with other previously reported methods that describe the preparation of hydrophilic MIPs.23–283.2. Characterization
Fig. 2 shows the FT-IR spectra of MHNTs, MHNTs@NH2, MHNTs@Br, MMINs and HMMINs. As shown in the spectrum of the MHNTs, the characteristic peaks at 1087 and 1032 cm−1 correspond to the Si–O and Si–O–Si stretching vibrations, respectively. The sharp peaks at 3707 and 3622 cm−1 are attributed to stretching vibration of the inner surface –OH and the peak for the deformation vibration of the inner surface –OH appeared at 912 cm−1. The deformation vibration of the Al–O–Si bond was clearly observed at 534 cm−1.37 The peak intensity of the Fe–O bond was too weak to be detected. The spectrum of the MHNTs@NH2 showed a weak peak at 2954 cm−1, which was assigned to the –CH2 stretching vibration of APTES. The broad peak at 3452 cm−1 corresponded to –NH2 stretching vibrations. After the amidation reaction, the peak of the amide carbonyl group was observed at 1649 cm−1 in the spectrum of MHNTs@Br, indicating that the initial group was successfully grafted onto the surface of MHNTs. In the spectra of MMINs, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N characteristic peak of pyridine ring occurred at 1601 and 1558 cm−1, and a C
N characteristic peak of pyridine ring occurred at 1601 and 1558 cm−1, and a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration peak was observed at 1725 cm−1, demonstrating that 4-VP and EGDMA were successfully grafted from the surface of MHNTs. It could be seen that the intensity of the peaks at 1158 and 1245 cm−1 were enhanced, which appeared due to the C–O–C stretching vibrations. Moreover, the intensity of the –OH characteristic peak at 3435 cm−1 increased. Thus, it can be inferred that the PHEMA brushes were efficiently introduced onto the surface of MMINs.
O stretching vibration peak was observed at 1725 cm−1, demonstrating that 4-VP and EGDMA were successfully grafted from the surface of MHNTs. It could be seen that the intensity of the peaks at 1158 and 1245 cm−1 were enhanced, which appeared due to the C–O–C stretching vibrations. Moreover, the intensity of the –OH characteristic peak at 3435 cm−1 increased. Thus, it can be inferred that the PHEMA brushes were efficiently introduced onto the surface of MMINs.
 | ||
| Fig. 2 (A) FT-IR spectra of MHNTs (a), MHNTs@NH2 (b) and MHNTs@Br (c). (B) FT-IR spectra of MMINs (d) and HMMINs (e). | ||
Fig. 3 shows the SEM images of HNTs, MHNTs, MMINs and HMMINs. HNTs had a tubular structure with a length of 1.0–3.0 μm and an external diameter of 50–180 nm. As compared with HNTs, the overall morphology of MHNTs did not change. The surface of MHNTs became a little more rough, which might be caused by the loading of magnetic nanoparticles. After grafting of the imprinted polymers, both the length and diameter of MMINs increased slightly. In Fig. 3d, HMMINs exhibit a smooth surface and a little cross-linking.
To further obtain their morphology and structural information, TEM was used to characterize HNTs, MHNTs, MMINs and HMMINs, as shown in Fig. 4. HNTs exhibited a hollow tubular structure with non-uniform length and diameter. From Fig. 4b, it could be clearly seen that the magnetic nanoparticles were uniformly dispersed into the lumen and onto the surface of HNTs, and have an average size of 11 nm. In Fig. 3c, MMINs show an evident core–shell structure, with an imprinted polymer nanoshell thickness of about 12 nm. After surface grafting of PHEMA, the polymer nanoshell thickness increased to 14–16 nm, thus the average thickness of the polymer brushes was in the range of 2–4 nm. From the SEM and TEM images, it can be inferred that the imprinted nanofilm and polymer brushes were successfully grafted onto the surface of MHNTs.
Fig. 5A presents the magnetization curves for MHNTs, MMINs and HMMINs. It was obvious that in all the three samples, there were no hysteresis, and remanence and coercivity were near zero in the hysteresis loop, suggesting that the samples were superparamagnetic. Magnetization saturation (Ms) value for MHNTs was 2.81 ± 0.071 emu g−1 at 298 K. After surface grafting of the imprinted polymer, the Ms value for MMINs was reduced to 2.51 ± 0.056 emu g−1. Moreover, the Ms value for HMMINs was 2.29 ± 0.042 emu g−1. With an increase in the polymer shell thickness, the Ms value decreased gradually, which efficiently matches with the results of the TEM analysis. Although the Ms value for HMMINs was not very large, it was enough to achieve magnetic separation. As shown in Fig. 5B, HMMINs were uniformly dispersed in pure water placed in a glass bottle. When a magnet was close to the bottle, HMMINs moved quickly to the wall of the bottle, and the solution became clear immediately. HMMINs thus show a good separation performance in an aqueous environment.
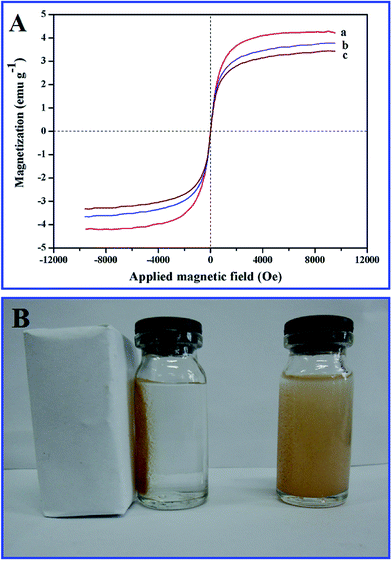 | ||
| Fig. 5 (A) Magnetization curves for MHNTs (a), MMINs (b) and HMMINs (c). (B) An image of the magnetic separation of HMMINs using an external magnet. | ||
Fig. 6 shows the TGA curves for MHNTs, MHNTs@NH2, MHNTs@Br, MMINs and HMMINs. At temperatures below 200 °C, only a low proportion of mass loss was observed for MHNTs, due to the evaporation of physically absorbed water. In the temperature range of 400–800 °C, because of the dehydration of Al–OH group in the lattice, the mass loss ratio for MHNTs at 800 °C was 11.83%. After silanization reaction, the mass loss ratio for MHNTs@NH2 was 14.52%. The mass loss for MHNTs@Br increased to 15.52%, indicating that the initiator was grafted onto the surface by the amidation reaction. For MMINs and HMMINs, the mass loss mainly occurred at a temperature range of 300–600 °C, due to the thermal decomposition of the polymers. The mass loss ratios for MMINs and HMMINs were about 29.78% and 43.62%, respectively. As obviously described by the TGA curves, the weight loss of the samples gradually increased stepwise, which demonstrated that each reaction step was completed successfully.
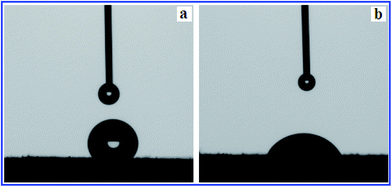
Fig. 7 shows the photographs of the static water contact angles of MMINs and HMMINs. It can be clearly seen that HMMINs showed a much lower static water contact angle than MMINs. The static water contact angles for MMINs and HMMINs were 122.34° ± 0.4° and 45.14° ± 0.2°, respectively, which indicated that the PHEMA brushes were grafted successfully onto the surface of MMINs and improved the surface hydrophilicity.
3.3. Adsorption isotherms
Fig. 8A shows adsorption isotherm curves for MMINs, MNINs, HMMINs and HMNINs towards SMZ at 298 K. Over the entire concentration range, the adsorption amount increased with an increase in the initial concentration and gradually reached adsorption equilibrium. The adsorption capacities of MMINs and HMMINs were much higher than that of MNINs and HMNINs, indicating that the imprinted sites exist in the polymer network. The adsorption capacities of SMZ onto MMINs, MNINs, HMMINs and HMNINs in an aqueous solution were 43.82 ± 1.85, 20.18 ± 0.625, 37.64 ± 1.36 and 14.42 ± 0.649 μmol g−1, respectively. Here, the HMMINs nanoadsorbent obtained by us shows a higher adsorption affinity for the SMZ template as compared to the other previously SMZ-imprinted adsorbents reported.38–41 It can be clearly observed in Fig. 8A that the adsorption amount decreased after the surface grafting of the hydrophilic polymer brushes, which could inhibit the hydrophobic interactions. The imprinted factors enhanced the absorption amount over the entire concentration range studied. Previous SMZ-imprinted adsorbents could only achieve relatively good recognition ability in an organic solvent, but were poor in a pure aqueous system. Through the surface grafting of the hydrophilic polymer without blocking the effective imprinted sites, this novel imprinted nanoadsorbent exhibited good selective recognition for the SMZ template in water. | ||
| Fig. 8 Adsorption isotherm curves for MMINs, MNINs, HMMINs and HMNINs (A) and the imprinted factor before and after surface-grafting (B). | ||
Langmuir and Freundlich isotherm models42 were used to analyze adsorption experimental data, the non-linear forms of which are listed as eqn (3) and (4), respectively:
 | (3) |
| Qe = KFCe1/n | (4) |
Fig. 9 shows the non-linear fitting curves of the adsorption isotherm for MMINs, MNINs, HMNINs and HMNINs towards SMZ, and the isotherm constants are listed in Table 1. Fig. 9 shows that the Langmuir fitting curves deviated from the experimental data for all the four nanoadsorbents. However, the fitting correlation coefficients (R2) of the Freundlich model for the four nanoadsorbents were all higher than 0.997, exhibiting good correlations. Moreover, the fitting curves of the Freundlich isotherm agreed well with the experimental data. The n values were all higher than 1.0, suggesting the procedure was in favor of SMZ adsorption. Thus, the Freundlich model could better describe the adsorption data, and multi-molecular layer adsorption was dominant in the adsorption process.
 | ||
| Fig. 9 The non-linear fitting curves for the Langmuir and Freundlich isotherms of MMINs and MNINs (A) and HMNINs and HMNINs (B) toward SMZ at 298 K. | ||
| Isotherm models | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| Nanoadsorbents | Qe (μmol g−1) | Qm (μmol g−1) | KL (L mg−1) | R2 | KF ((μmol g−1) (L μmol−1)1/n) | 1/n | R2 |
| MMINs | 43.82 ± 1.85 | 50.76 | 0.04039 | 0.9461 | 4.633 | 0.4829 | 0.9988 |
| MNINs | 20.18 ± 0.625 | 26.60 | 0.02664 | 0.9626 | 1.535 | 0.5591 | 0.9975 |
| HMMINs | 37.64 ± 1.36 | 42.19 | 0.047239 | 0.9627 | 4.561 | 0.4521 | 0.9994 |
| HMNINs | 14.42 ± 0.649 | 17.92 | 0.026399 | 0.9598 | 1.721 | 0.5429 | 0.9979 |
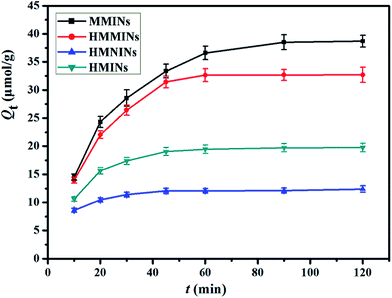
3.4. Adsorption kinetics
Batch adsorption experiments were performed to investigate the kinetic properties of MMINs, MNINs, HMMINs and HMNINs. The kinetic curves for the four nanoadsorbents are shown in Fig. 10. In the initial stage, owing to the existence of large empty binding sites, the adsorption amount quickly increased. With an increase in the contact time, the effective binding sites were gradually captured and the adsorption amount slowly achieved equilibrium. Due to the ultrathin polymer film, the adsorption equilibrium time was very short, which for MMINs, MNINs, HMMINs and HMNINs was only 60, 45, 45, and 30 min, respectively. Obviously, after grafting of the PHEMA brushes, the adsorption equilibrium time was shorter, which was attributed to the improvement in the surface hydrophilicity and an increase in the contact probability in water. Moreover, the adsorption amounts of MMINs and HMMINs were higher than that of the corresponding MNINs and HMNINs over the entire time range studied.To study adsorption kinetics, pseudo-first-order and pseudo-second-order kinetic equations43 are commonly used to analyze the kinetics data, the linear forms of which are shown in eqn (5) and (6), respectively:
ln(Qe − Qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe − k1t Qe − k1t
| (5) |
 | (6) |
The linear fitting curves for the adsorption kinetics of MMINs, MNINs, HMNINs and HMNINs towards SMZ are shown in Fig. 11 with the kinetic constants being listed in Table 2. For all the four nanoadsorbents, the R2 values of the pseudo-second-order kinetic equation were higher than that of the pseudo-first-order kinetic equation, which were all higher than 0.996, exhibiting good linear correlations. As shown in Table 2, the Qe,c values obtained from the pseudo-second-order kinetic equation are closer to the experimental values. All the results suggested that the adsorption processes were better described by the pseudo-second-order kinetic model. Moreover, it is obviously seen that k2 values increase after surface-grafting of the hydrophilic polymer brushes, which is attributed to the reduction in the binding time between the SMZ molecules and nanoadsorbents.
 | ||
| Fig. 11 The linear fitting curves of the pseudo-first-order (A) and pseudo-second-order (B) rate equations for MMINs, MNINs, HMINs and HMNINs toward SMZ. | ||
| Kinetic models | Pseudo-first-order | Pseudo-second-order | |||||
|---|---|---|---|---|---|---|---|
| Nanoadsorbents | Qe,exp (μmol g−1) | Qe,c (μmol g−1) | k1 (min−1) | R2 | Qe,c (μmol g−1) | k2 (g μmol−1 min−1) | R2 |
| MMINs | 39.09 ± 1.36 | 32.44 | 0.0386 | 0.9866 | 45.25 | 0.001255 | 0.9961 |
| MNINs | 19.81 ± 0.564 | 10.55 | 0.0513 | 0.9682 | 21.23 | 0.006563 | 0.9981 |
| HMMINs | 33.44 ± 1.22 | 17.73 | 0.0432 | 0.9446 | 37.74 | 0.003153 | 0.9977 |
| HMNINs | 12.40 ± 0.567 | 4.791 | 0.0449 | 0.8877 | 12.80 | 0.019049 | 0.9996 |
3.5. Selective adsorption ability
In this section, CIP and TC were selected as reference antibiotics to investigate the specific adsorption performance of the selective nanoadsorbents. The initial concentration of each antibiotic was 100 μmol L−1, and the process was carried out at 25 °C for 12 h to reach adsorption equilibrium. The adsorption capacities of HMMINs and HMNINs toward the three antibiotics are shown in Fig. 12. The adsorption amounts of HMNINs towards SMZ, TC and CIP were 12.6 ± 0.567, 17.24 ± 0.64 and 11.42 ± 0.52 μmol g−1, respectively, showing no specific recognition. Moreover, HMMINs exhibited a larger adsorption amount of SMZ (33.44 ± 1.22 μmol g−1) than that of TC (19.77 ± 0.78 μmol g−1) and CIP (15.63 ± 0.63 μmol g−1). The adsorption amounts of TC and CIP onto HMMINs and HMNINs had a slight difference. However, the adsorption amount of HMMINs was a lot larger than that found for HMNINs towards SMZ. The phenomenon could be explained by the fact that HMMINs possessed imprinted recognition sites in the polymer network, which matched with the molecular size, space structure and functional groups of the SMZ template. There were no imprinted sites in HMNINs, thus they showed no selectivity. The abovementioned results demonstrated that the imprinted nanoadsorbents were successfully synthesized and the specific recognition ability was satisfactory. | ||
| Fig. 12 Chemical structures of three antibiotics (insets) and selective adsorption data for HMMINs and HMNINs. | ||
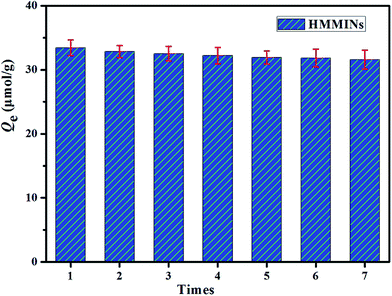
3.6. Regeneration performance of HMMINs
The regeneration ability is one of the key factors for the application performance of adsorbents. A batch adsorption experiment for HMMINs was first conducted to reach saturation adsorption. The SMZ adsorbed onto HMMINs was completely eluted using a solvent mixture of methanol and acetic acid (v/v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Adsorption–desorption cycles were carried out to test the reusability of HMMINs, and the experimental results are shown in Fig. 13. After seven cycles, the adsorption amount of HMMINs toward SMZ in an aqueous solution exhibited only a reduction of 7.62%, exhibiting a good stability and regeneration performance.
1). Adsorption–desorption cycles were carried out to test the reusability of HMMINs, and the experimental results are shown in Fig. 13. After seven cycles, the adsorption amount of HMMINs toward SMZ in an aqueous solution exhibited only a reduction of 7.62%, exhibiting a good stability and regeneration performance.
4. Conclusions
In conclusion, HMMINs was successfully prepared via a two-step surface-initiated ATRP from the surface of MHNTs. The characterization results showed that the thickness of the imprinted film and hydrophilic polymer brushes were about 12 and 2–4 nm, respectively. HMMINs exhibited good thermal and chemical stability, as well as ideal magnetic properties. After the surface grafting of the hydrophilic polymer brushes, the static water contact angle was obviously reduced to 45.14° ± 0.2° with the hydrophobic interactions being inhibited. Although the adsorption amount decreased, the imprinted factor increased over the entire concentration range studied. The adsorption isotherm data was well described by the Freundlich model. Due to the ultra-thin polymer nanofilm, the adsorption process for HMMINs could reach equilibrium within 45 min, and the kinetics data was better fitted to the pseudo-second-order rate equation. In addition, HMMINs exhibited a good specific recognition to the SMZ template molecule and good regeneration performance. This approach not only developed a simple and general synthesis technique for molecular imprinting in an aqueous system, but also for obtaining multifunctional (e.g. hydrophilic, thermosensitive and photosensitive) imprinted nanoadsorbents without affecting the recognition sites to achieve the highly efficient and selective removal of various pollutants from water.Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 21176107, 21174057, 21277063, 21446015 and U1407123), the National Basic Research Program of China (973 Program, 2012CB821500), the Natural Science Foundation of Jiangsu Province (BK20140534), Ph.D, the Innovation Programs Foundation of Jiangsu Province (No. CXZZ13_0668), Research Fund for the Doctoral Program of Higher Education of China (20133227110022 and 20133227110010) and Jiangsu Planned Projects for Postdoctoral Research Funds (1102119C).References
- A. U. Rajapaksha, M. Vithanage, M. Ahmad, D. C. Seo, J. S. Cho, S. E. Lee, S. S. Lee and Y. Sik Ok, Enhanced sulfamethazine removal by steam-activated invasive plant-derived biochar, J. Hazard. Mater., 2015, 290, 43–50 CrossRef CAS PubMed.
- M. Pan, C. K. C. Wong and L. M. Chu, Distribution of antibiotics in wastewater-irrigated soils and their, accumulation in vegetable crops in the pearl river delta, Southern China, J. Agric. Food Chem., 2014, 62, 11062–11069 CrossRef PubMed.
- M. Teixidó, J. J. Pignatello, J. L. Beltrán, M. Granados and J. Peccia, Speciation of the ionizable antibiotic sulfamethazine on black carbon (biochar), Environ. Sci. Technol., 2011, 45, 10020–10027 CrossRef PubMed.
- H. Chena, B. Gaoa and H. Li, Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide, J. Hazard. Mater., 2015, 282, 201–207 CrossRef PubMed.
- A. U. Rajapaksha, M. Vithanage, M. Zhang, M. Ahmad, D. Mohan, S. X. Chang and Y. S. Ok, Pyrolysis condition affected sulfamethazine sorption by tea waste biochars, Bioresour. Technol., 2014, 166, 303–308 CrossRef CAS PubMed.
- M. Shi, F. Y. Ma, Y. L. Han, X. Y. Zhang and H. B. Yu, Removal of sulfonamide antibiotics by oriented immobilized laccase on Fe3O4 nanoparticles with natural mediators, J. Hazard. Mater., 2014, 279, 203–211 CrossRef PubMed.
- R. M. P. Leal, L. R. F. Alleoni, V. L. Tornisielo and J. B. Regitano, Sorption of fluoroquinolones and sulfonamides in 13 Brazilian soils, Chemosphere, 2013, 92, 979–985 CrossRef CAS PubMed.
- R. Schirhagl, Bioapplications for molecularly imprinted polymers, Anal. Chem., 2014, 86, 250–261 CrossRef CAS PubMed.
- J. He, L. X. Song, S. Chen, Y. Y. Li, H. L. Wei, D. X. Zhao, K. R. Gu and S. S. Zhang, Novel restricted access materials combined to molecularly imprinted polymers for selective solid-phase extraction of organophosphorus pesticides from honey, Food Chem., 2015, 187, 331–337 CrossRef CAS PubMed.
- P. Lenain, S. De Saeger, B. Mattiasson and M. Hedström, Affinity sensor based on immobilized molecular imprinted synthetic recognition elements, Biosens. Bioelectron., 2015, 69, 34–39 CrossRef CAS PubMed.
- N. Li, L. Qi, Y. Shen, J. Qiao and Y. Chen, Novel oligo(ethylene glycol)-based molecularly imprinted magnetic nanoparticles for thermally modulated capture and release of lysozyme, ACS Appl. Mater. Interfaces, 2014, 6, 17289–17295 CAS.
- S. Muratsugu and M. Tada, Molecularly imprinted Ru complex catalysts integrated on oxide surfaces, Acc. Chem. Res., 2013, 46, 300–311 CrossRef CAS PubMed.
- D. S. Wang, D. Y. Xie, W. B. Shi, S. D. Sun and C. S. Zhao, Designing a photoresponsive molecularly imprinted system on a silicon wafer substrate surface, Langmuir, 2013, 29, 8311–8319 CrossRef CAS PubMed.
- C. H. Hu, J. Deng, Y. B. Zhao, L. S. Xia, K. H. Huang, S. Q. Ju and N. Xiao, A novel core–shell magnetic nano-sorbent with surface molecularly imprinted polymer coating for the selective solid phase extraction of dimetridazole, Food Chem., 2014, 158, 366–373 CrossRef CAS PubMed.
- J. D. Dai, J. S. He, A. T. Xie, L. Gao, J. M. Pan, X. Chen, Z. P. Zhou, X. Wei and Y. S. Yan, Novel Pitaya-Inspired Well-Defined Core–Shell Nanospheres with Ultrathin Surface Imprinted Nanofilm from Magnetic Mesoporous Nanosilica for Highly Efficient Chloramphenicol Removal, Chem. Eng. J., 2016, 284, 812–822 CrossRef CAS.
- P. Yuan, D. Y. Tan and F. Annabi-Bergaya, Properties and applications of halloysite nanotubes: recent research advances and future prospects, Appl. Clay Sci., 2015, 112–113, 75–93 CrossRef CAS.
- W. O. Yah, H. Xu, H. Soejima, W. Ma, Y. R. Lvov and A. Takahara, Biomimetic dopamine derivative for selective polymer modification of halloysite nanotube lumen, J. Am. Chem. Soc., 2012, 134, 12134–12137 CrossRef CAS PubMed.
- G. S. Machado, K. A. D. de Freitas Castro, F. Wypychb and S. Nakagaki, Immobilization of metalloporphyrins into nanotubes of natural halloysite toward selective catalysts for oxidation reactions, J. Mol. Catal. A: Chem., 2008, 283, 99–107 CrossRef CAS.
- H. J. Bai, H. Q. Zhang, Y. K. He, J. D. Liu, B. Zhang and J. T. Wang, Enhanced proton conduction of chitosan membrane enabled by halloysite nanotubes bearing sulfonate polyelectrolyte brushes, J. Membr. Sci., 2014, 454, 220–232 CrossRef CAS.
- S. X. Zuo, W. J. Liu, C. Yao, X. Z. Li, Y. Kong, X. H. Liu, H. H. Mao and Y. R. Li, Preparation of polyaniline–polypyrrole binary composite nanotube using halloysite as hard-template and its characterization, Chem. Eng. J., 2013, 228, 1092–1097 CrossRef CAS.
- H. Hemmatpour, V. Haddadi-Asl and H. Roghani-Mamaqani, Synthesis of pH-sensitive poly(N,N-dimethylaminoethyl methacrylate)-grafted halloysite nanotubes for adsorption and controlled release of DPH and DS drugs, Polymer, 2015, 65, 143–153 CrossRef CAS.
- J. D. Dai, X. Wei, Z. J. Cao, Z. P. Zhou, P. Yu, J. M. Pan, T. B. Zou, C. X. Li and Y. S. Yan, Highly-controllable imprinted polymer nanoshell at the surface of magnetic halloysite nanotubes for selective recognition and rapid adsorption of tetracycline, RSC Adv., 2014, 4, 7961–7978 Search PubMed.
- T. Jing, H. Xia, J. W. Niu, Y. S. Zhou, Q. Dai, Q. L. Hao, Y. K. Zhou and S. R. Mei, Determination of trace 2,4-dinitrophenol in surface water samples based on hydrophilic molecularly imprinted polymers/nickel fiber electrode, Biosens. Bioelectron., 2011, 26, 4450–4456 CrossRef CAS PubMed.
- K. C. Hua, L. Zhang, Z. H. Zhang, Y. Guo and T. Y. Guo, Surface hydrophilic modification with a sugar moiety for a uniform-sized polymer molecularly imprinted for phenobarbital in serum, Acta Biomater., 2011, 7, 3086–3093 CrossRef CAS PubMed.
- Y. Yoshimi and N. Ishii, Improved gate effect enantioselectivity of phenylalanine-imprinted polymers in water by blending crosslinkers, Anal. Chim. Acta, 2015, 862, 77–85 CrossRef CAS PubMed.
- G. Q. Pan, Y. Zhang, Y. Ma, C. X. Li and H. Q. Zhang, Efficient one-pot synthesis of water-compatible molecularly imprinted polymer microspheres by facile RAFT precipitation polymerization, Angew. Chem., Int. Ed., 2011, 50, 11731–11734 CrossRef CAS PubMed.
- G. Q. Pan, Y. Zhang, X. Z. Guo, C. X. Li and H. Q. Zhang, An efficient approach to obtaining water-compatible and stimuli-responsive molecularly imprinted polymers by the facile surface-grafting of functional polymer brushes via RAFT polymerization, Biosens. Bioelectron., 2010, 26, 976–982 CrossRef CAS PubMed.
- M. X. Yang, Y. Y. Zhang, S. Lin, X. L. Yang, Z. J. Fan, L. X. Yang and X. C. Dong, Preparation of a bifunctional pyrazosulfuron-ethyl imprinted polymer with hydrophilic external layers by reversible addition–fragmentation chain transfer polymerization and its application in the sulfonylurea residue analysis, Talanta, 2013, 114, 143–151 CrossRef CAS PubMed.
- Y. L. Liu, Y. Y. Huang, J. Z. Liu, W. Z. Wang, G. Q. Liu and R. Zhao, Superparamagnetic surface molecularly imprinted nanoparticles for water-soluble pefloxacin mesylate prepared via surface initiated atom transfer radical polymerization and its application in egg sample analysis, J. Chromatogr., 2012, A1246, 15–21 CrossRef PubMed.
- J. D. Dai, J. M. Pan, L. C. Xu, X. X. Li, Z. P. Zhou, R. X. Zhang and Y. S. Yan, Preparation of molecularly imprinted nanoparticles with superparamagnetic susceptibility through atom transfer radical emulsion polymerization for the selective recognition of tetracycline from aqueous medium, J. Hazard. Mater., 2012, 205–206, 179–188 CrossRef CAS PubMed.
- L. J. Fang, S. J. Chen, X. Z. Guo, Y. Zhang and H. Q. Zhang, Azobenzene-containing molecularly imprinted polymer microspheres with photo- and thermoresponsive template binding properties in pure aqueous media by atom transfer radical polymerization, Langmuir, 2012, 28, 9767–9777 CrossRef CAS PubMed.
- Z. Adali-Kaya, B. Tse Sum Bui, A. Falcimaigne-Cordin and K. Haupt, Molecularly Imprinted Polymer Nanomaterials and Nanocomposites: Atom-Transfer Radical Polymerization with Acidic Monomers, Angew. Chem., Int. Ed., 2015, 54, 5192–5195 CrossRef CAS PubMed.
- C. M. Hui, J. Pietrasik, M. Schmitt, C. Mahoney, J. Choi, M. R. Bockstaller and K. Matyjaszewski, Surface-initiated polymerization as an enabling tool for multifunctional (nano-) engineered hybrid materials, Chem. Mater., 2014, 26, 745–762 CrossRef CAS.
- K. Matyjaszewski, Atom transfer radical polymerization (ATRP): current status and future perspectives, Macromolecules, 2012, 45, 4015–4039 CrossRef CAS.
- J. D. Dai, Y. L. Zou, Z. P. Zhou, X. H. Dai, J. M. Pan, P. Yu, T. B. Zou, Y. S. Yan and C. X. Li, Narrowly dispersed imprinted microspheres with hydrophilic polymer brushes for the selective removal of sulfamethazine, RSC Adv., 2014, 4, 1965–1973 RSC.
- H. Nakajima, A. Ishihara, Y. J. Sawa and E. Sakuno, 3-(4-Methylfuran-3-yl)propan-1-ol: a white-spotted stinkbug (Eysarcoris ventralis) repellent produced by an endophyte isolated from green foxtail, J. Agric. Food Chem., 2010, 58, 2882–2885 CrossRef CAS PubMed.
- P. Yuan, P. D. Southon, Z. W. Liu, M. E. R. Green, J. M. Hook, S. J. Antill and C. J. Kepert, Functionalization of halloysite clay nanotubes by grafting with γ-aminopropyltriethoxysilane, J. Phys. Chem. C, 2008, 112, 15742–15751 CAS.
- J. X. He, S. Wang, G. Z. Fang, H. P. Zhu and Y. Zhang, Molecularly imprinted polymer online solid-phase extraction coupled with high-performance liquid chromatography-UV for the determination of three sulfonamides in pork and chicken, J. Agric. Food Chem., 2008, 56, 2919–2925 CrossRef CAS PubMed.
- S. C. Lee, F. L. Chuang, Y. L. Tsai and H. Chen, Studies on the preparation and properties of sol–gel molecularly imprinted polymer based on tetraethoxysilane for recognizing sulfonamides, J. Polym. Res., 2010, 17, 737–744 CrossRef CAS.
- M. Valtchev, B. S. Palm, M. Schiller and U. Steinfeld, Development of sulfamethoxazole-imprinted polymers for the selective extraction from waters, J. Hazard. Mater., 2009, 170, 722–728 CrossRef CAS PubMed.
- R. X. Gao, J. J. Zhang, X. W. He, L. X. Chen and Y. K. Zhang, Selective extraction of sulfonamides from food by use of silica-coated molecularly imprinted polymer nanospheres, Anal. Bioanal. Chem., 2010, 398, 451–461 CrossRef CAS PubMed.
- S. Ghorai, A. Sarkar, M. Raou, A. B. Panda, H. Schönherr and S. Pal, Enhanced removal of methylene blue and methyl violet dyes from aqueous solution using a nanocomposite of hydrolyzed polyacrylamide grafted xanthan gum and incorporated nanosilica, ACS Appl. Mater. Interfaces, 2014, 6, 4766–4777 CAS.
- H. L. Zhang, X. C. Li, G. H. He, J. J. Zhan and D. Liu, Preparation of magnetic composite hollow microsphere and its adsorption capacity for basic dyes, Ind. Eng. Chem. Res., 2013, 52, 16902–16910 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |