Facile fabrication of thermally reduced graphene oxide–platinum nanohybrids and their application in catalytic reduction and dye-sensitized solar cells†
Nguyen Tri Khoa‡
a,
Doan Van Thuan‡a,
Soon Wook Kima,
Sujung Parka,
Tran Van Tamb,
Won Mook Choib,
Shinuk Choa,
Eui Jung Kim*b and
Sung Hong Hahn*a
aDepartment of Physics and Energy Harvest-storage Research Center, University of Ulsan, Ulsan 680-749, South Korea. E-mail: shhahn@ulsan.ac.kr; Tel: +82-52-259-2330
bDepartment of Chemical Engineering, University of Ulsan, Ulsan 680-749, South Korea. E-mail: ejkim@ulsan.ac.kr; Tel: +82-52-259-2832
First published on 21st December 2015
Abstract
We report the fast synthesis of thermally reduced graphene oxide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) platinum (TRGO
platinum (TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt) nanohybrids by simply spraying a GO:Pt4+ solution on a hot plate. X-ray photoelectron spectroscopy and atomic force microscopy analyses are performed to investigate the thermal reduction of GO:Pt4+ and the morphologies of the TRGO
Pt) nanohybrids by simply spraying a GO:Pt4+ solution on a hot plate. X-ray photoelectron spectroscopy and atomic force microscopy analyses are performed to investigate the thermal reduction of GO:Pt4+ and the morphologies of the TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt hybrid monolayer, respectively. The catalytic performance of TRGO
Pt hybrid monolayer, respectively. The catalytic performance of TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt is evaluated for the reduction of o-nitroaniline. A significant increase in the reaction rate constant for TRGO
Pt is evaluated for the reduction of o-nitroaniline. A significant increase in the reaction rate constant for TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt compared with pure Pt is due to facilitated electron transfer at the TRGO
Pt compared with pure Pt is due to facilitated electron transfer at the TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt interface and enhanced catalytic active sites. Effective electron transfer from TRGO to Pt and significantly increased catalytic active sites in hybrids suggest that TRGO
Pt interface and enhanced catalytic active sites. Effective electron transfer from TRGO to Pt and significantly increased catalytic active sites in hybrids suggest that TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt is a highly potential counter electrode material in dye-sensitized solar cells (DSSCs). The hybrid provides numerous electrons to I−/I3− electrolyte to reduce the recombination at the interface. As a result, the performance of DSSCs with the TRGO
Pt is a highly potential counter electrode material in dye-sensitized solar cells (DSSCs). The hybrid provides numerous electrons to I−/I3− electrolyte to reduce the recombination at the interface. As a result, the performance of DSSCs with the TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt hybrid electrode is significantly increased by 34% in comparison with a pure Pt electrode.
Pt hybrid electrode is significantly increased by 34% in comparison with a pure Pt electrode.
Introduction
Numerous efforts have been recently made to improve the efficiency of dye-sensitized solar cells (DSSCs), which are promising photovoltaic devices, and to reduce their manufacturing costs.1–7 Typically, the operational principles of DSSCs are based on the generation and diffusion of electrons at interfaces such as injection of photoinduced electrons from dyes, diffusion of electrons through semiconductors, and regeneration of oxidized dyes (receiving electrons from electrolyte) and reduced electrolytes at counter electrode interfaces (charge transfer reaction through catalytic activity at counter electrode).8–11 The interfacial structure of TiO2/ruthenium-based dye/iodide-triiodide redox couple electrolyte/platinum (Pt) counter electrode has been mostly used in the fabrication of high-performance DSSCs.12–15 Especially, catalytic reduction at the counter electrode surface is crucial in producing surplus electrons that are transferred to the electrolyte to regenerate oxidized dye, which greatly affects the conversion efficiency of DSSCs. Several materials have been employed in the fabrication of the counter electrode of DSSCs such as a thin catalytic Pt layer on conducting glass substrate,16,17 carbon material (carbon black, graphene, carbon nanotube),18–20 and graphene-based composite material.21–25 Graphene-based composites have recently attracted attention in fabricating low-cost and high-performance counter electrode of DSSCs due to its large specific surface area, excellent catalytic activity support, and high conductivity of graphene.26–31 Ahn et al. reported a superior performance of p-doped 3D graphene/Pt hybrid as the counter electrode of a DSSC.26 The DSSC efficiency of graphene-based hybrid counter electrode (8.46%) was 6% greater than that of Pt counter electrode (7.98%). Lin et al. developed nitrogen-doped graphene/platinum hybrid for use as the counter electrode to enhance the DSSCs efficiency.27 The hybrid electrode showed the best performance of 9.38% which increased by 25% in comparison with the Pt counter electrode (7.53%).In this article, we synthesize thermally reduced graphene oxide (TRGO)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt hybrid using a novel and simple spray pyrolysis method and test the hybrid as the counter electrode of DSSCs. X-ray photoelectron spectroscopy (XPS) and atomic force microscopy (AFM) are used to study the bond interactions and surface morphologies of the hybrid. The synthesized TRGO
Pt hybrid using a novel and simple spray pyrolysis method and test the hybrid as the counter electrode of DSSCs. X-ray photoelectron spectroscopy (XPS) and atomic force microscopy (AFM) are used to study the bond interactions and surface morphologies of the hybrid. The synthesized TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt hybrid exhibits enhanced catalytic performance and high durability for catalytic hydrogenation. The TRGO
Pt hybrid exhibits enhanced catalytic performance and high durability for catalytic hydrogenation. The TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt hybrid provides surplus electrons to reduce a redox couple in the electrolyte resulting in a significant increase the efficiency of DSSCs to 5.74% (34% increase) compared with the Pt counter electrode (4.31%). We demonstrate that the TRGO
Pt hybrid provides surplus electrons to reduce a redox couple in the electrolyte resulting in a significant increase the efficiency of DSSCs to 5.74% (34% increase) compared with the Pt counter electrode (4.31%). We demonstrate that the TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt hybrid is a promising counter electrode material of DSSCs.
Pt hybrid is a promising counter electrode material of DSSCs.
Experimental
Preparation of TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) Pt hybrid
Pt hybrid
0.1 mL of 8%wt H2PtCl6 in H2O (Sigma Aldrich) was diluted in 5 mL of ethanol. 1, 3, 5, and 7 mL of colloidal GO (0.3 g L−1) synthesized using a modified Hummers method were added to the diluted solution, which was followed by stirring for 30 min. The final solution was sprayed on FTO substrate (for DSSCs study) and quartz substrate (for catalytic measurement) on a hot plate at 150 °C. The sprayed sample was burned on the hot plate at 350 °C for 20 min to thermally reduce graphene oxide to TRGO and Pt4+ to Pt and to improve the adhesion of the thin film coated on the substrate.
Fabrication of DSSCs
A TiO2 paste (Ti-Nanoxide T/SP, Solaronix) was coated on FTO substrate using a doctor-blade technique and was then annealed at 500 °C for 60 min in air. To adsorb a sensitizing dye, the annealed mesoporous TiO2 film was immersed in 0.3 mM Z907 dye solution (C42H52N6O4RuS2, Sigma-Aldrich) in a mixture of acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tert-butyl alcohol (1
tert-butyl alcohol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) at room temperature for 6 h. DSSCs were assembled onto two substrates of photoanode and counter electrode using a thermoplastic foil. The iodide/tri-iodide electrolyte (I−/I3−, Iodolyte AN-50, Solaronix) was injected into the space between the photoanode and photocathode through a hole in the counter electrode substrate.
1 v/v) at room temperature for 6 h. DSSCs were assembled onto two substrates of photoanode and counter electrode using a thermoplastic foil. The iodide/tri-iodide electrolyte (I−/I3−, Iodolyte AN-50, Solaronix) was injected into the space between the photoanode and photocathode through a hole in the counter electrode substrate.
Catalytic hydrogenation measurement
The catalytic hydrogenation performance of TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt hybrids was evaluated for the reduction of o-nitroaniline to 1,2-benzenediamine. Sodium borohydride (NaBH4) was used as a hydrogen source for hydrogenation reaction. A mixture of 3 mL of 0.75 mM o-nitroaniline and 2 mL of 0.22 M NaBH4 was stirred in 17 mL of deionized water for 5 min. The prepared TRGO
Pt hybrids was evaluated for the reduction of o-nitroaniline to 1,2-benzenediamine. Sodium borohydride (NaBH4) was used as a hydrogen source for hydrogenation reaction. A mixture of 3 mL of 0.75 mM o-nitroaniline and 2 mL of 0.22 M NaBH4 was stirred in 17 mL of deionized water for 5 min. The prepared TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt on quartz substrate was placed in the o-nitroaniline solution to initiate the reduction reaction. UV-vis spectroscopy (HP8453) was employed to monitor the concentration of o-nitroaniline by measuring the absorption intensity at 410 nm.
Pt on quartz substrate was placed in the o-nitroaniline solution to initiate the reduction reaction. UV-vis spectroscopy (HP8453) was employed to monitor the concentration of o-nitroaniline by measuring the absorption intensity at 410 nm.
Photo-conversion efficiency measurement
The photo-conversion efficiency of the DSSCs was evaluated using a PEC-L11 model 13 (Pecell Technologies Inc.) under simulated AM 1.5 illumination at an intensity of 100 mW cm−2. The intensity of sunlight illumination was calibrated using a standard Si-photodiode detector with a KG-5 filter.Characterization
Chemical bonding was investigated using a X-ray photoelectron spectroscopy (Thermal Fisher, K-alpha, XPS) and a FT-IR spectrometer (Varian 670/620). The surface morphologies of the samples were examined using field emission scanning electron microscopy (SEM, JEOL, JSM6500F) with a 10 kV operating voltage, atomic force microscopy (AFM, Veeco, Multimode V) with RTESP probe in a tapping mode, and transmission electron microscopy (TEM, JOEL, JEM-2200FS) with a 200 keV electron gun. The cyclic voltammetry with three electrodes including the Pt counter electrode, the Ag/AgCl reference electrode, and the working electrode (TRGO, Pt and TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt) was performed using an electrochemical workstation (CHI 660E) in a solution of I−/I3− and 0.1 M LiCl in ethanol at a scan rate of 50 mV s−1. The electrochemical impedance of the samples was measured using an impedance spectroscopy (IviumStat, Ivium Tech) in the frequency range of 1–106 Hz.
Pt) was performed using an electrochemical workstation (CHI 660E) in a solution of I−/I3− and 0.1 M LiCl in ethanol at a scan rate of 50 mV s−1. The electrochemical impedance of the samples was measured using an impedance spectroscopy (IviumStat, Ivium Tech) in the frequency range of 1–106 Hz.
Results and discussion
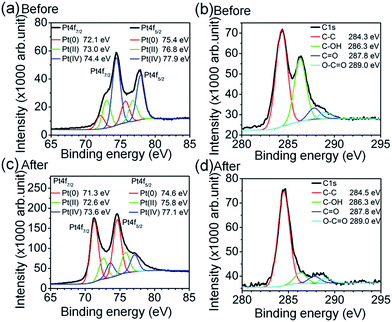
Fig. 1 shows the XPS spectra of GO:Pt4+ (before thermal treatment) and TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt (after thermal treatment at 350 °C for 20 min). The Pt4f surveys (Fig. 1a and c) reveal the presence of Pt(0), Pt(II), and Pt(IV) and the C1s scans (Fig. 1b and d) show the presence of C–C and other carbon groups in graphene network. As shown in Fig. 1a, the bonding energies of metallic Pt(0) are 72.1 eV (Pt4f7/2) and 75.4 eV (Pt4f5/2).32 Ionic Pt(II) and Pt (IV) peaks appear at 73.0 and 74.4 eV for Pt4f7/2; 76.8 eV and 77.9 eV for Pt4f5/2, respectively.32 After thermal treatment, the binding energy of Pt decreases and the metallic Pt(0) peak intensity significantly increases. The ratios of Pt(0)
Pt (after thermal treatment at 350 °C for 20 min). The Pt4f surveys (Fig. 1a and c) reveal the presence of Pt(0), Pt(II), and Pt(IV) and the C1s scans (Fig. 1b and d) show the presence of C–C and other carbon groups in graphene network. As shown in Fig. 1a, the bonding energies of metallic Pt(0) are 72.1 eV (Pt4f7/2) and 75.4 eV (Pt4f5/2).32 Ionic Pt(II) and Pt (IV) peaks appear at 73.0 and 74.4 eV for Pt4f7/2; 76.8 eV and 77.9 eV for Pt4f5/2, respectively.32 After thermal treatment, the binding energy of Pt decreases and the metallic Pt(0) peak intensity significantly increases. The ratios of Pt(0)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt(II) and Pt(0)
Pt(II) and Pt(0)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt(IV) in Pt4f7/2 binding peak intensity are significantly increased from 0.46 and 0.18 (before annealing) to 2.45 and 3.06 (after annealing), respectively. These results indicate the formation of metallic Pt in the composite.
Pt(IV) in Pt4f7/2 binding peak intensity are significantly increased from 0.46 and 0.18 (before annealing) to 2.45 and 3.06 (after annealing), respectively. These results indicate the formation of metallic Pt in the composite.
The thermal treatment of GO reduces the intensity of peaks related to oxygen functional groups as shown in Fig. 1b and d. The C–C binding energy slightly increases from 284.3 to 284.5 eV after thermal reduction process. Hydroxyl (C–OH), carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and carboxylate (HO–C
O), and carboxylate (HO–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) groups have their binding energies at 286.3, 287.8, 289.0 eV, respectively.33 The oxygen functional groups in graphene network such as C–OH, C
O) groups have their binding energies at 286.3, 287.8, 289.0 eV, respectively.33 The oxygen functional groups in graphene network such as C–OH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and HO–C
O, and HO–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O are greatly reduced after thermal reduction process. The C–OH, C
O are greatly reduced after thermal reduction process. The C–OH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and O–C
O, and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O to C–C peak intensity ratio is decreased from 0.71, 0.20, and 0.15 to 0.13, 0.09, and 0.06 after annealing, respectively. Thus, by simply placing the sample on a hot plate at 350 °C for 20 min, GO and Pt4+ are easily reduced to TRGO and metallic Pt, respectively. The FT-IR spectra of GO and TRGO are shown in Fig. S1† indicating that the oxygen-related functional groups of GO are greatly reduced after thermal reduction.
O to C–C peak intensity ratio is decreased from 0.71, 0.20, and 0.15 to 0.13, 0.09, and 0.06 after annealing, respectively. Thus, by simply placing the sample on a hot plate at 350 °C for 20 min, GO and Pt4+ are easily reduced to TRGO and metallic Pt, respectively. The FT-IR spectra of GO and TRGO are shown in Fig. S1† indicating that the oxygen-related functional groups of GO are greatly reduced after thermal reduction.
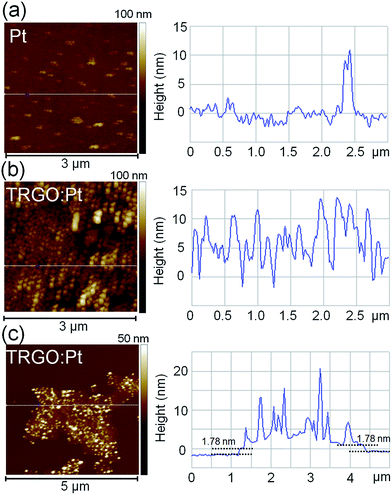
The AFM images of the samples are shown in Fig. 2. After thermal treatment of the as-sprayed H2PtCl6 solution, a smooth Pt film is formed on the substrate (Fig. 2a). One can see that the Pt film consists of Pt nanoparticles that are produced from the thermal reduction of Pt4+ precursor. Some Pt clusters are observed that result from the aggregation of nanoparticles. The surface roughness of the TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt film is higher than that of the Pt film (Fig. 2b). The AFM image of Pt-decorated TRGO monolayer is illustrated in Fig. 2c. The thickness of the TRGO monolayer is found to be about 1.78 nm and Pt nanoparticles decorated onto the TRGO monolayer are 3–15 nm in size. In the absence of GO, spraying H2PtCl6 colloidal on the substrate results in a smooth Pt film which consists of aggregated Pt4+ particles. In the TRGO
Pt film is higher than that of the Pt film (Fig. 2b). The AFM image of Pt-decorated TRGO monolayer is illustrated in Fig. 2c. The thickness of the TRGO monolayer is found to be about 1.78 nm and Pt nanoparticles decorated onto the TRGO monolayer are 3–15 nm in size. In the absence of GO, spraying H2PtCl6 colloidal on the substrate results in a smooth Pt film which consists of aggregated Pt4+ particles. In the TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt hybrid, GO serves as a stabilizer that adsorbs Pt4+ particles onto its negative network,34 thus preventing the aggregation of Pt particles and forming nanoparticle structure during thermal reduction process. Because of the excellent adsorption ability and hydrophilic properties of colloidal GO, Pt4+ is easily adsorbed onto the surface of GO nanosheets. After thermal reduction, Pt nanoparticles formed are evenly distributed over TRGO nanosheets as shown in Fig. 2c.
Pt hybrid, GO serves as a stabilizer that adsorbs Pt4+ particles onto its negative network,34 thus preventing the aggregation of Pt particles and forming nanoparticle structure during thermal reduction process. Because of the excellent adsorption ability and hydrophilic properties of colloidal GO, Pt4+ is easily adsorbed onto the surface of GO nanosheets. After thermal reduction, Pt nanoparticles formed are evenly distributed over TRGO nanosheets as shown in Fig. 2c.
Fig. 3 shows the TEM images of TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt hybrid catalyst. As shown in Fig. 3, the size of Pt nanoparticles is 3–15 nm, which is consistent with the AFM results. One can see that the Pt nanoparticles are evenly distributed over TRGO nanosheets. High resolution TEM image and corresponding FFT pattern in Fig. S2† indicate the formation of crystalline TRGO
Pt hybrid catalyst. As shown in Fig. 3, the size of Pt nanoparticles is 3–15 nm, which is consistent with the AFM results. One can see that the Pt nanoparticles are evenly distributed over TRGO nanosheets. High resolution TEM image and corresponding FFT pattern in Fig. S2† indicate the formation of crystalline TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt hybrids. The (111) and (200) diffraction spots of Pt nanoparticles and (002) spot of graphene are clearly observed in the FFT pattern.
Pt hybrids. The (111) and (200) diffraction spots of Pt nanoparticles and (002) spot of graphene are clearly observed in the FFT pattern.
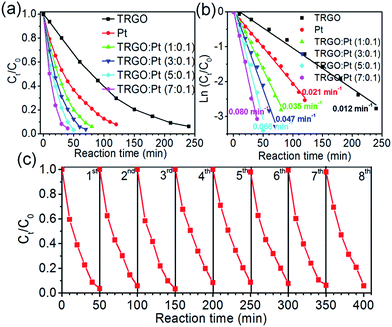
The catalytic reduction efficiency of o-nitroaniline into 1,2-benzenediamine is shown in Fig. 4. The ratio in the TRGO–Pt nanohybrid sample name refers to the volume ratio of colloidal GO to H2PtCl6 in H2O before diluted with ethanol. To test the stability and durability of the synthesized catalysts, they are prepared in the form of thin film on quartz substrate using a spray pyrolysis method. As the content of TRGO in the hybrid increases, the catalytic performance is considerably improved as shown in Fig. 4a and b. If the reduction reaction is assumed to follow first-order kinetics, the reaction rate constant is found to be as 0.012 min−1 for TRGO and 0.021 min−1 for pure Pt, and it increases to 0.036, 0.047, 0.066, and 0.080 min−1 for TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt hybrids prepared with 1, 3, 5, and 7 mL of aqueous GO precursor, respectively. Fig. 4c illustrates the stability and durability of the TRGO
Pt hybrids prepared with 1, 3, 5, and 7 mL of aqueous GO precursor, respectively. Fig. 4c illustrates the stability and durability of the TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt hybrid catalyst for the reduction of o-nitroaniline for eight cycles. Each cycle runs for 50 min. The catalytic efficiency is maintained at 90–97% for each cycle, which shows that the synthesized catalyst is highly stable and durable.
Pt hybrid catalyst for the reduction of o-nitroaniline for eight cycles. Each cycle runs for 50 min. The catalytic efficiency is maintained at 90–97% for each cycle, which shows that the synthesized catalyst is highly stable and durable.
Pt has been well-known as an excellent catalyst for hydrogenation reaction. In TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt hybrids, Pt nanoparticles accept electrons from TRGO to enhance the ability of hydrogen chemisorption for catalytic reactivity. The reduction mechanism of o-nitroaniline to 1,2-benzenediamine using a TGRO
Pt hybrids, Pt nanoparticles accept electrons from TRGO to enhance the ability of hydrogen chemisorption for catalytic reactivity. The reduction mechanism of o-nitroaniline to 1,2-benzenediamine using a TGRO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt catalyst is described in Fig. 5. NaBH4 reacts with water to generate hydrogen molecules which are attracted to the Pt surface by physisorption process and then are dissociated on the Pt surface by chemisorption process.34,35 Hydrogen atoms produced reduce the O
Pt catalyst is described in Fig. 5. NaBH4 reacts with water to generate hydrogen molecules which are attracted to the Pt surface by physisorption process and then are dissociated on the Pt surface by chemisorption process.34,35 Hydrogen atoms produced reduce the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+–O− group in o-nitroaniline to the NH2 group as follows:
N+–O− group in o-nitroaniline to the NH2 group as follows:
| NH2(C6H4)NO2 + 6H → NH2(C6H4)NH2 + 2H2O |
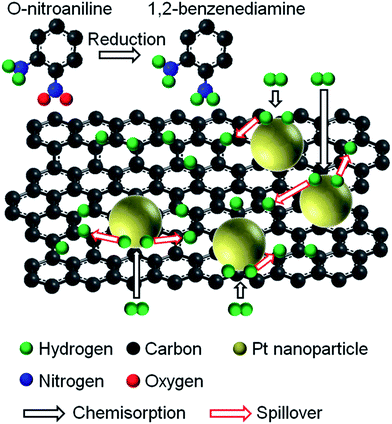 | ||
Fig. 5 A schematic illustration of the reduction of o-nitroaniline to 1,2-benzenediamine over TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt catalyst. Pt catalyst. | ||
Due to a difference in work function between TRGO and Pt, electrons can transport from TRGO nanosheets to Pt nanoparticles,36,37 thus enhancing the electron perturbation at the Pt surface which easily dissociates hydrogen molecules chemisorbed on the Pt surface for catalytic reaction. Furthermore, an enhanced catalytic performance with an increase in TRGO amount in Fig. 4 indicates that TRGO works as the active site for catalytic reaction. The chemisorbed hydrogen atoms tend to spillover to TRGO network.38 The negative surface of TRGO could attract the NO2 terminal group of o-ntroaniline,34,39 reducing it to the NH2 group. Thus, TRGO acts as a ESI† in improving the catalytic performance of Pt by providing electrons to the Pt surface and it also acts as an active site in catalytic reduction. The absorption spectra of o-nitroaniline reduced over TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt catalysts with different composition are shown in Fig. S3† to monitor the reduction of o-nitroanilline to 1,2-benzendiamine. The absorption peaks appearing at 280 and 415 nm are indicative of the presence of o-nitroaniline. As the catalytic reduction proceeds, the intensity of the main peak at 415 nm is decreased and the peak at 280 nm is found to be red-shifted. A red-shifted of the peak from 280 to 290 nm with reaction time indicates that the reduction of o-nitroaniline to 1,2-benzenediamine has taken place.40
Pt catalysts with different composition are shown in Fig. S3† to monitor the reduction of o-nitroanilline to 1,2-benzendiamine. The absorption peaks appearing at 280 and 415 nm are indicative of the presence of o-nitroaniline. As the catalytic reduction proceeds, the intensity of the main peak at 415 nm is decreased and the peak at 280 nm is found to be red-shifted. A red-shifted of the peak from 280 to 290 nm with reaction time indicates that the reduction of o-nitroaniline to 1,2-benzenediamine has taken place.40
Taking advantage of its highly stable and effective catalytic activity, we fabricate DSSCs using TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt as the counter electrode material. Fig. 6a shows the cross-sectional SEM image of a TiO2 photoanode which is prepared by applying TiO2 paste to a FTO glass substrate using a doctor-blade technique. The thickness of the TiO2 photoanode is 11 μm. The cross-sectional SEM images of a TRGO
Pt as the counter electrode material. Fig. 6a shows the cross-sectional SEM image of a TiO2 photoanode which is prepared by applying TiO2 paste to a FTO glass substrate using a doctor-blade technique. The thickness of the TiO2 photoanode is 11 μm. The cross-sectional SEM images of a TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt counter electrode with various TRGO/Pt ratios are shown in Fig. 6b–f. One can see that the TRGO
Pt counter electrode with various TRGO/Pt ratios are shown in Fig. 6b–f. One can see that the TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt is tightly adhered to the FTO glass substrate. The thickness of the TRGO
Pt is tightly adhered to the FTO glass substrate. The thickness of the TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt slightly increases with an increase in the amount of colloidal GO precursor. The film thickness is 58 nm for Pt and increases to 67, 72, 81, and 86 nm for TRGO
Pt slightly increases with an increase in the amount of colloidal GO precursor. The film thickness is 58 nm for Pt and increases to 67, 72, 81, and 86 nm for TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt (1, 3, 5, and 7
Pt (1, 3, 5, and 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1), respectively.
0.1), respectively.
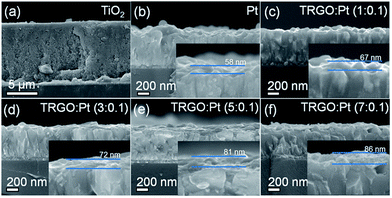 | ||
Fig. 6 Cross-sectional FESEM images of TiO2 photoanode (a), Pt (b), and TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt counter electrodes (c–f). Pt counter electrodes (c–f). | ||
Fig. 7 shows the cyclic voltammograms for the I−/I3− redox reaction of different working electrodes. Positive current peaks are attributed to the oxidation reaction, while negative current peaks are ascribed to the reduction reaction.41,42 The reduction peak at a lower voltage for TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt has a higher current-response and is red-shifted by 0.1 V in comparison with Pt. This result implies that TRGO
Pt has a higher current-response and is red-shifted by 0.1 V in comparison with Pt. This result implies that TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt hybrid exhibits stronger and faster reduction reaction than Pt. Accordingly, the TRGO
Pt hybrid exhibits stronger and faster reduction reaction than Pt. Accordingly, the TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt hybrid is an efficient counter electrode material for DSSCs to enhance the I−/I3− electrolyte reduction process.
Pt hybrid is an efficient counter electrode material for DSSCs to enhance the I−/I3− electrolyte reduction process.
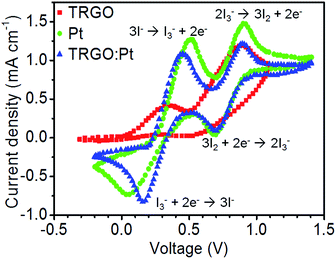 | ||
Fig. 7 The cyclic voltammograms for the I−/I3− redox reaction of different working electrodes including TRGO, Pt, and TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt. Pt. | ||
The electrochemical impedance spectra of DSSCs with different counter electrodes are shown in Fig. 8 to probe charge transfer at the interface between I−/I3− electrolyte and counter electrode. The semicircle radius of TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt hybrids is found to be smaller (indicating a lower charge transfer resistance) than that of Pt counter electrode. This result demonstrates that the TRGO
Pt hybrids is found to be smaller (indicating a lower charge transfer resistance) than that of Pt counter electrode. This result demonstrates that the TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt is suitable for use as a counter electrode in DSSCs to increase a charge transfer rate at TRGO
Pt is suitable for use as a counter electrode in DSSCs to increase a charge transfer rate at TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt–I−/I3− interface and reduce internal cell resistance.
Pt–I−/I3− interface and reduce internal cell resistance.
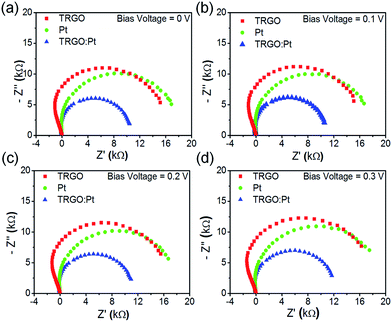 | ||
Fig. 8 Nyquist plots of electrochemical impedance spectroscopy of TRGO, Pt, and TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt at a bias voltage of 0 V (a), 0.1 V (b), 0.2 V (c), and 0.3 V (d). Pt at a bias voltage of 0 V (a), 0.1 V (b), 0.2 V (c), and 0.3 V (d). | ||
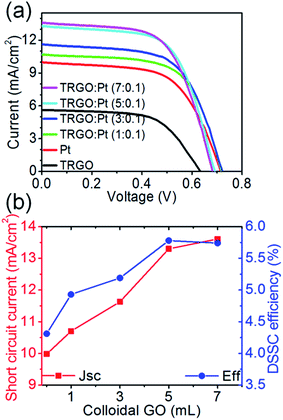
Fig. 9 shows the photovoltaic performance of DSSCs with different counter electrodes. The photoconversion efficiency (η) is 2.15% for the TRGO electrode and 4.31% for the Pt electrode. The η increases to 4.93, 5.19, 5.78, and 5.74% for the TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt (1, 3, 5 and 7
Pt (1, 3, 5 and 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1), respectively. It becomes saturated as 5.78% for the TRGO
0.1), respectively. It becomes saturated as 5.78% for the TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt (5
Pt (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) electrode. The short circuit current density of DSSCs, Jsc, increases as the amount of colloidal GO precursor is increased. The TRGO
0.1) electrode. The short circuit current density of DSSCs, Jsc, increases as the amount of colloidal GO precursor is increased. The TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt (5
Pt (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) electrode is found to exhibit the best performance as shown in Fig. 9b and Table 1. TRGO
0.1) electrode is found to exhibit the best performance as shown in Fig. 9b and Table 1. TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt hybrids are also evaluated for the catalytic reduction of I3− to I− in the electrolyte of DSSCs to enhance photoconversion efficiency. As shown in Fig. 9, the short circuit current, Jsc, of the DSSCs increases with an increase in the amount of TRGO from 9.98 for pure Pt to 13.61 mA cm−2 for TRGO
Pt hybrids are also evaluated for the catalytic reduction of I3− to I− in the electrolyte of DSSCs to enhance photoconversion efficiency. As shown in Fig. 9, the short circuit current, Jsc, of the DSSCs increases with an increase in the amount of TRGO from 9.98 for pure Pt to 13.61 mA cm−2 for TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt hybrid (7
Pt hybrid (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1). These results indicate that TRGO improves electron transport in the photoanode resulting in enhanced photovoltaic performance.
0.1). These results indicate that TRGO improves electron transport in the photoanode resulting in enhanced photovoltaic performance.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt counter electrode
Pt counter electrode
| Sample | Voc V | Jsc mA cm−2 | FF | Eff% |
|---|---|---|---|---|
| TRGO | 0.64 | 5.61 | 0.60 | 2.15 |
| Pt | 0.71 | 9.98 | 0.61 | 4.31 |
TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt (1 Pt (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) 0.1) |
0.72 | 10.70 | 0.64 | 4.93 |
TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt (3 Pt (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) 0.1) |
0.72 | 11.63 | 0.62 | 5.19 |
TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt (5 Pt (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) 0.1) |
0.69 | 13.30 | 0.63 | 5.78 |
TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt (7 Pt (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) 0.1) |
0.68 | 13.61 | 0.62 | 5.74 |
Fig. 10 schematically illustrates the role of TRGO in supplying electrons to reduce I3− thus increasing Jsc in a DSSC. Under solar irradiation, electrons are generated from dye and are diffused to the conduction band of TiO2. At the same time, the dye is oxidized (D+) and is then recovered by accepting an electron from I− in the electrolyte.10,43 I− loses electrons to be I3−, and I3− receives electrons from the counter electrode to form I−.10,11 A number of D+ and I3− ions in the DSSC may recombine with electrons in dye and TiO2, reducing the performance of the DSSC. When the Pt film is used as the counter electrode (Fig. 10a), electrons may not be supplied from Pt enough to reduce I3−, so the electron recombination would considerably occur. However, when the TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt hybrid is used as the counter electrode (Fig. 10b), rapid electron transfer from TRGO to Pt at the interface and electron transfer from TRGO to the electrolyte greatly increase the reduction rate of I3−, thus reducing the recombination of photo-excited electrons in dye and TiO2 photoanode, which enhances Jsc in the DSSC. Furthermore, the decoration of Pt nanoparticles on the TRGO surface increases the number of active sites for catalytic reduction of I3−.
Pt hybrid is used as the counter electrode (Fig. 10b), rapid electron transfer from TRGO to Pt at the interface and electron transfer from TRGO to the electrolyte greatly increase the reduction rate of I3−, thus reducing the recombination of photo-excited electrons in dye and TiO2 photoanode, which enhances Jsc in the DSSC. Furthermore, the decoration of Pt nanoparticles on the TRGO surface increases the number of active sites for catalytic reduction of I3−.
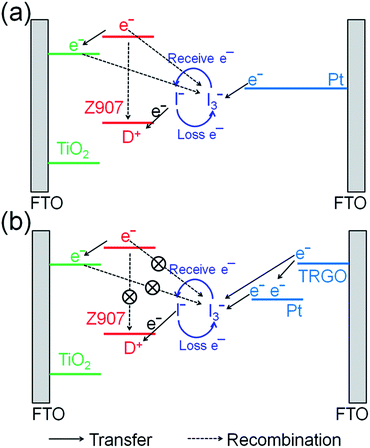 | ||
Fig. 10 A schematic illustration of electron transfer in a DSSC using Pt (a) and TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt (b) as the counter electrode. Pt (b) as the counter electrode. | ||
Conclusions
We synthesize TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt hybrids via a novel and facile solution method and evaluate them for use as a catalyst for the hydrogenation reaction of o-nitroaniline and a counter electrode in DSSCs. We demonstrate that TRGO with its negative network provides electrons to Pt and that it serves as a stabilizer for the uniform decoration of Pt nanopaticles on the Pt surface, which increases the number of catalytic active sites for the reduction of o-nitroaniline and I3−. The excellent catalytic performance of TRGO
Pt hybrids via a novel and facile solution method and evaluate them for use as a catalyst for the hydrogenation reaction of o-nitroaniline and a counter electrode in DSSCs. We demonstrate that TRGO with its negative network provides electrons to Pt and that it serves as a stabilizer for the uniform decoration of Pt nanopaticles on the Pt surface, which increases the number of catalytic active sites for the reduction of o-nitroaniline and I3−. The excellent catalytic performance of TRGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt hybrids results in a significant increase in Jsc enhancing the efficiency of DSSCs.
Pt hybrids results in a significant increase in Jsc enhancing the efficiency of DSSCs.
Acknowledgements
This research was financially supported by Priority Research Centers Program (2009-0093818) and by the National Research Foundation of Korea (NRF-2014R1A4A1071686).Notes and references
- B. E. Hardin, H. J. Snaith and M. D. McGehee, Nat. Photonics, 2012, 6, 162 CrossRef CAS.
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef.
- N. A. Ludin, A. M. A. Mahmoud, A. B. Mohamad, A. A. H. Kadhum, K. Sopian and N. S. A. Karim, Renewable Sustainable Energy Rev., 2014, 31, 386 CrossRef CAS.
- A. Nattestad, A. J. Mozer, M. K. R. Fischer, Y. B. Cheng, A. Mishra, P. Bäuerle and U. Bach, Nat. Mater., 2010, 9, 31 CrossRef CAS PubMed.
- J. Wu, Z. Lan, J. Lin, M. Huang, Y. Huang, L. Fan and G. Luo, Chem. Rev., 2015, 115, 2136 CrossRef CAS PubMed.
- M. Urbani, M. Grätzel, M. K. Nazeeruddin and T. Torres, Chem. Rev., 2014, 114, 12330 CrossRef CAS PubMed.
- Z. Chai, J. Gu, J. Khan, Y. Yuan, L. Du, X. Yu, M. Wu and W. Mai, RSC Adv., 2015, 5, 88052 RSC.
- A. Akimov, A. J. Neukirch and O. V. Prezhdo, Chem. Rev., 2013, 113, 4496 CrossRef CAS PubMed.
- J. A. Anta, E. Guillén and R. Tena-Zaera, J. Phys. Chem. C, 2012, 116, 11413 CAS.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595 CrossRef CAS PubMed.
- S. Thomas, T. G. Deepak, G. S. Anjusree, T. A. Arun, S. V. Nair and A. S. Nair, J. Mater. Chem. A, 2014, 2, 4474 CAS.
- H. Kim and J. S. Suh, RSC Adv., 2015, 5, 74107 RSC.
- D. Bhattacharyya, P. K. Sarswat, M. Islam, G. Kumar, M. Misra and M. L. Free, RSC Adv., 2015, 5, 70361 RSC.
- Y. S. Kwon, I. Song, J. C. Lim, I. Y. Song, A. Siva and T. Park, ACS Appl. Mater. Interfaces, 2012, 4, 3141 CAS.
- T. Kinoshita, J. T. Dy, S. Uchida, T. Kubo and H. Segawa, Nat. Photonics, 2013, 7, 535 CrossRef CAS.
- G. Calogero, P. Calandra, A. Irrera, A. Sinopoli, I. Citro and G. D. Marco, Energy Environ. Sci., 2011, 4, 1838 CAS.
- Z. Zheng, J. Chen, Y. Hu, W. Wu, J. Hua and H. Tian, J. Mater. Chem. C, 2014, 2, 8497 RSC.
- J. D. Roy-Mayhew, D. J. Bozym, C. Punckt and I. A. Aksay, ACS Nano, 2010, 4, 6203 CrossRef CAS PubMed.
- W. J. Lee, E. Ramasamy, D. Y. Lee and J. S. Song, ACS Appl. Mater. Interfaces, 2009, 1, 1145 CAS.
- M. Chen, L. L. Shao, X. Qian, L. Liu, T. Z. Ren and Z. Y. Yuan, Chem. Eng. J., 2014, 256, 23 CrossRef CAS.
- M. H. Yeh, L. Y. Lin, C. L. Sun, Y. A. Leu, J. T. Tsai, C. Y. Yeh, R. Vittal and K. C. Ho, J. Phys. Chem. C, 2014, 118, 16626 CAS.
- G. Li, X. Chen and G. Gao, Nanoscale, 2014, 6, 3283 RSC.
- J. D. Roy-Mayhew and I. A. Aksay, Chem. Rev., 2014, 114, 6323 CrossRef CAS PubMed.
- Q. Luo, F. Hao, S. Wang, H. Shen, L. Zhao, J. Li, M. Gratzel and H. Lin, J. Phys. Chem. C, 2014, 118, 17010 CAS.
- S. P. Lim, A. Pandikumar, Y. S. Lim, N. M. Huang and H. N. Lim, Sci. Rep., 2014, 4, 5305 Search PubMed.
- H. J. Ahn, I. H. Kim, J. C. Yoon, S. I. Kim and J. H. Jang, Chem. Commun., 2014, 50, 2412 RSC.
- C. A. Lin, C. P. Lee, S. T. Ho, T. C. Wei, Y. W. Chi, K. P. Huang and J. H. He, ACS Photonics, 2014, 1, 1264 CrossRef CAS.
- K. Saranya, N. Sivasankar and A. Subramania, RSC Adv., 2014, 4, 36226 RSC.
- H. Bi, S. Sun, F. Huang, X. Xie and M. Jiang, J. Mater. Chem., 2012, 22, 411 RSC.
- G. Wang, Y. Fang, Y. Lin, W. Xing and S. Zhuo, Mater. Res. Bull., 2012, 47, 4252 CrossRef CAS.
- Y. C. Hsu, G. L. Chen and R. H. Lee, J. Polym. Res., 2014, 21, 440 CrossRef.
- K. C. Park, I. Y. Jang, W. Wongwiriyapan, S. Morimoto, Y. J. Kim, Y. C. Jung, T. Toya and M. Endo, J. Mater. Chem., 2010, 20, 5345 RSC.
- S. Stankovich, R. D. Piner, X. Chen, N. Wu, S. T. Nguyen and R. S. Ruoff, J. Mater. Chem., 2006, 16, 155 RSC.
- N. T. Khoa, S. W. Kim, D. H. Yoo, E. J. Kim and S. H. Hahn, Appl. Catal., A, 2014, 469, 159 CrossRef CAS.
- J. Huang, L. Zhang, B. Chen, N. Ji, F. Chen, Y. Zhang and Z. Zhang, Nanoscale, 2010, 2, 2733 RSC.
- N. T. Khoa, S. W. Kim, D. H. Yoo, S. Cho, E. J. Kim and S. H. Hahn, ACS Appl. Mater. Interfaces, 2015, 7, 3524 CAS.
- G. Giovannetti, P. A. Khomyakov, G. Brocks, V. M. Karpan, J. van den Brink and P. J. Kelly, Phys. Rev. Lett., 2008, 101, 026803 CrossRef CAS PubMed.
- C. E. Hunt, J. Catal., 1971, 23, 93 CrossRef CAS.
- Y. Choi, H. S. Bae, E. Seo, S. Jang, K. H. Park and B. S. Kim, J. Mater. Chem., 2011, 21, 15431 RSC.
- D. V. Thuan, N. T. Khoa, S. W. Kim, E. J. Kim and S. H. Hahn, J. Catal., 2015, 329, 144 CrossRef.
- W. B. Dai, Y. F. Lei, P. Li and L. F. Xu, J. Mater. Chem. A, 2015, 3, 4875 CAS.
- S. Shukla, N. H. Loc, P. P. Boix, T. M. Koh, R. R. Prabhakar, H. K. Mulmudi, J. Zhang, S. Chen, C. F. Ng, C. H. A. Huan, N. Mathews, T. Sritharan and Q. Xiong, ACS Nano, 2014, 8, 10597 CrossRef CAS PubMed.
- A. Nattestad, A. J. Mozer, K. M. R. Fischer, Y. B. Cheng, A. Mishra, P. Bäuerle and U. Bach, Nat. Mater., 2010, 9, 31 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra21896a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |