Structural analysis of ginseng polysaccharides extracted by EDTA solution
Yan Zheng,
Guang Yang,
Zihan Zhao,
Te Guo,
Huimin Shi,
Yifa Zhou and
Lin Sun*
Jilin Province Key Laboratory on Chemistry and Biology of Natural Drugs in Changbai Mountain, School of Life Sciences, Northeast Normal University, Changchun 130024, P. R. China. E-mail: sunl925@nenu.edu.cn; Tel: +86-431-85099350
First published on 23rd December 2015
Abstract
In the present study, polysaccharides were extracted from ginseng with ethylene diamine tetraacetic acid (EDTA) after consecutive hot water and α-amylase extraction. The yield of EDTA-soluble ginseng polysaccharide (EGP) was 6.0%. Using anion exchange and gel permeation chromatography, EGP was fractionated into one major starch-like glucan (EGP-N), two minor glucan fractions (EGP-1a and EGP-1b) and four pectic fractions (EGP-2a, EGP-2b, EGP-3a and EGP-3b). High-performance liquid chromatography, fourier transform-infrared and nuclear magnetic resonance analyses demonstrated that the four pectic fractions were composed of both homogalacturonan and type-I rhamnogalacturonan domains. EGP had a better stimulation effect on lymphocyte proliferation in vivo than hot water and α-amylase extracted polysaccharides, demonstrating that some novel and active polysaccharides can be extracted from ginseng by EDTA. These results provide new insight into the preparation of ginseng polysaccharides that may have potential use in the food and pharmaceutical industries.
1. Introduction
Panax ginseng C. A. Meyer (ginseng) is a well-known traditional medicinal plant that has been known in the Orient for more than 3000 years to have “mysterious powers”. In recent years, various ginseng preparations have been marketed as dietary supplements. Polysaccharides are one of the active components of ginseng.1 Since first reported in 1966,2 many studies have been performed on polysaccharides from ginseng in terms of their isolation, structural analysis, and bioactivities. In most reports, ginseng polysaccharides have been extracted primarily from ginseng roots by using distilled water.3–8 In recent years, our lab has completely fractionated ginseng polysaccharides by extraction with hot water, and has systematically studied their structure–activity relationships. Our results showed that hot water-extracted ginseng polysaccharides contained starch-like glucans, homogalacturonan (HG), rhamnogalacturonan-I (RG-I) and arabinogalactans (AG)-type pectin.9–11 Different types of ginseng polysaccharides exhibit different activities, including inhibitory effects against galectin-3,12 cancer cell anti-proliferative effects,13 immunomodulatory effects,14 inhibition of cell migration,10 anti-fatigue activity15 and anti-depressant-like effects.16Enzyme-assisted extraction is an emerging approach that has been used to extract polysaccharides from many plant and fungal species, such as Astragalus membranaceus,17 Hericium erinaceus,18 Cornus officinalis19 and Tricholoma matsutake.20 Based on these reports, our lab has employed α-amylase treatment post hot water extraction to improve extraction of polysaccharides from ginseng roots.21 Although this procedure also produced polysaccharides in high yield, including starch-like glucans, HG, RG-I and AG-type pectins, they had different monosaccharide compositions and weight-average molecular weights compared to hot water extracted polysaccharides. This demonstrated that α-amylase treatment following hot water extraction could yield polysaccharides with different chemical structures.21
Low-methyl esterified HG type pectin can bind Ca2+ ions within the lamella of cell walls, forming so called “egg-box” structures.22 These polysaccharides can not be extracted easily with water alone; however, they can be solubilized by using chelating agents, like ethylene diamine tetra-acetic acid (EDTA) or 1,2-diaminocyclo-hexane-N,N,N′,N′-tetra-acetic acid (CDTA).22 To our knowledge, few published reports have used EDTA to extract polysaccharides from ginseng. Therefore, in this study, we modified our ginseng root extraction procedure to include a EDTA extraction step, and compared the yields and structures of polysaccharides with those from hot water and α-amylase extraction. Our results will provide new insight into the content and chemical structure of polysaccharides from ginseng, findings that will be useful for better exploration of these polysaccharides in functional foods or medicine.
2. Experimental section
2.1 Materials
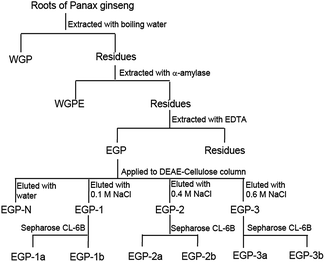
Ginseng were cultivated and collected from Changbai Mountain, Jilin Province, China. DEAE-cellulose and Sepharose CL-6B were purchased from Amersham Pharmacia Biotech (Uppsala, Sweden). Concanavalin A (ConA), lipopolysaccharide (LPS), 3-(4,5-dimethylthyiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were of analytical grade.2.2 Extraction of polysaccharides from ginseng roots by EDTA
Ginseng roots (500 g) were first extracted with hot water, yielding a polysaccharide fraction referred to as Water-soluble Ginseng Polysaccharide (WGP). Ginseng root residues were then extracted with the assistance of α-amylase, giving the next polysaccharide fraction called Water-soluble Ginseng Polysaccharide extracted by Enzyme (WGPE), as described in our previous papers.21 Ginseng root residues were further extracted with 4 L of 50 mM EDTA solution at 60 °C for 3 h, and this procedure was performed in duplicate. After filtration, the residues were washed twice with distilled water. The filtrates and washings were combined and centrifuged, following which the extracts were dialyzed against distilled water (MWCO = 3500 Da) and freeze-dried, yielding EDTA-soluble ginseng polysaccharide (EGP).2.3 Anion exchange chromatography
EGP (10 g) was dissolved in distilled water (100 mL), centrifuged and the supernatant was loaded on a DEAE-cellulose preparative column (8.0 × 20 cm, Cl−). Four fractions were produced upon stepwise elution with aqueous NaCl solutions of increasing ionic strength (0.0, 0.1, 0.4 and 0.6 M) at a flow rate of 13 mL min−1. Fractions of interest were pooled, concentrated, dialyzed and freeze-dried to give EGP-N, EGP-1, EGP-2 and EGP-3.2.4 Size exclusion chromatography
The three fractions (EGP-1, EGP-2, and EGP-3) were further fractionated on Sepharose CL-6B (1.5 × 90 cm) column. Each fraction was dissolved in 0.15 M NaCl, loaded onto the column and eluted with the same buffer at a flow rate of 0.15 mL min−1. Fractions (3 mL per tube) were collected and assayed for total sugar and uronic acid contents. The appropriate fractions were combined, concentrated, dialyzed and freeze-dried.2.5 General methods
Total carbohydrate content was determined by the phenol-sulfuric acid method, using glucose (Glc) as the standard.23 Uronic acid content was determined by the m-hydroxydiphenyl method, using galacturonic acid (GalA) as the standard.24 Protein content was determined by the Bradford assay, using bovine serum albumin (BSA) as the standard.25 Starch was detected by the I2-KI assay. Monosaccharide composition analysis was performed by high performance liquid chromatography (HPLC) as previously described.9 Weight-average molecular weights (Mw) were estimated by using high performance gel-permeation chromatography (HPGPC) with a TSK-gel G-3000PWXL column (7.8 × 300 mm, TOSOH, Japan) coupled to a Shimadzu HPLC system.92.6 Determination of the degree of methyl esterification (DM)
The DM was determined by using Fourier transform infrared spectroscopy (FT-IR, ThermoScientific, Waltham, MA, USA) with a Nicolet 560 FT-IR spectrometer and a DTGS detector over the range of 400 to 4000 cm−1.26 Specific bands at 1740 and 1630 cm−1 correspond to the absorption of the esterified carbonyl groups (–COOCH3) and carboxylic ions (–COO−), respectively. The DM was calculated as the ratio of the area under the 1740 cm−1 band to the sum of the areas under 1740 and 1630 cm−1 bands.2.7 NMR spectroscopy
Samples (20 mg) were dissolved in D2O (1 mL, 99.8%) and stirred overnight at room temperature. 13C NMR spectra were obtained using a Bruker AV600 NMR spectrometer (Bruker Inc., Rheinstetten, Germany) operating at 150 MHz. Spectra were recorded at 25 °C with 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 transients. Acetone was used as an internal standard.9
000 transients. Acetone was used as an internal standard.9
2.8 In vivo lymphocyte proliferation assay
Male ICR mice (18–22 g) were purchased from the Pharmacology Experimental Center of Jilin University, Changchun, China. Mice were housed and maintained on a light–dark cycle of 12/12 h with ad lib access to food and water. Animal handling procedures were conducted under the National Institutes of Health animal care and use guidelines. Polysaccharide samples were dissolved in physiological saline solution and administered intra-peritoneally into mice at doses of 1, 5, 10 and 50 mg kg−1, with each dose (0.2 mL) being administered for 14 consecutive days. Control mice were injected with physiological saline alone.After the 14 day treatment, mice were sacrificed by cervical dislocation. Spleen cells were extracted from each group and seeded (5 × 106 per mL) in a 96-well plate in the presence of ConA (5.0 μg mL−1) or LPS (10.0 μg mL−1). Cells were then incubated for 44 h at 37 °C in an atmosphere of 5% CO2. MTT (10 μL, 5 mg mL−1) was added to each well, and the plate was incubated for another 4 h. Lymphocyte proliferation was expressed as the absorption at 570 nm, measured by a microplate reader (Bio-Tek, Winooski, USA).9 Proliferation activities of different polysaccharides were compared to the control group by statistical analysis.
2.9 Statistical analyses
Results were expressed as the mean ± S.D. of the indicated number of experiments, and data were analyzed using the Student's t-test. p-Values of <0.05 and <0.01 were considered statistically significant.3. Results and discussion
3.1 Extraction of ginseng polysaccharide EGP by EDTA
In our previous reports, ginseng roots were sequentially extracted by using hot water and α-amylase, yielding polysaccharide fractions called WGP and WGPE,21 respectively. In this study, ginseng root residues were further extracted by using 50 mM EDTA solution to produce fraction EGP (Fig. 1). EGP had a lower yield (6.0%) than either WGP (10.7%) or WGPE (9.0%), and contained less Glc (50.4%) and more GalA (24.2%), Gal (10.5%), Ara (11.6%) and Rha (3.2%) than either WGP or WGPE (Table 1). The I2-KI assay indicated that EGP contained starch-like polysaccharides, which could not be hydrolyzed during the α-amylase extraction process. Based on GalA content, we concluded that EGP has a higher content of pectic polysaccharides than WGP or WGPE. Within the cell wall, these pectic polysaccharides might be low methyl esterified and Ca2+ cross-linked, and thus could be extracted with the use of chelating agent EDTA, something that does not work with hot water or α-amylase treatment.3.2 Fractionation of EGP by anion-exchange and size-exclusion chromatographies
EGP was applied to a DEAE-cellulose column (Cl−), and fractions were eluted step-wise with water, 0.1, 0.4, and 0.6 M NaCl. One neutral polysaccharide (EGP-N, 41.5%) and three acidic polysaccharides (EGP-1, 2.7%; EGP-2, 32.6%, and EGP-3, 2.7%) were obtained (Fig. 1). The three acidic polysaccharides were further fractionated on a Sepharose CL-6B column (Fig. 2). EGP-1 was separated into two fractions: EGP-1a (21.3%) and EGP-1b (21.0%) (Fig. 2A). EGP-2 was separated into two fractions: EGP-2a (57.1%) and EGP-2b (18.5%) (Fig. 2B). EGP-3 was also separated into two fractions: EGP-3a (21.0%) and EGP-3b (30.6%) (Fig. 2C).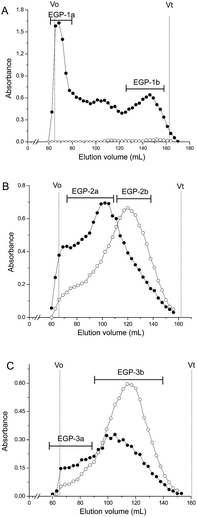 | ||
| Fig. 2 Elution profiles of (A) EGP-1, (B) EGP-2 and (C) EGP-3 on Sepharose CL-6B column (-●- total sugar; -○- uronic acid). | ||
3.3 Structural features of the EGP fractions
EGP-N was found to contain 88.7% Glc as the major component, and 3.9% Gal and 4.6% Ara as minor components (Table 2). The I2-KI assay indicated that EGP-N was mainly composed of starch-like glucan, with perhaps the presence of a few arabinogalactans.| Fraction | Yielda (w/w%) | Yieldb (w/w%) | DM (mol%) | Mw (Da) | PIc | Sugar composition (mol%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| GalA | Rha | Gal | Ara | Glc | ||||||
| a Yield in relation to fraction applied onto column.b Yield in relation to EGP.c PI: polydispersity index. | ||||||||||
| EGP-N | 41.5 | 41.5 | — | — | — | — | — | 3.9 | 4.6 | 88.7 |
| EGP-1a | 21.3 | 0.6 | — | 4.5 × 105 | 1.2 | — | — | — | — | 100 |
| EGP-1b | 21.0 | 0.6 | — | 6.2 × 103 | 1.8 | 2.3 | 1.1 | 11.2 | 9.9 | 75.5 |
| EGP-2a | 57.1 | 18.6 | 1.7 | 4.2 × 105 | 1.4 | 32.7 | 8.0 | 27.6 | 27.7 | 3.4 |
| EGP-2b | 18.5 | 6.0 | 7.6 | 1.5 × 105 | 1.7 | 46.5 | 7.0 | 20.7 | 21.9 | 3.9 |
| EGP-3a | 21.0 | 0.6 | 4.5 | 4.3 × 105 | 1.5 | 52.8 | 8.7 | 16.9 | 17.7 | 3.9 |
| EGP-3b | 30.6 | 0.8 | 3.1 | 1.1 × 105 | 1.9 | 64.5 | 7.4 | 12.5 | 9.5 | 6.1 |
EGP-1a was totally composed of Glc and could react with I2-KI suggesting that it also is a starch-like glucan. However, it was not eluted along with EGP-N on the DEAE-cellulose column, possibly the result of incomplete elution with water. Although EGP-1b also contained mostly Glc (75.5%), it did not react with I2-KI, indicating that another type of glucan, other than starch, comprised this fraction. In addition, EGP-1b might contain minor RG-I type pectin, as GalA (2.3%), Rha (1.1%), Gal (11.2%) and Ara (9.9%) were present, which were typical for RG-I type pectin.9
EGP-2a and EGP-3a had high weight average molecular weights of 4.2 × 105 and 4.3 × 105 Da, respectively, whereas EGP-2b and EGP-3b had low molecular weights of 1.5 × 105 and 1.1 × 105 Da, respectively. These four fractions were all primarily composed of GalA, Gal, Ara and Rha, with minor Glc, suggesting they were all pectic polysaccharides. Although their Rha contents were similar (7–8.7%), their GalA composition was significantly different (32.7% to 64.5%, Table 2). Because their GalA/Rha molar ratios were all higher than 1.0 (4.1, 6.6, 6.1 and 8.7 for EGP-2a, EGP-2b, EGP-3a and EGP-3b, respectively), we concluded that both RG-I and HG were present in these fractions. The molar ratio of GalA/Rha is usually considered to reflect the ratio of HG/RG-I present within a pectin sample.27 In our case, the GalA/Rha molar ratios increased in the order EGP-2a < EGP-3a < EGP-2b < EGP-3b, indicating an increase in HG content and a decrease in RG-I content through the series.
The compositions of Gal and Ara in EGP-2a and EGP-2b were high, whereas they were low in EGP-3a and EGP-3b (Table 2). The molar ratio of Gal + Ara/Rha could roughly reflect the length of side chain in RG-I domain.11 For EGP-2a, EGP-2b, EGP-3a and EGP-3b, we found that the Gal + Ara/Rha ratios were 6.9, 6.1, 4.0 and 3.0, respectively, indicating the length of side chains in these fractions were in the same order. In this regard, among these four fractions, EGP-2a contained higher proportion of RG-I domains with longer side chains and lower proportion of HG domains. In contrast, EGP-3b contained lower proportion of RG-I domains with shorter side chains and higher proportion of HG domains. EGP-2b and EGP-3a had moderate proportion of RG-I and HG domains. In these four pectic polysaccharides, we found that the DM was relatively low (≤7.6%), which is consistent with the fact that HG-type pectin with relatively low DM may readily cross-link via Ca2+ bridges and therefore could be extracted by chelating agent.22 Meanwhile, RG-I domains with neutral side chains were also extracted by EDTA, which might be due to the covalent linkage between RG-I and HG or to some specific effect of EDTA other than the chelating effects.28 Similar results were reported for mature orange fruit albedo29 and apple cell walls,30 where branched RG domains were extracted with chelating agent.
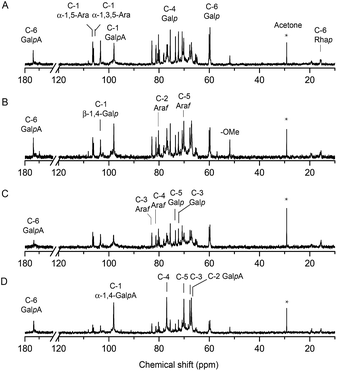
The structure of EGP-2a, EGP-2b, EGP-3a and EGP-3b were further analyzed by 13C NMR spectrum (Fig. 3), and the chemical shift assignments were listed in Table 3. The spectra of the four fractions were similar to each other. As can be seen, six signals at around 97.95, 67.09, 67.76, 76.88, 70.18 and 174.12 ppm were attributed to C-1, C-2, C-3, C-4, C-5 and C-6 of α-1,4-GalA, respectively,31 whereas the two signals at 98.15 and 15.47 ppm were assigned to C-1 and C-6 of α-1,2-Rha.11 The presence of these resonances confirmed the existence of both HG and RG-I domains in these fractions. We attributed the very weak signal at 51.81 ppm to methyl groups from methyl-esterified α-1,4-GalA residues,32 consistent with the relatively low DM content determined by FT-IR. The two anomeric carbon signals at about 106.39 and 106.05 ppm were attributed to the C-1 of α-1,5-Ara and α-1,3,5-Ara, respectively, and the signals at 82.85 ppm and 78.09 ppm were assigned to the C-3 carbons of α-1,3,5-Ara and α-1,5-Ara, respectively. We associated the low intensity signal at 108.18 ppm in EGP-2b and EGP-3b with the presence of a t-α-Araf anomer. β-1,4-Linked Gal residues displayed six signals at 103.31, 70.48, 72.26, 75.52, 73.46 and 60.06 ppm, associated with their C-1 to C-6 carbons. These results confirmed that the RG-I domains in these fractions branched with α-1,5/1,3,5-arabinan and β-1,4-galactan side chains.33
| Fraction | Sugar residues | Chemical shifts, δ (ppm) | |||||
|---|---|---|---|---|---|---|---|
| C-1 | C-2 | C-3 | C-4 | C-5 | C-6 | ||
| EGP-2a | →4)-α-GalpA-(1→ | 97.95 | 67.09 | 67.76 | 76.88 | 70.18 | 174.12 |
| →4)-β-Galp-(1→ | 103.31 | 70.48 | 72.26 | 75.52 | 73.46 | 60.06 | |
| →5)-α-Araf-(1→ | 106.39 | 80.22 | 78.09 | 81.18 | 70.79 | ||
| →3,5)-α-Araf-(1→ | 106.05 | 79.82 | 82.85 | 80.55 | 70.79 | ||
| →2)-α-Rhap-(1→ | 98.15 | — | — | — | — | 15.47 | |
| EGP-2b | →4)-α-GalpA-(1→ | 98.00 | 67.07 | 67.71 | 76.89 | 70.14 | 174.02 |
| →4)-β-Galp-(1→ | 103.32 | 70.48 | 72.27 | 75.52 | 73.46 | 60.06 | |
| →5)-α-Araf-(1→ | 106.39 | 80.22 | 78.07 | 81.19 | 70.79 | ||
| →3,5)-α-Araf-(1→ | 106.05 | 79.83 | 82.86 | 80.56 | 70.79 | ||
| t-α-Araf-(1→ | 108.18 | 80.50 | 77.40 | 80.39 | 59.70 | ||
| →2)-α-Rhap-(1→ | 99.33 | — | — | — | — | 15.51 | |
| EGP-3a | →4)-α-GalpA-(1→ | 98.10 | 67.09 | 67.65 | 76.95 | 70.05 | 173.67 |
| →4)-β-Galp-(1→ | 103.32 | 70.48 | 72.28 | 75.53 | 73.47 | 60.07 | |
| →5)-α-Araf-(1→ | 106.40 | 80.23 | 78.09 | 81.20 | 70.80 | ||
| →3,5)-α-Araf-(1→ | 106.06 | 79.83 | 82.86 | 80.30 | 70.80 | ||
| →2)-α-Rhap-(1→ | 98.16 | — | — | — | — | 15.50 | |
| EGP-3b | →4)-α-GalpA-(1→ | 98.07 | 67.01 | 67.72 | 76.95 | 70.10 | 173.75 |
| →4)-β-Galp-(1→ | 103.31 | 70.50 | 72.27 | 75.75 | 73.46 | 60.09 | |
| →5)-α-Araf-(1→ | 106.41 | 80.23 | 78.12 | 81.19 | 70.81 | ||
| →3,5)-α-Araf-(1→ | 106.07 | 79.84 | 82.87 | 80.86 | 70.81 | ||
| t-α-Araf-(1→ | 108.14 | 80.83 | 77.50 | 80.40 | 59.70 | ||
| →2)-α-Rhap-(1→ | 98.12 | — | — | — | — | 15.51 | |
The chemical structures of polysaccharides depend on how these glycans were extracted from their source material.34 In the present study, chelating reagent EDTA was used to extract polysaccharides from ginseng root after hot water and α-amylase extraction. The obtained polysaccharide EGP was fractionated by ion-exchange and size-exclusion chromatography, like hot water extracted polysaccharide WGP9 and α-amylase extracted polysaccharide WGPE21 in our previous studies, giving a major neural fraction EGP-N and four major pectic polysaccharides. EGP-N was identified to be a starch-like glucan, similar to the neutral fraction WGPN in WGP and WGPE-N in WGPE. The content of EGP-N was 41.5% in EGP, which was lower than that of WGPN (60.5%) in WGP and WGPE-N (76.0%) in WGPE, suggesting that less starch-like glucans were extracted by EDTA. It is possible that most of the starch-like glucans had already been extracted by using hot water and α-amylase.
Structural analysis indicated that the four major pectic polysaccharides EGP-2a, EGP-2b, EGP-3a and EGP-3b contained both HG and RG-I domains, that were likely to be covalently linked. The total contents of HG and RG-I domains in EGP were 26.0%. These fractions contained GalA in the range of 32.7% to 64.5%, with DM lower than 7.6%. The molar ratios of GalA/Rha in these fractions were from 4.1 to 8.7. Their molecular weights were between 1.1 × 105 and 4.3 × 105 Da. Compared with the pectic polysaccharides in EGP, two AG domains (WGPA-1-RG and WGPA-2-RG), two RG-I domains (WGPA-3-RG and WGPA-4-RG), and four HG domains (WGPA-1-HG to WGPA-4-HG) were purified from WGP, that accounted for only 1.2%, 1.4% and 6.1% in WGP, respectively. Therefore, the total pectin content in WGP was lower than that in EGP. The percentage of GalA in pectic fractions from WGP ranges widely from 1.8% to 92.1%, and the GalA/Rha molar ratios were between 1.3 and 184. The DMs of the HG fractions were from 5.0% to 30.0%, higher than those in EGP fractions. Their molecular weights were between 3.5 × 103 and 3.3 × 105 Da, lower than some of the fractions in EGP. For WGPE, two RG-I domains (WGPE-2a and WGPE-3a) and two HG domains (WGPE-2b and WGPE-3b) were purified, which accounted for 4.8% and 5.1% in WGPE, respectively, also lower than the contents of pectic polysaccharides in EGP. The contents of GalA in these fractions were between 13.7% and 81.7%, with DM from 5.0% to 32.0%, higher than those in EGP fractions. Their molecular weights were between 1.2 × 104 and 4.3 × 105 Da, a little lower than some of the fractions in EGP. The structural information of EGP, WGP and WGPE were concluded in Table 4.
| Fraction | GalA (%) | GalA/Rha | DM (%) | Mw (Da) | Pectin typeb | Contentc (%) | |
|---|---|---|---|---|---|---|---|
| a EGP: EDTA-soluble Ginseng Polysaccharide; WGP: Water-soluble Ginseng Polysaccharide extracted by hot water; WGPE: Water-soluble Ginseng Polysaccharide extracted by Enzyme.b The dominant pectin type in different fractions.c The contents of different fractions were calculated in relation to EGP, WGP or WGPE.d HG–RG-I: both HG and RG-I were present and dominant in one fraction.e —: not detected.f ND: not determined. | |||||||
| EGP | EGP-2a | 32.7 | 4.1 | 1.7 | 4.2 × 105 | HG-RG-Id | 18.6 |
| EGP-2b | 46.5 | 6.6 | 7.6 | 1.5 × 105 | HG-RG-I | 6.0 | |
| EGP-3a | 52.8 | 6.1 | 4.5 | 4.3 × 105 | HG-RG-I | 0.6 | |
| EGP-3b | 64.5 | 8.7 | 3.1 | 1.1 × 105 | HG-RG-I | 0.8 | |
| WGP9 | WGPA-1-RG | 1.8 | 9.0 | —e | 1.0 × 105 | AG | 0.3 |
| WGPA-2-RG | 5.3 | 1.3 | —e | 1.1 × 105 | AG | 0.9 | |
| WGPA-3-RG | 20.2 | 2.8 | NDf | 3.1 × 105 | RG-I | 0.7 | |
| WGPA-4-RG | 38.4 | 3.3 | NDf | 3.3 × 105 | RG-I | 0.7 | |
| WGPA-1-HG | 62.4 | 39.0 | 30.0 | 3.5 × 103 | HG | 0.7 | |
| WGPA-2-HG | 83.6 | 27.9 | 20.0 | 6.5 × 103 | HG | 2.5 | |
| WGPA-3-HG | 90.9 | 60.6 | 10.0 | 1.6 × 104 | HG | 2.2 | |
| WGPA-4-HG | 92.1 | 184.0 | 5.0 | 4.5 × 104 | HG | 0.7 | |
| WGPE21 | WGPE-2a | 13.7 | 1.7 | 32.0 | 4.3 × 105 | RG-I | 3.9 |
| WGPE-2b | 62.2 | 13.5 | 27.0 | 1.2 × 104 | HG | 3.2 | |
| WGPE-3a | 34.9 | 3.1 | 10.0 | 4.2 × 105 | RG-I | 0.9 | |
| WGPE-3b | 81.7 | 24.0 | 5.0 | 5.0 × 104 | HG | 1.9 | |
According to our results, more pectin could be extracted from ginseng by EDTA compared to hot water and α-amylase extraction. EDTA-extracted pectin contained moderate contents of GalA with lower DM and displayed higher weight average molecular weights. HG and RG-I domains might be linked together to be extracted with EDTA, unlike some free HG domains could be extracted by hot water or α-amylase, such as WGPA-4-HG in WGP and WGPE-3b in WGPE. It is known that some free pectin with relatively high DM, generally located in the middle lamella of the cell walls, can be easily extracted with water.35 Previous result showed that abundant starch granules existed in ginseng roots which interfered with the extraction of some pectin,36 and α-amylase extraction could release these pectin by destroying starch granules. Moreover, extraction with chelating reagent is thought to release low DM pectin bound by Ca2+ in the cell walls.35 Therefore, due to the extraction conditions were various, different structures of pectin could be extracted from ginseng roots.
3.4 In vivo lymphocyte proliferation activity
Lymphocyte proliferation is a crucial event in the activation cascade of both humoral and cellular immune responses. Here, we used ConA-induced and LPS-induced spleen lymphocyte proliferation to evaluate T and B lymphocyte activities,37 respectively, and assessed the effects from treatment with EGP, WGP and WGPE in vivo. As shown in Table 5, all three polysaccharide fractions could significantly enhance T lymphocyte proliferation at 10 and 50 mg kg−1; for B lymphocyte proliferation, WGP showed significant stimulating activity at 50 mg kg−1, while WGPE and EGP showed significant stimulating activity at both 10 and 50 mg kg−1. A dose-dependent induction of T and B lymphocyte proliferation was observed over the dose range tested, with potency being ranked as EGP > WGPE > WGP. This ranking may be caused by the structural differences among these fractions. Previous results indicated that starch-like polysaccharides and RG-I-type pectin with AG side chains might be responsible for lymphocyte proliferation activity of polysaccharides.9 Although EGP contained less starch-like polysaccharides than WGPE and WGP, it contained the most abundant content of RG-I-type pectin. As EGP showed relatively higher lymphocyte proliferation activity than the other two polysaccharide fractions, we speculated that RG-I-type pectin might play a major role in lymphocyte proliferation.| Dose (mg kg−1) | Lymphocyte | ||
|---|---|---|---|
| T cell (A570) | B cell (A570) | ||
| a Lymphocyte proliferation was expressed as absorption at 570 nm. Data are mean ± SD values based on 10 mice in each group. Significant differences from the control group were evaluated using Student's t test. *P < 0.05, significantly different from the control; **P < 0.01, significantly different from the control. | |||
| Control | NaCl | 0.174 ± 0.02 | 0.140 ± 0.05 |
| WGP | 1 | 0.186 ± 0.02 | 0.149 ± 0.03 |
| 5 | 0.193 ± 0.03 | 0.167 ± 0.02 | |
| 10 | 0.221 ± 0.02* | 0.197 ± 0.01 | |
| 50 | 0.254 ± 0.01** | 0.238 ± 0.02* | |
| WGPE | 1 | 0.198 ± 0.02 | 0.151 ± 0.03 |
| 5 | 0.215 ± 0.03 | 0.163 ± 0.02 | |
| 10 | 0.232 ± 0.02* | 0.248 ± 0.01* | |
| 50 | 0.291 ± 0.01** | 0.274 ± 0.02** | |
| EGP | 1 | 0.217 ± 0.04 | 0.179 ± 0.02 |
| 5 | 0.237 ± 0.02 | 0.219 ± 0.02 | |
| 10 | 0.303 ± 0.02** | 0.287 ± 0.03** | |
| 50 | 0.362 ± 0.01** | 0.327 ± 0.02** | |
4. Conclusions
In summary, the polysaccharide fraction EGP (6.0% yield) was extracted from hot water and α-amylase-extracted ginseng roots by EDTA. EGP was fractionated into one major neutral fraction (EGP-N) and four major acidic fractions (EGP-2a, EGP-2b, EGP-3a and EGP-3b). EGP-N was found to be a starch-like glucan, with minor AG. The four pectic fractions contained both RG-I and HG domains, but with different monosaccharide composition and weight average molecular weight. EGP could significantly stimulate T and B lymphocyte proliferation in vivo. Our results provide new insight into the extraction of polysaccharides from ginseng roots and highly useful information that could be exploited in the food and pharmaceutical industries.Acknowledgements
This work was supported by National Natural Science Foundation of China (No.: 31470798 and 31500274), the Doctoral Fund of Ministry of Education of China (20120043130001) and the Fundamental Research Funds for the Central Universities (No.: 2412015KJ019).References
- T. K. Yun, Lancet Oncol., 2001, 2, 49–55 CrossRef CAS PubMed.
- Y. S. Ovodov and T. F. Solov’eva, Khim. Prir. Soedin., 1966, 2, 299–303 CAS.
- J. Y. Song, S. K. Han, E. H. Son, S. N. Pyo, Y. S. Yun and S. Y. Yi, Int. Immunopharmacol., 2002, 2, 857–865 CrossRef CAS PubMed.
- J. H. Lee, J. S. Shim, J. S. Lee, M. K. Kim, M. S. Chung and K. H. Kim, Carbohydr. Res., 2006, 341, 1154–1163 CrossRef CAS PubMed.
- D. H. Luo and B. S. Fang, Carbohydr. Polym., 2008, 72, 376–381 CrossRef CAS.
- S. H. Baek, J. G. Lee, S. Y. Park, O. N. Bae, D. H. Kim and J. H. Park, Biomacromolecules, 2010, 11, 2044–2052 CrossRef CAS PubMed.
- C. Li, J. P. Cai, J. S. Geng, Y. H. Li, Z. Y. Wang and R. Li, Int. J. Biol. Macromol., 2012, 51, 968–973 CrossRef CAS PubMed.
- X. Zhou, H. Y. Shi, G. N. Jiang, Y. G. Zhou and J. F. Xu, Tumor Biol., 2014, 35, 12561–12566 CrossRef CAS PubMed.
- X. Zhang, L. Yu, H. T. Bi, X. H. Li, W. H. Ni, H. Han, N. Li, B. Q. Wang, Y. F. Zhou and G. H. Tai, Carbohydr. Polym., 2009, 77, 544–552 CrossRef CAS.
- Y. Y. Fan, H. R. Cheng, S. S. Li, J. Wang, D. Liu, M. Hao, X. G. Gao, E. X. Fan, G. H. Tai and Y. F. Zhou, Carbohydr. Polym., 2010, 81, 340–347 CrossRef CAS.
- L. Yu, X. Zhang, S. S. Li, X. Y. Liu, L. Sun, H. B. Liu, J. Iteku, Y. F. Zhou and G. H. Tai, Carbohydr. Polym., 2010, 79, 811–817 CrossRef CAS.
- X. G. Gao, Y. Zhi, L. Sun, X. X. Peng, T. Zhang, H. T. Xue, G. H. Tai and Y. F. Zhou, J. Biol. Chem., 2013, 288, 33953–33965 CrossRef CAS PubMed.
- H. R. Cheng, S. S. Li, Y. Y. Fan, X. G. Gao, M. Hao, J. Wang, X. Y. Zhang, G. H. Tai and Y. F. Zhou, Med. Oncol., 2011, 28, 175–181 CrossRef CAS PubMed.
- W. H. Ni, X. Zhang, B. Wang, Y. Chen, H. Han, Y. Y. Fan, Y. F. Zhou and G. H. Tai, J. Med. Food, 2010, 13, 270–277 CrossRef CAS PubMed.
- J. Wang, S. S. Li, Y. Y. Fan, Y. Chen, D. Liu, H. R. Cheng, X. G. Gao and Y. F. Zhou, J. Ethnopharmacol., 2010, 130, 421–423 CrossRef CAS PubMed.
- J. Wang, S. Flaisher-Grinberg, S. S. Li, H. B. Liu, L. Sun, Y. F. Zhou and H. Einat, J. Ethnopharmacol., 2010, 132, 65–69 CrossRef CAS PubMed.
- H. G. Chen, X. Zhou and J. Z. Zhang, Carbohydr. Polym., 2014, 111, 567–575 CrossRef CAS PubMed.
- Y. Zhu, Q. Li, G. H. Mao, Y. Zou, W. W. Feng, D. H. Zheng, W. Wang, L. L. Zhou, T. X. Zhang, J. Yang, L. Q. Yang and X. Y. Wu, Carbohydr. Polym., 2014, 101, 606–613 CrossRef CAS PubMed.
- Q. H. You, X. L. Yin and Y. P. Zhao, Carbohydr. Polym., 2013, 98, 607–610 CrossRef CAS PubMed.
- X. L. Yin, Q. H. You and Z. H. Jiang, Carbohydr. Polym., 2011, 86, 1358–1364 CrossRef CAS.
- L. Sun, D. Wu, X. Ning, G. Yang, Z. H. Lin, M. H. Tian and Y. F. Zhou, Int. J. Biol. Macromol., 2015, 75, 152–157 CrossRef CAS PubMed.
- X. J. Guo, H. Y. Duan, C. Wang and X. S. Huang, J. Agric. Food Chem., 2014, 62, 6354–6361 CrossRef CAS PubMed.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. A. Rebers and F. Smith, Anal. Chem., 1956, 28, 350–356 CrossRef CAS.
- N. Blumenkrantz and G. Asboe-Hansen, Anal. Biochem., 1973, 54, 484–489 CrossRef CAS PubMed.
- J. J. Sedmark and S. E. Grossberg, Anal. Biochem., 1979, 79, 544–552 CrossRef.
- M. A. Monsoor, U. Kalapathy and A. Proctor, Food Chem., 2001, 74, 233–238 CrossRef CAS.
- M. Kaya, A. G. Sousa, M. J. Crepeau, S. O. Sørensen and M. C. Ralet, Ann. Bot., 2014, 114, 1319–1326 CrossRef PubMed.
- M. M. H. Huisman, C. T. M. Fransen, J. P. Kamerling, J. F. G. Vliegenthart, H. A. Schols and A. G. J. Voragen, Biopolymers, 2001, 58, 279–294 CrossRef CAS PubMed.
- I. Prabasari, F. Pettolino, M. L. Liao and A. Bacic, Carbohydr. Polym., 2011, 84, 484–494 CrossRef CAS.
- H. A. Schols, E. Vierhuis, E. J. Bakx and A. G. J. Voragen, Carbohydr. Res., 1995, 275, 343–360 CrossRef CAS PubMed.
- Q. Dong, X. Liu, J. Yao, X. T. Dong, C. Ma, Y. X. Xu, J. N. Fang and K. Ding, Phytochemistry, 2010, 71, 1430–1437 CrossRef CAS PubMed.
- Y. J. Wang, X. Q. Liu, J. Z. Zhang, G. P. Liu, Y. Liu, K. M. Wang, M. Yang, H. L. Cheng and Z. X. Zhao, RSC Adv., 2015, 5, 7860–7867 RSC.
- D. Wefers, C. E. Tyl and M. Bunzel, J. Agric. Food Chem., 2015, 63, 707–715 CrossRef CAS PubMed.
- M. Y. Li, S. Pattathil, M. G. Hahncde and D. B. Hodge, RSC Adv., 2014, 4, 17282–17292 RSC.
- E. Taboada, P. Fisher, R. Jara, E. Zúñiga, M. Gidekel, J. C. Cabrera, E. Pereira, A. Gutiérrez-Moraga, R. Villalong and G. Cabrera, Food Chem., 2010, 123, 669–678 CrossRef CAS.
- L. Yu, Y. F. Zhou and J. P. Knox, Planta, 2011, 234, 487–499 CrossRef CAS PubMed.
- D. K. Sharma, M. R. Lambu, T. Sidiq, A. Khajuria, A. K. Tripathi, S. K. Yousuf and D. Mukherjee, RSC Adv., 2013, 3, 11450–11455 RSC.
| This journal is © The Royal Society of Chemistry 2016 |